DOI:
10.1039/C7RA08817E
(Paper)
RSC Adv., 2017,
7, 49810-49816
Fused multifunctionalized isoindole-1,3-diones via the coupled oxidation of imidazoles and tetraynes†
Received
9th August 2017
, Accepted 13th October 2017
First published on 25th October 2017
Abstract
The facile synthesis of fused multifunctionalized isoindole-1,3-diones by a hexadehydro-Diels–Alder domino reaction of various substituted tetraynes and imidazole derivatives is reported. The overall transformation involved the formation of three new C–C bonds and two new Caryl–O bonds via intramolecular cyclization and intermolecular coupling oxidation reactions.
Introduction
Isoindole-1,3-dione derivatives, commonly known as phthalimides, represent an important class of biological and pharmaceutical compounds, including indole alkaloids.1 Multifunctionalized isoindole-1,3-dione derivatives are widespread structural motifs in a plethora of different natural products.2 They are usually synthesized by the condensation of a phthalic anhydride with primary amines. An efficient strategy to construct isoindole-1,3-dione building blocks is convenient for the preparation of multifunctionalized isoindole-1,3-dione cores,3 and various synthetic methods have been documented in the literature.4 Sheykhan reported a method to form two C–C bonds via consecutive activation of four C–H bonds in the reaction of maleimides/maleic anhydride with styrenes.5 Bhanage presented a CO-free protocol for the synthesis of isoindole-1,3-diones.6 Zhang reported the reaction of benzyne with N-substituted imidazoles to afford arylamines containing anthracene.7 Zhang reported the cobalt-catalyzed carbonylation of C(sp2)–H bonds with azodicarboxylate as the carbonyl source.8 Although alkylarenes are generally thought to be relatively easy to oxygenate, the development of a selective, high-yield reaction to convert isoindole-1,3-dione into fused highly substituted isoindole-1,3-dione derivatives remains challenge.9 The skeletal representation of the fused heterocyclic ring system shown in Scheme 1 depicts the common planes in the A–B and B–C ring system.10 Surprisingly, the benzannulation of tetraynes and imidazole derivatives in a green, waste-free transformation with a high atom economy in toluene yielded isoindole-1,3-dione derivatives as the major product. Compared with ordinary isoindole-1,3-dione derivatives, tricyclic isoindole-1,3-dione derivatives prepared in the present report have multiple rings, complex and variable structures, and a broad potential for use in chemical production and clinical medicine.
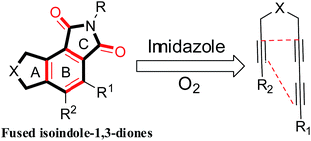 |
| | Scheme 1 Target multifunctionalized isoindole-1,3-dione core structures. | |
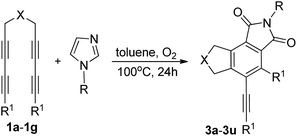
Here, we report the reaction of tetraynes with imidazole derivatives and oxygen to produce various multifunctionalized tricyclic isoindole-1,3-diones in good to excellent yields in the absence of metals, catalysts, and bases. This approach did not require directing groups and can produce highly substituted isoindole-1,3-diones in good to excellent yields via a hexadehydro-Diels–Alder (HDDA) reaction11 and intermolecular oxygen-coupling oxidation (1a–g) (Table 1). Thus, this method provides a direct, efficient, and economical way of constructing tricyclic isoindole-1,3-dione compounds.
Table 1 One-pot formation of fused isoindole-1,3-dionesa,b
|
| Reaction conditions: 1a–g (1.0 equiv.), imidazole (1.1 equiv.), toluene (2 mL), 100 °C. Yield of the isolated product after flash column chromatography. |
 |
Results and discussion
The reaction of tetrayne 1a with N-ethylimidazole was used as a test experiment (Table 1). Several phenomena were observed when the experimental conditions were modified. The efficiency of the reaction was greatly enhanced by increasing the reaction temperature to 100 °C. An investigation of various catalysts indicated that palladium(II) acetate was the most effective catalytic system in the cross-coupling reactions; however, the reaction proceeded well without metal catalysts or other additives, such as bases. In addition, the reaction also worked well if not protected. But we could hardly get any product under oxygen-free conditions. By blowing oxygen through the system the best results were obtained. Thus, the following standard reaction conditions were used for the subsequent experiments: 1 equiv. of 1 was reacted with 1.1 equiv. of N-ethylimidazole vent with O2 (1 atm) at 100 °C for 24 h.
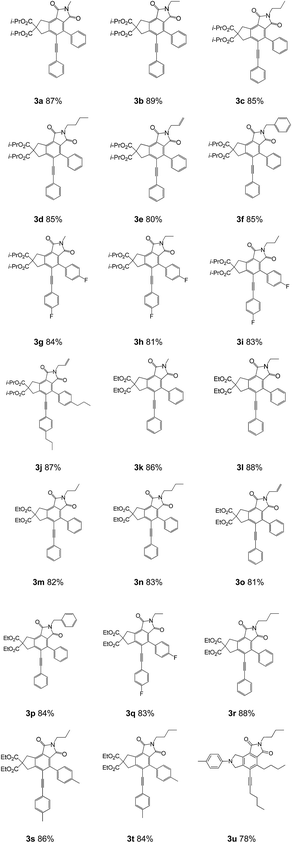
Illustrative examples of the scope of this study are presented in Table 1. Various substituted tetraynes were compatible with this reaction. Compounds ranging from diisopropyl 2-methyl-1,3-dioxo-4-phenyl-5-(phenylethynyl)-2,3,6,8-tetrahydrocyclopenta[e]isoindole-7,7(1H)-dicarboxylate to 2,4-dibutyl-5-(hex-1-yn-1-yl)-7-(p-tolyl)-7,8-dihydropyrrolo[3,4-e]isoindole-1,3(2H,6H)-dione were readily isolated with good to excellent yields from various substituted tetraynes. The substituents in the aryl ring of the tetraynes could be either electron donating or electron withdrawing. For instance, methyl, n-propyl and fluoro groups were all acceptable. Substituents, such as methyl, ethyl, propyl, n-butyl, benzyl, and allyl, in the N-substituted imidazoles were also suitable for the reaction. Reactions of different tetraynes with various substituted imidazoles and oxygen afforded the 4-phenyl-5-(phenylethynyl)-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione cores in excellent yields (for instance, Table 1 indicates that 3a, 3b, 3j, 3k, 3l, 3r, and 3s had yields up to 85%). Compound 3b was produced in the highest isolated yield (89%) among the examined products. Compounds 3e, 3h, 3i, 3m, 3o, 3q, and 3t were also obtained in good yields between 80-85%. These results demonstrate the potential of the direct functionalization of existing unsaturated hydrocarbons with substituted imidazoles and oxygen for the synthesis of multifunctionalized cyclopenta[e]isoindole-1,3(2H,6H)-diones. Using another substrate, alkyl-substituted N-tetrayne (3u, 78%), instead of alkyl-substituted C-tetrayne also generated the corresponding product in good yield.
All the resulting tricyclic isoindole-1,3-dione compounds were verified via various spectroscopic techniques (1H NMR, 13C NMR, IR spectroscopy and/or HRMS). The molecular structures and relative configurations of 3g and 4a were confirmed unambiguously by X-ray diffraction (Fig. 1). Further details are provided in the ESI.†12
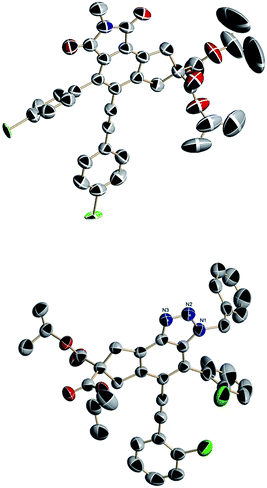 |
| | Fig. 1 Molecular structure of the fused isoindole-1,3-dione 3g (top) and the molecular structure of the trapped intermediate 4a (bottom). | |
Scheme 2 indicates the sequence of steps involved in the synthesis of highly substituted tricyclic isoindole-1,3-diones by a cascade HDDA reaction, the formation of an intermolecular cycloaddition and an oxidation by oxygen. A HDDA reaction of tetrayne 1 produces the aryne13 intermediate A, which subsequently reacts with the imidazole derivative by a formal [4 + 2] cycloaddition to produce the unstable bridge-ring intermediate B.14 An elimination reaction occurs on the C–N double bond in B, which was easy to be oxidized to take off HCN for isoindolones skeleton structure, followed by an oxidation reaction by oxygen to afford the final product 3. Fortunately, the aryne-trapped15 product was isolated by adding a small quantity of RN3 to the reaction system, and this product was characterized by X-ray crystallography (Fig. 1, bottom, 4a).
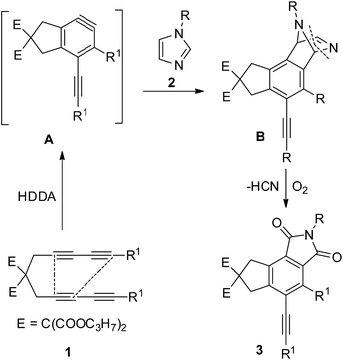 |
| | Scheme 2 Tentative mechanism for the HDDA annulation to produce tricyclic isoindole-1,3-diones 3. | |
Conclusions
In conclusion, we developed a convenient and general method for synthesizing highly substituted tricyclic isoindole-1,3-diones via the reaction of tetraynes with imidazoles and oxygen. The formation of C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds involved a cascade HDDA reaction, the formation of an intermolecular imidazole cycloaddition and an oxygen-coupling oxidation. This approach required no metals, catalysts, additives, or directing groups and could apply to a range of substrates. The reaction also exhibited excellent regioselectivity, producing all types of highly substituted tricyclic isoindole-1,3-diones under mild conditions in good to excellent yields. The primary evaluation of the photoluminescence property of the novel extended p-systems indicated that these heteroarenes could be potentially used in the field of optoelectronic materials.
O bonds involved a cascade HDDA reaction, the formation of an intermolecular imidazole cycloaddition and an oxygen-coupling oxidation. This approach required no metals, catalysts, additives, or directing groups and could apply to a range of substrates. The reaction also exhibited excellent regioselectivity, producing all types of highly substituted tricyclic isoindole-1,3-diones under mild conditions in good to excellent yields. The primary evaluation of the photoluminescence property of the novel extended p-systems indicated that these heteroarenes could be potentially used in the field of optoelectronic materials.
Experiment
General information
All the catalytic reactions were performed under an argon atmosphere using the oven-dried Schlenk flask. The chemicals were purchased from Alfa Aesar and Acros Chemicals. All solvents and materials were pre-dried, redistilled or recrystallized before use. 1H NMR (300 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker Avance 300 spectrometer with CDCl3 as the solvent. Chemical shifts are reported in ppm by assigning TMS resonance in the 1H NMR spectra as 0.00 ppm and CDCl3 resonance in the 13C spectra as 77.0 ppm. All coupling constants (J values) were reported in Hertz (Hz). Column chromatography was performed on silica gel 300–400 mesh. Melting points were determined using a Gallenkamp melting point apparatus and are uncorrected. The FT-IR spectra were recorded from KBr pellets or thin film from CHCl3 on the NaCl window in the 4000–400 cm−1 ranges on a Nicolet 5DX spectrometer. All HRMS spectra were record using EI at 70 eV. X-ray crystallography diffraction data of 3g and 4a were collected at room temperature with a Bruker SMART Apex CCD diffractometer with Mo-Kα radiation (λ = 0.71073 Å) with a graphite monochromator using the ω-scan mode. Data reductions and absorption corrections were performed with SAINT and SADABS software, respectively. The structure was solved by direct methods and refined on F2 by full-matrix least squares using SHELXTL. All non-hydrogen atoms were treated anisotropically. The positions of hydrogen atoms were generated geometrically.
General procedures
Typical experimental procedure: tetraynes (1.0 equiv.), imidazole (1.1 equiv.), were added to toluene (2 mL), the reaction was vented 3 times to eliminate argon which was connected to a tank of O2 and pressurized to 1 atm, the mixture was stirred at room temperature for half an hour and then heated at 100 °C for 10 hours. The reaction mixture was cooled to room temperature, and the solvent was evaporated in vacuo. The residue was purified by preparative thin-layer chromatography (TLC) on silica gel with the appropriate mixture of petroleum ether and ethyl acetate to give the fused multifunctionalized isoindole-1,3-diones.
Diisopropyl 2-methyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3a). White solid, 478 mg (87% yield); m.p. 210–211 °C; FT-IR (KBr): 3481, 2983, 2360, 1701, 1654, 1433, 1382, 1269, 1101, 910, 688, 486 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 2H), 7.36–7.16 (m, 8H), 5.09 (dt, J = 12.3, 6.1 Hz, 2H), 3.97 (s, 2H), 3.80 (s, 2H), 3.09 (s, 3H), 1.28 (d, J = 6.1 Hz, 12H); 13C NMR (75 MHz, C6D6) δ 170.63, 150.67, 137.13, 134.84, 131.66, 129.81, 129.04, 128.43, 127.59, 126.31, 125.39, 122.34, 100.42, 85.75, 77.48, 77.06, 76.63, 69.80, 59.96, 40.85, 38.94, 23.83, 21.57 ppm; HRMS (APCI): m/z [M + H]+ calcd for C34H31NO6: 550.2220; found: 551.2251.
Diisopropyl 2-ethyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3b). White solid, 501 mg (89% yield); m.p. 220–221 °C; FT-IR (KBr): 3456, 2980, 2208, 1766, 1710, 1442, 1400, 1261, 1190, 1101, 908, 758, 688, 493 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 2H), 7.26 (dd, J = 13.4, 7.5 Hz, 8H), 5.10 (dt, J = 12.4, 6.2 Hz, 2H), 3.97 (s, 1H), 3.80 (s, 1H), 3.65 (dd, J = 14.1, 6.9 Hz, 4H), 1.39–1.10 (m, 15H); 13C NMR (75 MHz, MeOD) δ 170.67, 167.45, 150.61, 141.30, 137.09, 134.86, 131.66, 129.84, 129.03, 128.41, 127.70, 126.35, 125.34, 122.36, 100.35, 85.79, 77.71, 76.76, 76.62, 69.79, 59.94, 40.84, 38.92, 32.84, 21.57, 13.87 ppm; HRMS (APCI): m/z [M + H]+ calcd for C35H33NO6: 564.2375; found: 565.2407.
Diisopropyl 1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2-propyl-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3c). White solid, 491 mg (85% yield); m.p. 164–165 °C; FT-IR (KBr): 3431, 2980, 2935, 2360, 2341, 1718, 1708, 1400, 1259, 1192, 1101, 908, 881, 756, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.65 (d, J = 6.6 Hz, 4H), 7.45 (d, J = 13.2 Hz, 2H), 7.26 (dd, J = 13.5, 6.2 Hz, 4H), 5.20–5.01 (m, 2H), 3.98 (s, 2H), 3.81 (s, 2H), 3.55 (t, J = 6.4 Hz, 2H), 1.64 (d, J = 6.9 Hz, 2H), 1.28 (d, J = 5.0 Hz, 12H), 0.95 (dt, J = 30.3, 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 170.78, 167.90, 150.75, 141.42, 137.21, 134.99, 131.77, 131.07, 129.97, 129.50, 129.12, 128.52, 128.34, 127.75, 127.36, 126.80, 122.51, 85.92, 77.39, 77.17, 77.01, 69.88, 69.42, 60.08, 45.50, 41.41, 40.99, 39.69, 39.06, 22.82, 21.93, 21.68, 11.70, 11.48 ppm; HRMS (APCI): m/z [M + H]+ calcd for C36H35NO6: 577.2504; found: 577.6726.
Diisopropyl 2-butyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3d). White solid, 503 mg (85% yield); m.p. 187–188 °C; FT-IR (KBr): 3466, 2980, 2939, 2875, 2374, 1708, 1400, 1708, 1259, 1190, 1107, 921, 752, 684, 478 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 2H), 7.26 (dd, J = 13.2, 7.4 Hz, 8H), 5.10 (dt, J = 12.3, 6.1 Hz, 2H), 3.97 (s, 1H), 3.80 (s, 1H), 3.59 (t, J = 7.1 Hz, 4H), 1.67–1.51 (m, 2H), 1.28 (d, J = 6.2 Hz, 14H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, C6D6) δ 170.68, 167.79, 167.52, 150.62, 141.29, 137.08, 134.86, 131.66, 129.86, 129.03, 128.42, 127.66, 126.30, 125.34, 122.37, 100.35, 85.82, 77.50, 77.08, 76.65, 69.79, 59.93, 40.86, 38.93, 37.78, 30.56, 21.58, 20.15, 13.66 ppm; HRMS (APCI): m/z [M + H]+ calcd for C37H37NO6: 592.2687; found: 593.2721.
Diisopropyl 2-allyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3e). White solid, 460 mg (80% yield); m.p. 205–206 °C; FT-IR (KBr): 3448, 2980, 2924, 2360, 2341, 2208, 1766, 1712, 1419, 1390, 1282, 1261, 1192, 1101, 1055, 933, 918, 756, 690 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 2H), 7.31–7.17 (m, 8H), 5.82 (ddd, J = 16.0, 10.0, 5.0 Hz, 1H), 5.28–5.02 (m, 2H), 4.20 (d, J = 5.0 Hz, 4H), 3.98 (s, 2H), 3.81 (s, 2H), 1.31–1.25 (m, 12H); 13C NMR (126 MHz, CDCl3) δ 170.75, 167.37, 167.06, 150.89, 141.60, 137.39, 134.92, 131.75, 129.95, 129.15, 128.53, 127.76, 126.31, 125.65, 122.47, 118.18, 100.61, 85.88, 77.39, 77.05, 76.88, 69.90, 60.06, 41.00, 40.23, 39.06, 21.67 ppm; HRMS (APCI): m/z [M + H]+ calcd for C36H33NO6: 575.2394; found: 575.6586.
Diisopropyl 2-benzyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3f). White solid, 531 mg (85% yield); m.p. 123–125 °C; FT-IR (KBr): 3446, 2978, 2926, 2854, 1734, 1701, 1490, 1386, 1340, 1257, 1190, 1103, 1068, 910, 756, 688, 486, 459 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.81–7.16 (m, 15H), 5.08 (dd, J = 7.8, 4.6 Hz, 2H), 4.75 (s, 1H), 4.44 (s, 1H), 3.97 (s, 2H), 3.80 (s, 2H), 1.29–0.82 (m, 12H); 13C NMR (126 MHz, CDCl3) δ 171.16, 170.72, 169.62, 150.90, 132.71, 132.00, 131.64, 130.10, 129.15, 128.92, 128.91, 126.79, 127.83, 127.74, 127.83, 126.79, 127.74, 126.95, 126.31, 126.05, 125.68, 122.04, 77.28, 77.14, 76.88, 69.87, 69.41, 60.06, 41.81, 40.98, 39.06, 29.81, 21.65, 21.38 ppm; HRMS (APCI): m/z [M + H]+ calcd for C40H35NO6: 625.2519; found: 625.7156.
Diisopropyl 4-(4-fluorophenyl)-5-(2-(4-fluorophenyl)ethynyl)-2-methyl-1,3-dioxo-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3g). White solid, 491 mg (84% yield); m.p. 234–235 °C; FT-IR (KBr): 3446, 2981, 2360, 1722, 1629, 1382, 1269, 1101, 833, 484 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.42 (dd, J = 19.0, 8.2 Hz, 4H), 7.35–7.22 (m, 2H), 7.16 (d, J = 8.2 Hz, 2H), 5.09 (dt, J = 12.2, 6.0 Hz, 2H), 3.97 (s, 2H), 3.77 (s, 2H), 3.09 (s, 3H), 1.28 (d, J = 6.1 Hz, 12H); 13C NMR (75 MHz, C6D6) δ 170.53, 167.42, 150.99, 137.56, 135.37, 134.67, 133.12, 132.81, 131.30, 128.88, 127.89, 126.55, 124.86, 120.55, 86.30, 77.46, 77.04, 76.62, 69.90, 59.94, 40.81, 38.92, 23.90, 21.56 ppm; HRMS (APCI): m/z [M + H]+ calcd for C34H29F2NO6: 586.2033; found: 587.2065.
Diisopropyl 2-ethyl-4-(4-fluorophenyl)-5-(2-(4-fluorophenyl)ethynyl)-1,3-dioxo-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3h). White solid, 485 mg (81% yield); m.p. 223–225 °C; FT-IR (KBr): 3448, 2981, 2933, 1764, 1726, 1708, 1506, 1436, 1400, 1350, 1259, 1232, 1190, 1101, 1053, 840, 761, 520 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.49–7.39 (m, 4H), 7.30–7.10 (m, 2H), 6.99 (t, J = 8.2 Hz, 2H), 5.09 (dt, J = 12.1, 6.0 Hz, 2H), 3.96 (s, 2H), 3.77 (s, 2H), 3.65 (dd, J = 13.7, 6.7 Hz, 2H), 1.26 (t, J = 8.8 Hz, 12H), 1.20 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 170.72, 167.46, 150.86, 140.13, 137.46, 133.72, 131.91, 128.02, 126.54, 125.29, 116.06, 115.89, 114.87, 114.68, 99.52, 85.44, 77.40, 77.15, 76.89, 69.97, 60.07, 40.93, 39.02, 33.00, 21.66, 13.94 ppm; HRMS (APCI): m/z [M + H]+ calcd for C35H31F2NO6: 599.2124; found: 599.6244.
Diisopropyl 4-(4-fluorophenyl)-5-(2-(4-fluorophenyl)ethynyl)-1,3-dioxo-2-propyl-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3i). White solid, 509 mg (83% yield); m.p. 184–186 °C; FT-IR (KBr): 3448, 2978, 2937, 2210, 1718, 1602, 1458, 1436, 1400, 1382, 1344, 1269, 1193, 1155, 1101, 1060, 906, 881, 839, 756, 657, 524, 399 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.51–7.37 (m, 2H), 7.29–7.11 (m, 4H), 7.05–6.94 (m, 2H), 5.09 (dd, J = 7.7, 4.5 Hz, 2H), 3.97 (s, 2H), 3.78 (s, 2H), 3.56 (d, J = 7.7 Hz, 2H), 1.64 (d, J = 7.0 Hz, 2H), 1.28 (dd, J = 5.8, 3.1 Hz, 12H), 0.90 (dd, J = 8.8, 5.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 170.71, 167.73, 150.87, 137.47, 133.69, 131.88, 130.78, 127.93, 125.30, 118.43, 115.97, 114.76, 77.38, 77.00, 76.76, 76.41, 69.95, 60.07, 40.96, 39.74, 39.04, 21.92, 21.66, 11.46 ppm; HRMS (APCI): m/z [M + H]+ calcd for C36H33F2NO6: 613.2313; found: 613.6587.
Diisopropyl 2-allyl-1,3-dioxo-4-(4-propylphenyl)-5-(2-(4-propylphenyl)ethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3j). White solid, 574 mg (87% yield); m.p. 138–139 °C; FT-IR (KBr): 3431, 2981, 2374, 1724, 1674, 1394, 1259, 1101, 920, 677, 480 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.37 (d, J = 7.6 Hz, 21H), 7.27 (d, J = 10.9 Hz, 2H), 7.10 (dd, J = 16.7, 7.7 Hz, 4H), 5.82 (d, J = 6.5 Hz, 1H), 5.30–5.03 (m, 2H), 4.20 (d, J = 4.9 Hz, 4H), 3.96 (s, 2H), 3.79 (s, 2H), 2.68 (t, J = 7.2 Hz, 2H), 2.56 (t, J = 7.3 Hz, 2H), 1.73 (dd, J = 14.4, 7.2 Hz, 2H), 1.60 (dd, J = 15.8, 8.4 Hz, 2H), 1.28 (d, J = 6.1 Hz, 12H), 0.95 (dt, J = 25.7, 7.2 Hz, 6H); 13C NMR (75 MHz, C6D6) δ 170.69, 167.25, 150.48, 144.13, 142.88, 141.64, 137.00, 132.09, 131.63, 129.77, 128.52, 127.70, 119.62, 118.00, 100.84, 85.59, 77.47, 77.04, 76.62, 69.76, 59.91, 40.88, 40.09, 38.93, 38.00, 24.43, 21.56, 13.83 ppm; HRMS (APCI): m/z [M + H]+ calcd for C42H45NO6: 660.3314; found: 661.3346.
Diethyl 2-methyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3k). White solid, 448 mg (86% yield); m.p. 195–196 °C; FT-IR (KBr): 3462, 1708, 1637, 1425, 1382, 1261, 1070, 761, 692, 472 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 2H), 7.26 (dd, J = 12.8, 7.0 Hz, 8H), 4.27 (dd, J = 14.0, 6.9 Hz, 4H), 4.01 (s, 2H), 3.84 (s, 2H), 3.09 (s, 3H), 1.30 (t, J = 7.0 Hz, 6H); 13C NMR (75 MHz, C6D6) δ 171.07, 167.76, 150.53, 141.36, 136.97, 134.80, 131.67, 129.81, 129.08, 128.45, 127.60, 126.34, 122.29, 100.48, 85.74, 77.49, 77.07, 76.64, 62.24, 59.92, 40.90, 38.97, 23.84, 14.07 ppm; HRMS (APCI): m/z [M + H]+ calcd for C32H27NO6: 522.1914; found: 523.1945.
Diethyl 2-ethyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3l). White solid, 471 mg (88% yield); m.p. 178–180 °C; FT-IR (KBr): 2926, 2854, 2360, 2341, 1734, 1707, 1637, 1400, 1384, 1257, 1186, 1105, 754, 704, 688, 478 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 4H), 7.34–7.17 (m, 6H), 4.33–4.17 (m, 4H), 4.01 (s, 2H), 3.83 (s, 2H), 3.67 (dt, J = 21.4, 7.1 Hz, 2H), 1.24 (ddd, J = 31.7, 17.1, 10.0 Hz, 9H); 13C NMR (126 MHz, CDCl3) δ 171.20, 131.77, 129.94, 129.14, 128.51, 127.69, 77.25, 77.11, 77.10, 76.87, 62.30, 41.02, 39.09, 32.96, 32.03, 29.63, 29.39, 28.78, 27.32, 22.79, 14.15, 13.95 ppm; HRMS (APCI): m/z [M + H]+ calcd for C33H29NO6: 535.2334; found: 535.5987.
Diethyl 1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2-propyl-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3m). White solid, 450 mg (82% yield); m.p. 177–178 °C; FT-IR (KBr): 3446, 2968, 2935, 2875, 1759, 1734, 1707, 1440, 1400, 1367, 1253, 1184, 1087, 1068, 866, 744, 688, 520, 476 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.48 (s, 4H), 7.26 (dd, J = 13.0, 6.2 Hz, 6H), 4.27 (dd, J = 14.0, 6.9 Hz, 4H), 4.02 (s, 2H), 3.84 (s, 2H), 3.56 (t, J = 7.1 Hz, 2H), 1.64 (dd, J = 14.2, 7.1 Hz, 2H), 1.30 (t, J = 7.0 Hz, 6H), 0.90 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 171.13, 167.66, 150.50, 141.32, 136.92, 134.78, 131.67, 129.86, 129.08, 128.44, 127.59, 126.30, 125.37, 122.31, 100.40, 85.79, 77.50, 77.07, 76.65, 62.25, 59.87, 40.89, 39.59, 38.96, 21.85, 14.09, 11.41 ppm; HRMS (APCI): m/z [M + H]+ calcd for C34H31NO6: 549.2223; found: 549.6165.
Diethyl 2-butyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3n). White solid, 467 mg (83% yield); m.p. 185–186 °C; FT-IR (KBr): 3446, 2960, 2935, 2873, 1757, 1730, 1707, 1440, 1398, 1363, 1278, 1253, 1186, 1159, 1089, 1068, 933, 864, 744, 688, 522, 478 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.65 (d, J = 7.4 Hz, 2H), 7.45 (d, J = 13.2 Hz, 4H), 7.32–7.20 (m, 4H), 4.26 (p, J = 6.8 Hz, 4H), 4.02 (s, 2H), 3.84 (s, 2H), 3.59 (t, J = 7.2 Hz, 2H), 1.59 (dt, J = 15.0, 7.4 Hz, 2H), 1.32 (dd, J = 14.3, 7.2 Hz, 8H), 0.89 (dd, J = 15.4, 8.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.21, 167.86, 167.57, 150.60, 141.44, 137.03, 134.96, 131.78, 129.97, 129.15, 128.52, 127.80, 126.45, 125.49, 122.47, 100.53, 85.91, 77.40, 77.15, 76.89, 62.31, 60.03, 41.03, 39.09, 37.90, 30.67, 20.25, 14.21, 13.95 ppm; HRMS (APCI): m/z [M + H]+ calcd for C35H33NO6: 563.2376; found: 563.6448.
Diethyl 2-allyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3o). White solid, 443 mg (81% yield); m.p. 196–197 °C; FT-IR (KBr): 3448, 2987, 2926, 2360, 2341, 1761, 1734, 1707, 1436, 1394, 1255, 1184, 1091, 931, 862, 758, 688, 474 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.47 (s, 4H), 7.32–7.18 (m, 6H), 5.82 (ddd, J = 15.9, 11.0, 5.8 Hz, 1H), 5.19 (dd, J = 21.2, 13.6 Hz, 2H), 4.24 (dt, J = 12.4, 6.4 Hz, 6H), 4.02 (s, 2H), 3.84 (s, 2H), 1.30 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 171.19, 167.20, 150.76, 141.63, 137.22, 134.87, 131.74, 129.95, 129.18, 128.54, 127.78, 126.33, 125.68, 122.43, 118.18, 100.67, 85.86, 77.39, 77.14, 76.88, 62.32, 60.03, 41.05, 40.23, 39.10, 14.16 ppm; HRMS (APCI): m/z [M + H]+ calcd for C34H29NO6: 547.2328; found: 547.6339.
Diethyl 2-benzyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3p). White solid, 502 mg (84% yield); m.p. 124–125 °C; FT-IR (KBr): 3448, 2956, 2924, 2852, 1718, 1707, 1637, 1560, 1396, 1259, 1078, 756, 690, 472 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.53–7.19 (m, 15H), 4.75 (s, 2H), 4.26 (dd, J = 14.2, 7.1 Hz, 4H), 4.01 (s, 2H), 3.83 (s, 2H), 1.36–1.21 (m, 6H); 13C NMR (126 MHz, CDCl3) δ 171.16, 167.50, 167.10, 150.77, 141.64, 137.25, 136.53, 134.90, 132.72, 131.78, 131.54, 129.98, 129.45, 129.31, 129.10, 129.00, 128.97, 128.82, 128.55, 128.21, 127.79, 127.69, 127.52, 126.34, 125.70, 122.42, 100.69, 85.87, 77.40, 77.15, 76.90, 62.31, 60.03, 41.71, 41.04, 39.10, 32.04, 29.82, 29.52, 27.33, 22.81, 21.91, 20.33, 14.16 ppm; HRMS (APCI): m/z [M + H]+ calcd for C38H31NO6: 597.2227; found: 597.6606.
Diethyl 2-ethyl-4-(4-fluorophenyl)-5-(2-(4-fluorophenyl)ethynyl)-1,3-dioxo-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3q). White solid, 474 mg (83% yield); m.p. 198–199 °C; FT-IR (KBr): 3446, 2374, 1718, 1637, 1500, 1400, 1085, 837, 489 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 5.6 Hz, 2H), 7.33–7.12 (m, 4H), 7.01 (d, J = 8.3 Hz, 2H), 4.26 (dd, J = 14.0, 6.9 Hz, 4H), 4.00 (s, 2H), 3.81 (s, 2H), 3.65 (d, J = 7.0 Hz, 2H), 1.30 (t, J = 6.8 Hz, 6H), 1.21 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, C6D6) δ 214.59, 214.32, 191.40, 191.13, 171.02, 167.38, 164.67, 161.34, 150.60, 140.05, 137.17, 133.62, 131.79, 130.62, 130.35, 128.44, 128.44, 124.71, 125.07, 124.71, 118.38, 117.11, 115.87, 115.20, 115.09, 114.81, 114.52, 99.46, 85.31, 79.24, 78.98, 77.73, 76.81, 76.59, 62.24, 59.91, 40.87, 38.94, 34.47, 34.21, 32.90, 13.94 ppm; HRMS (APCI): m/z [M + H]+ calcd for C33H27F2NO6: 572.1874; found: 573.1906.
Diethyl 2-butyl-1,3-dioxo-4-phenyl-5-(2-phenylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3r). White solid, 496 mg (88% yield); m.p. 153–154 °C; FT-IR (KBr): 3446, 2935, 2374, 1710, 1602, 1508, 1398, 1236, 1087, 835, 669, 468 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.43 (d, J = 5.3 Hz, 2H), 7.22 (dt, J = 17.7, 12.2 Hz, 4H), 7.00 (t, J = 8.2 Hz, 2H), 4.27 (dd, J = 13.9, 6.9 Hz, 4H), 4.01 (s, 2H), 3.81 (s, 2H), 3.59 (t, J = 7.0 Hz, 2H), 1.67–1.51 (m, 2H), 1.30 (t, J = 6.9 Hz, 8H), 0.90 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, DMSO) δ 171.03, 167.60, 167.34, 164.67, 161.47, 161.21, 150.61, 140.04, 137.16, 133.63, 131.80, 131.00, 130.61, 127.85, 126.40, 125.19, 118.43, 118.16, 116.39, 116.17, 115.87, 115.08, 114.67, 99.45, 85.31, 77.44, 76.83, 76.59, 62.24, 59.89, 52.54, 52.27, 40.88, 38.39, 37.38, 36.75, 30.55, 20.02, 14.05, 13.62 ppm; HRMS (APCI): m/z [M + H]+ calcd for C35H33NO6: 600.2186; found: 601.2217.
Diethyl 1,3-dioxo-2-propyl-4-p-tolyl-5-(2-p-tolylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3s). White solid, 496 mg (86% yield); m.p. 175–177 °C; FT-IR (KBr): 2360, 2341, 1734, 1701, 1627, 1400, 1257, 1186, 1105, 1085, 1085, 1051, 817, 759, 659, 474 cm−1; 1H NMR (300 MHz, C6D6) δ 7.37 (d, J = 7.7 Hz, 2H), 7.27 (d, J = 10.2 Hz, 2H), 7.13 (dd, J = 17.2, 7.7 Hz, 4H), 4.26 (dd, J = 14.0, 6.9 Hz, 4H), 4.00 (s, 2H), 3.82 (s, 2H), 3.55 (t, J = 7.0 Hz, 2H), 2.44 (s, 3H), 2.34 (s, 3H), 1.63 (dd, J = 14.1, 7.1 Hz, 2H), 1.30 (t, J = 7.0 Hz, 6H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 171.15, 167.73, 150.39, 141.32, 139.35, 138.20, 136.62, 131.65, 129.82, 129.13, 128.25, 127.96, 124.07, 124.71, 124.71, 119.38, 100.66, 98.74, 96.92, 98.74, 70.78, 76.59, 76.59, 62.17, 59.87, 40.94, 39.55, 38.96, 22.11, 20.90, 14.05, 11.38 ppm; HRMS (APCI): m/z [M + H]+ calcd for C36H35NO6: 577.2514; found: 577.6746.
Diethyl 2-butyl-1,3-dioxo-4-p-tolyl-5-(2-p-tolylethynyl)-2,3-dihydrocyclopenta[e]isoindole-7,7(1H,6H,8H)-dicarboxylate (3t). White solid, 497 mg (84% yield); m.p. 157–159 °C; FT-IR (KBr): 3446, 2933, 2868, 1734, 1707, 1500, 1438, 1398, 1251, 1184, 1151, 1082, 1049, 933, 858, 813, 761, 731, 663, 509 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.37 (d, J = 7.6 Hz, 2H), 7.27 (d, J = 10.7 Hz, 2H), 7.12 (dd, J = 17.0, 7.7 Hz, 4H), 4.26 (dd, J = 13.9, 6.9 Hz, 4H), 4.00 (s, 2H), 3.79 (m, 2H), 3.58 (t, J = 7.0 Hz, 2H), 2.44 (s, 3H), 2.34 (s, 3H), 1.59 (m, 2H), 1.30 (t, J = 6.9 Hz, 8H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.25, 167.93, 167.71, 150.49, 141.43, 139.45, 138.29, 136.73, 131.87, 131.69, 129.93, 129.23, 128.37, 126.29, 125.72, 119.51, 100.77, 85.58, 77.39, 77.13, 76.88, 62.27, 60.00, 41.06, 39.08, 37.87, 30.66, 21.65, 20.25, 14.16, 13.75 ppm; HRMS (APCI): m/z [M + H]+ calcd for C37H37NO6: 591.2651; found: 591.6923.
2,4-Dibutyl-5-(hex-1-ynyl)-7-N-p-tolyl-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione (3u). White solid, 389 mg (78% yield); m.p. 129–130 °C; 1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 7.9 Hz, 2H), 7.36–7.27 (m, 1H), 7.26 (s, 1H), 4.84 (s, 2H), 4.64 (s, 2H), 3.65–3.52 (m, 2H), 3.15 (d, J = 6.2 Hz, 2H), 2.57–2.44 (m, 2H), 2.40 (s, 3H), 1.65–1.24 (m, 16H), 1.01–0.83 (m, 9H); 13C NMR (126 MHz, CDCl3) δ 168.51, 167.33, 146.00, 144.81, 144.03, 133.76, 130.86, 130.06, 128.02, 125.33, 124.79, 124.45, 103.64, 102.72, 77.24, 76.81, 75.66, 75.42, 53.91, 52.76, 37.84, 32.64, 30.67, 29.13, 22.99, 21.87, 21.63, 19.89, 19.59, 14.29, 12.96 ppm; HRMS (APCI): m/z [M + H]+ calcd for C31H38N2O2: 470.2905; found: 470.6516.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors thank the National Natural Science Foundation of China (21572002, 21272005), The Research Culture Funds of Anhui Normal University (2016XJJ020), and Department of Human Resources of Anhui Province for financial support.
Notes and references
- For reviews on this topic, see:
(a) B. Baire, D. W. Niu, P. H. Willoughby, B. P. Woods and T. R. Hoye, Nat. Protoc., 2013, 8, 501–509 CrossRef CAS PubMed;
(b) S. W. M. Crossley and R. A. Shenvi, Chem. Rev., 2015, 115, 9465–9531 CrossRef CAS PubMed;
(c) S. Ziegler, V. Pries, C. Hedberg and H. Waldmann, Angew. Chem., Int. Ed., 2013, 52, 2744–2792 CrossRef CAS PubMed.
- For reviews on this topic, see:
(a) M. S. Chen and M. C. A. White, Science, 2007, 318, 783–787 CrossRef CAS PubMed;
(b) S. P. Ross and T. R. Hoye, Nat. Chem., 2017, 9, 523–530 CrossRef CAS PubMed;
(c) H. Pellissier and H. Clavier, Chem. Rev., 2014, 114, 2275–2823 CrossRef PubMed.
- For selected examples of syntheses of isoindole-1,3-diones derivatives, see:
(a) A. R. Katritzky and S. Rachwal, Chem. Rev., 2011, 111, 7063–7120 CrossRef CAS PubMed;
(b) J. R. Kong and M. J. Krische, J. Am. Chem. Soc., 2006, 128, 16040–16041 CrossRef CAS PubMed;
(c) J. Shen, T. T. Nguyen, Y. P. Goh, W. P. Ye, X. Fu, J. Y. Xu and C. H. Tan, J. Am. Chem. Soc., 2006, 128, 13692–13693 CrossRef CAS PubMed.
-
(a) X. S. Wu, Y. Zhao and H. B. Ge, J. Am. Chem. Soc., 2015, 137, 4924d–4927 CrossRef PubMed;
(b) S. Inoue, H. Shiota, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2009, 131, 6898–6899 CrossRef CAS PubMed;
(c) H. Li, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2014, 136, 6288–6296 CrossRef CAS PubMed.
- M. Sheykhan, M. Shafiee-Pour and M. Abbasnia, Org. Lett., 2017, 19, 1270–1273 CrossRef CAS PubMed.
-
(a) D. N. Sawant, Y. S. Wagh, K. D. Bhatte and B. M. Bhanage, Eur. J. Org. Chem., 2011, 33, 6719–6724 CrossRef;
(b) M. V. Khedkara, A. R. Shindea, T. Sasakib and B. M. Bhanage, J. Mol. Catal. A: Chem., 2014, 385, 91–97 CrossRef.
-
(a) C. S. Xie and Y. H. Zhang, Org. Lett., 2007, 9, 781–784 CrossRef CAS PubMed;
(b) R. Kranthikumar, R. Chegondi and S. Chandrasekhar, J. Org. Chem., 2016, 81, 2451–2459 CrossRef CAS PubMed;
(c) C. R. Wu, Y. S. Fang, R. C. Larock and F. Shi, Org. Lett., 2010, 12, 2234–2237 CrossRef CAS PubMed.
-
(a) J. Ni, J. Li, Z. Fan and A. Zhang, Org. Lett., 2016, 18, 5960–5963 CrossRef CAS PubMed;
(b) L. Grigorjeva and O. Daugulis, Angew. Chem., Int. Ed., 2014, 53, 10209–10212 CrossRef CAS PubMed.
-
(a) F. Sandfort, M. J. O'Neill, J. Cornella, L. Wimmer and P. S. Baran, Angew. Chem., Int. Ed., 2017, 56, 3319–3323 CrossRef CAS PubMed;
(b) X. G. Liu, Q. L. Qiao, W. M. Tian, W. J. Liu, J. Chen, M. J. Lang and Z. C. Xu, J. Am. Chem. Soc., 2016, 138, 6960–6963 CrossRef CAS PubMed;
(c) K. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. DiBenedetto, S. Kim, L. K. G. Ackerman and D. J. Weix, J. Am. Chem. Soc., 2016, 138, 5016–5019 CrossRef CAS PubMed.
-
(a) Y. M. Hu, Y. D. Hu, Q. Hu, J. Ma, S. Lv and S. Wang, Chem.–Eur. J., 2017, 23, 4065–4072 CrossRef CAS PubMed;
(b) Y. M. Hu, J. Ma, L. D Li, Q. Hu, S. Lv, B. H Liu and S. Wang, Chem. Commun., 2017, 53, 1542–1545 RSC;
(c) X. Z. Meng, S. Lv, D. Cheng, Q. Hu, J. Ma, B. H. Liu and Y. M. Hu, Chem.–Eur. J., 2017, 23, 6264–6271 CrossRef CAS PubMed;
(d) Y. M. Hu, C. L. Yu, D. Ren, Q. Hu, L. D. Zhang and D. Cheng, Angew. Chem., Int. Ed., 2009, 48, 5448–5451 CrossRef CAS PubMed.
-
(a) A. Z. Bradley and R. P. Johnson, J. Am. Chem. Soc., 1997, 119, 9917–9918 CrossRef CAS;
(b) T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, Nature, 2012, 490, 208–212 CrossRef CAS PubMed;
(c) S. Y. Yun, K. P. Wang, N. K. Lee, P. Mamidipalli and D. Lee, J. Am. Chem. Soc., 2013, 135, 4668–4671 CrossRef CAS PubMed;
(d) R. Karmakar, S. Y. Yun, K. P. Wang and D. Lee, Org. Lett., 2014, 16, 6–9 CrossRef CAS PubMed.
- CCDC-1520526 (3g), CCDC-992692 (4a) contain the supplementary crystallographic data for this paper.†.
-
(a) P. H. Willoughby, D. W. Niu, T. Wang, M. K. Haj, C. J. Cramer and T. R. Hoye, J. Am. Chem. Soc., 2014, 136, 13657–13665 CrossRef CAS PubMed;
(b) J.-A. Garcia-Lopez and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC.
-
(a) Z. J. Liu and R. C. Larock, J. Org. Chem., 2006, 71, 3198–3209 CrossRef CAS PubMed;
(b) L. Grigorjeva and O. Daugulis, Org. Lett., 2014, 16, 4688–4690 CrossRef CAS PubMed;
(c) E. Yoshioka and H. Miyabe, Tetrahedron, 2012, 68, 179–189 CrossRef CAS;
(d) E. Yoshioka, S. Kohtani and H. Miyabe, Molecules, 2014, 19, 863–880 CrossRef PubMed.
-
(a) T. Wang and H. R. Hoye, J. Am. Chem. Soc., 2016, 138, 13870–13873 CrossRef CAS PubMed;
(b) H. Miyabe, Molecules, 2015, 20, 12558–12575 CrossRef CAS PubMed;
(c) T. R. Hoye, B. Baire and T. Wang, Chem. Sci., 2014, 5, 545–550 RSC;
(d) A. E. Goetz, T. K. Shah and N. K. Garg, Chem. Commun., 2015, 51, 34–45 RSC;
(e) I. Pozo, A. Cobas, D. Pena, E. Guitianab and D. Perez, Chem. Commun., 2016, 52, 5534–5537 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of all new compounds. CCDC 1520526 and 992692. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra08817e |
| ‡ Q. H. and L. L. contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds involved a cascade HDDA reaction, the formation of an intermolecular imidazole cycloaddition and an oxygen-coupling oxidation. This approach required no metals, catalysts, additives, or directing groups and could apply to a range of substrates. The reaction also exhibited excellent regioselectivity, producing all types of highly substituted tricyclic isoindole-1,3-diones under mild conditions in good to excellent yields. The primary evaluation of the photoluminescence property of the novel extended p-systems indicated that these heteroarenes could be potentially used in the field of optoelectronic materials.
O bonds involved a cascade HDDA reaction, the formation of an intermolecular imidazole cycloaddition and an oxygen-coupling oxidation. This approach required no metals, catalysts, additives, or directing groups and could apply to a range of substrates. The reaction also exhibited excellent regioselectivity, producing all types of highly substituted tricyclic isoindole-1,3-diones under mild conditions in good to excellent yields. The primary evaluation of the photoluminescence property of the novel extended p-systems indicated that these heteroarenes could be potentially used in the field of optoelectronic materials.




