Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil†
Hongbiao Cuiab,
Xiong Yanga,
Lei Xubc,
Yuchao Fana,
Qitao Yi *a,
Ruyan Lia and
Jing Zhou*b
*a,
Ruyan Lia and
Jing Zhou*b
aSchool of Earth and Environment, Anhui University of Science and Technology, Huainan 232001, China. E-mail: yiqitao@163.com; Tel: +86 15215546045 Tel: +86 25 86881632
bKey Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy Sciences, Nanjing 210008, China. E-mail: zhoujing@issas.ac.cn
cCollege of Environmental Science and Tourism, NanYang Normal University, NanYang 473000, China
First published on 27th September 2017
Abstract
Goethite is of great importance as it affects the migration and transformation of heavy metals and phosphorus. To further understand the effect of goethite in soil on the immobilization efficiency of heavy metals and soil biological characteristics with the application of hydroxyapatite (HAP), the fractions of Cu, Cd, Pb, and P and soil enzyme activities were determined. The batch experiments indicated that single 1% HAP or 1% goethite treated soil evidently decreased amount of CaCl2-extractable, exchangeable fraction of Cu, Cd and Pb, compared to the control, and the treatment transformed the fractions from active to inactive ones. Goethite did not change the immobilization and bioaccessibility of Cu, Cd, and Pb in the presence of HAP. HAP application significantly increases soil resin-P, HCl–P, and residual-P, but goethite plus HAP decreases the labile-P, more pronounced than single HAP treatment. Moreover, soil catalase, urease, and acid phosphatase activities are increased markedly in HAP and composite additives soils. Our results suggest that goethite has little effects on the decreasing availability of heavy metals and the enhancing soil enzyme activities in the presence of HAP, but it decreases soil labile P significantly. These findings can provide important insights into the practical application of phosphate-based amendments for heavy metal-contaminated soils with considerable iron oxides.
1. Introduction
Soils contaminated by heavy metals produced as a result of both pedogenic and anthropogenic processes has become a global disaster.1 Heavy metals in soil cannot undergo microbial or chemical degradation, and thus more attention is paid on the decrease of their mobility and bioavailability for food security and human health risk.1 Chemical immobilization method could reduce heavy metals mobility and bioavailability by binding toxic heavy metals or changing their chemical speciation, and it has been widely implemented for heavy metals contaminated soils.2,3 Phosphate compounds, liming materials, organic composts, biochar, and metal oxides, have been applied to date to treat heavy metal-contaminated soils.1,3 Moreover, soil amendments have different immobilization efficiency for various heavy metals (Cu, Cd, Pb, As, etc.) and soils with different characteristics.2 Therefore, the development of effective amendments to decrease their availability and mobility for contaminated soils has become necessary.According to China's National Investigation of Soil Contamination (CNISC) status during the period of 2005 to 2013, the standard rates of Cd, Ni, As, Cu, Hg, Pb and Cr and Zn contamination were 7%, 4.8%, 2.7%, 2.1%, 1.6, 1.5%, 1.1% and 0.9%, respectively, among all sample sites,4 and soil pollution in the south of China is more serious than that in the north. Red soil is the typical soil in southern China, which is primarily derived from Quaternary red clay, tertiary red sandstone, granite and limestone. Area of red soil in China is approximately 2.18 × 106 km2,5 which is characteristics of acidic and nutrient deficiency (particularly phosphorus (P)).6 Therefore, P-rich amendments are just fit for the remediation of heavy metal-contaminated red soil. The immobilization method not only effectively decreases the availability of heavy metals, but also enhances soil P content. For example, hydroxyapatite (HAP, indissoluble) is advocated as a promising amendment for remediation of soils contaminated with heavy metals and a P fertilizer with slowing P release kinetics.7,8
Furthermore, red soil is also rich in iron oxides, such as goethite, hematite and ferrihydrite.9 Among them, goethite is a widespread soil mineral, and a primary component of soils and sediments and has been increasingly demonstrated to determine the mobility and transformation of soil contaminants (As and Cd, etc.).10,11 Goethite also plays pivotal role in the fate, bioavailability, and cycling of P due to their large sorption capacity for P.8 Ioannou et al.12 found that the maximum sorption amount of phosphate on goethite is 80 mmol kg−1, which would decrease the phosphate content during the immobilization of heavy metals with P-rich amendments. Thus, we hypothesized that the immobilization of phosphate-based amendments is closely related to the iron oxide content. The rationale is that large amount of iron phosphate such as vivianite may be formed with the application of phosphate-based amendments in red soil with a high content of iron oxide,13 and the formation process of iron phosphate would deplete the contents of phosphate and decrease the content of metal–phosphate and bioavailability of P. However, effects of goethite on the immobilization of heavy metals with HAP have not yet been realized.
Previous studies state that microorganisms are more sensitive to heavy metal stress than plants and soil macrofauna, and thus the soil enzyme activities could be used as an indicator or index in monitoring soil pollution by heavy metals.14 Moreover, the objective of immobilization is not just to remove contaminants from soil, but also to recover the biological characteristics. Therefore, the overall objectives of this research are to elucidate the roles of goethite on the immobilization of heavy metal-contaminated soils with hydroxyapatite by investigating the availability of Cu, Cd, Pb, and P, and soil enzyme activities. Our findings could provide valuable insights into the practical application of phosphate-based amendments for heavy metals-contaminated soils rich in iron oxides.
2. Materials and methods
2.1. Soil, HAP, and goethite
The soils were collected from the top 20 cm of abandoned paddy soil contaminated by drainage from slag-disposal sites. The study site is in Guixi City, Jiangxi Province, China, which is near a large copper smelter and has been contaminated for more than 30 years. Soils are primarily derived from Quaternary red clay and classified as Ultisols based on USDA Soil Taxonomy.15 After being air-dried, the soil samples were passed through a 2 mm sieve. Basic chemical characteristics of the tested soil are shown in Table 1.| pH | SOC, g kg−1 | CEC, mmol kg−1 | Fe2O3, g kg−1 | A–N, mg kg−1 | O–P, mg kg−1 | S–K, mg kg−1 | Total concentrations (mg kg−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Pb | P | |||||||
| a SOC, soil organic carbon; CEC, cation exchange capacity; A–N, alkali-hydrolyzable N; O–P, Olsen P; S–K, soil-test K. | ||||||||||
| 5.5 | 13.8 | 90.9 | 5.77 | 106 | 58.1 | 42.5 | 2225 | 17.5 | 1267 | 589 |
Hydroxyapatite (HAP, purity > 96%) was purchased from Nanjing emperornano material Co. Ltd. The Ca/P molar ratios of HAP (pH = 7.2) was 1.61, which is close to the ideal ratio of 1.67. The concentrations of Cu, Cd and Pb in HAP were 21.6 mg kg−1, 0.45 mg kg−1, and 8.94 mg kg−1, respectively. The transmission electron microscopy (TEM) image of hydroxyapatite is shown in Fig. S1.† Powder X-ray diffraction (XRD) patterns (Fig. S2†) indicate that the tested material was single pure hydroxyapatite.
Goethite was synthesized using the method of Brigante et al.16 Briefly, 5 mol L−1 KOH was added into 0.5 mol L−1 Fe(NO3)3 until the red colloid was generated. The synthesized ferrihydrite solid was aged at 60 °C in a capped Teflon container for 60 h and then was washed with deionized water until the supernatant reached a pH close to the point of zero charge. Afterwards, the solid was freeze-dried and was passed through a 0.75 μm sieve. The specific surface area measured by N2-BET analysis was 34.1 m2 g−1. Fig. S3† shows the TEM images of goethite. As shown in Fig. S4,† XRD patterns of goethite were consistent with the standard goethite sample (PDF#99-0055).
2.2. Experiment design
The experiments were conducted in 1000 mL plastic beakers containing 500 g of contaminated soil at 25 °C mixed well with different application amounts of HAP and goethite. Considering the high contamination of Cu, Cd, and Pb in soil, we chose 1% as the dosage rate for HAP herein.17,18 Moreover, the content of iron oxides is 5.77 g kg−1 (0.577%) for the contaminated soil, and our preliminary experiment shows that the soil color changes from gray to isabelline and becomes hard with increasing goethite. Thus, goethite was chosen to be 0.5% and 1% of soil mass based on our previously obtained adsorption capacity of HAP and goethite from the soil incubation experiment. Moreover, the mass ratio of HAP to goethite (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is consistent with the reports by Qian et al.19 and Wang et al.8 There were six treatments with three replicates: untreated soil (CK), soil plus (low rate) 0.5% goethite (LG), soil plus (high rate) 1% goethite (HG), soil plus 1% HAP (HAP), soil plus (low rate) 0.5% goethite and 1% HAP (LGH), and soil plus (high rate) 1% goethite and 1% HAP (HGH). All the beakers were covered with a plastic film to prevent moisture loss and then incubated for 60 d. Soil samples were collected at 7, 30, and 60 d for soil enzyme activities and CaCl2 extractable Cu, Cd and Pb analysis. Deionized water was added in order to maintain 60% of soil water holding capacity for 60 d. The fractions of Cu, Cd, Pb and P were analyzed after 60 d.
1) is consistent with the reports by Qian et al.19 and Wang et al.8 There were six treatments with three replicates: untreated soil (CK), soil plus (low rate) 0.5% goethite (LG), soil plus (high rate) 1% goethite (HG), soil plus 1% HAP (HAP), soil plus (low rate) 0.5% goethite and 1% HAP (LGH), and soil plus (high rate) 1% goethite and 1% HAP (HGH). All the beakers were covered with a plastic film to prevent moisture loss and then incubated for 60 d. Soil samples were collected at 7, 30, and 60 d for soil enzyme activities and CaCl2 extractable Cu, Cd and Pb analysis. Deionized water was added in order to maintain 60% of soil water holding capacity for 60 d. The fractions of Cu, Cd, Pb and P were analyzed after 60 d.
2.3. Analytical methods
The pH values of soil and HAP were measured by a pH electrode in suspension of distilled water at a liquid to solid ratio of 2.5 (E-201-C, Shanghai Truelab Instrument Company, China). Organic carbon in soil was measured by digesting soil with K2Cr2O7 and concentrated H2SO4 at 170–180 °C and then titrating with FeSO4.20 The cation exchange capacity was determined according to the ammonium acetate method.21 Soil alkali-hydrolyzable N was analyzed using the method described by Lu.22 Soil Olsen P and soil-test K were determined according to Olsen et al.23 and Pratt,24 respectively. Total P in soil was determined colorimetrically by acidic molybdate–ascorbic acid blue color method after the soil digestion with nitric acid/perchloric acid mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).25 Total Fe, Cu, Cd, and Pb in soil were measured by a flame or graphite furnace atomic absorption spectrophotometer (Hitachi Model Z-2000, Japan) after digestion with mixed nitric acid, hydrofluoric acid, and perchloric acid (5
1).25 Total Fe, Cu, Cd, and Pb in soil were measured by a flame or graphite furnace atomic absorption spectrophotometer (Hitachi Model Z-2000, Japan) after digestion with mixed nitric acid, hydrofluoric acid, and perchloric acid (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) on a hot plate (120–240 °C). A certified soil reference material (GBW07405, National Research Center for Certified Reference Materials, China) was used to ensure the accuracy of the analytical data and the accuracy ranged from 93.9 to 107.4%.
5) on a hot plate (120–240 °C). A certified soil reference material (GBW07405, National Research Center for Certified Reference Materials, China) was used to ensure the accuracy of the analytical data and the accuracy ranged from 93.9 to 107.4%.
The CaCl2-extracted heavy metals were analyzed by extracting soil samples with 0.01 mol L−1 CaCl2 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio and then shaking for 2 h at room temperature (25 °C).26 A simplified bioaccessibility extraction test (SBET) procedure described by Ruby et al.27 was used to evaluate the bioaccessibility of metals in soils to mammals (see text S1†). Five chemical speciations including those of exchangeable (EXC) Cu, Cd, and Pb, Cu, Cd, and Pb bound to carbonate (CA), their Fe–Mn oxides (Fe–Mn), organic matter (OM), and residual fraction (RES) were determined by the sequential extraction procedure of Tessier et al. (see text S2†).28
5 ratio and then shaking for 2 h at room temperature (25 °C).26 A simplified bioaccessibility extraction test (SBET) procedure described by Ruby et al.27 was used to evaluate the bioaccessibility of metals in soils to mammals (see text S1†). Five chemical speciations including those of exchangeable (EXC) Cu, Cd, and Pb, Cu, Cd, and Pb bound to carbonate (CA), their Fe–Mn oxides (Fe–Mn), organic matter (OM), and residual fraction (RES) were determined by the sequential extraction procedure of Tessier et al. (see text S2†).28
Soil P speciations including those of labile resin-P, labile inorganic NaHCO3–P (NaHCO3-Pi) and organic NaHCO3–P (NaHCO3-Po), moderately labile inorganic NaOH–P (NaOH-Pi) and organic NaOH–P (NaOH-Po), stable HCl–P and residual P were analyzed based on the modified method of Tiessen and Moir (see text S3†).29
Soil catalase was analyzed according to the method of Johnson and Temple.30 Briefly, 2 g soil with 5 mL of 0.3% H2O2 was incubated for 30 min at 30 °C. Then, the suspension was titrated with 0.1 mol L−1 KMnO4 solution, and the activity of catalase was expressed in milliliters of KMnO4 decomposed per g of soil. The activity of soil urease was assayed using 5 g soil with 10 mL of 10% urea solution and 20 mL citrate buffer (pH = 6.7) for 24 h at 37 °C. The formation of ammonium was determined using a spectrophotometer within 1 h at λ = 578 nm after a 30 min color development period. The activity of urease was reported in milligrams of NH3–N generated by 1 g soil.22 Soil acid phosphatase activity was measured by incubating 5 g soil with 5 mL of modified universal buffer (pH = 5) and 5 mL of p-nitrophenyl phosphate for 24 h at 37 °C. The complexes were analyzed with 4-aminoantipyrine colorimetric method at λ = 510 nm and the activity of acid phosphatases was expressed as milligrams of phenol hydrolyzed by 1 g soil.31
The specific surface areas of goethite and hydroxyapatite were measured by the Brunauer–Emmett–Teller (BET) method using the specific surface area automatic analyzer (Quantachrome Autosorb-iQ, America). The physical structures of goethite and hydroxyapatite were imaged via a transmission electron microscopy (TEM, JEOL TEM-2100, Japan) system. The mineral phases of soil samples were identified by a Rigaku X-ray diffractometer with CuKα radiation (40 kV/40 mA). The scan speed was 1° min−1 and the scan 2θ ranged from 10° to 60°. The XRD data were analyzed using MDI Jade 5.0 software (Materials Data Inc., Liverpool, CA).
2.4. Data analyses
Data were presented as mean ± standard error and were analyzed by one-way analysis of variance using SPSS (version 19.0 for Windows). The multiple comparisons of the means within the treatments were tested by Turkey's multiple range test at the 5% significance level.3. Results
3.1. Soil pH and CaCl2 extractable Cu, Cd and Pb
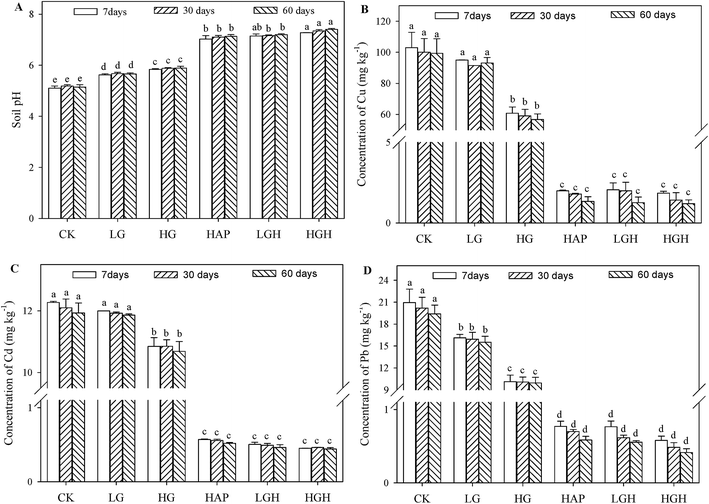
The pH of untreated soil was low (5.1–5.2) and did not change significantly during the incubation period (Fig. 1A). With increasing the application rates of goethite from 0.5% to 1%, the pH increased to 5.6–5.7 and 5.8–5.9. Soil pH in single HAP treated soils increased ∼2 units compared to the control soils. The highest pH (7.3–7.4) was found in HAP plus 1% goethite treated soils.As expected, CaCl2 extractable Cu (48.1–50.4 mg kg−1), Cd (6.15–6.46 mg kg−1) and Pb (17.4–18.9 mg kg−1) in the control soil were the highest during the incubation period (Fig. 1B–D). Compared with the control, the CaCl2 extractable Cu, Cd, and Pb decreased significantly to 12.1, 4.47, and 13.4 mg kg−1 in 1% goethite treated soil at 60 d. The concentrations of Cu, Cd, and Pb in 1% HAP treated soils decreased drastically to 5.42, 0.22 and 1.27 mg kg−1, respectively, decreasing by 89%, 97% and 93% than the control. Nevertheless, goethite plus HAP treatments had little effects on the CaCl2-extractable Cu, Cd, and Pb than the single HAP treated soils.
3.2. Fractions of Cu, Cd and Pb
The five fractions of Cu, Cd, and Pb are listed in Table 2, and the relative distributions of those five fractions are shown in Fig. S5.† In the untreated soil, Cu and Pb were dominated by the residual fraction with concentrations of 760 mg kg−1 (34.2%) for Cu and 435 mg kg−1 (34.9%) for Pb. However, the exchangeable fraction (12.4 mg kg−1, 71.1%) of Cd was predominant. Compared with the control and single goethite amended soils, HAP and composite additives decreased the exchangeable fractions of Cu, Cd, and Pb drastically. Particularly, exchangeable fraction of Cu and Pb decreased from 358 mg kg−1 (16.1%) and 428 mg kg−1 (34.4%) in the control to 32.7–38.9 mg kg−1 (1.45–1.74%) and 15.4–20.4 mg kg−1 (1.22–1.66%), respectively, in HAP and composite additives, respectively. However, exchangeable fraction of Cd remained at a high level (6.14–6.79 mg kg−1, 34.9–38.7%), which was higher than the residual fraction.| Treatment | EXC | CA | Fe–Mn | OM | RES |
|---|---|---|---|---|---|
| a CK = untreated soil, LG = 0.5% goethite plus soil, HG = 1% goethite plus soil, HAP = 1% HAP plus soil, LGH = 0.5% goethite and 1% HAP plus soil, HGH = 1% goethite and 1% HAP plus soil. Mean (n = 3) and standard error followed by different letters indicated significant differences (P < 0.05). | |||||
| Cu (mg kg−1) | |||||
| CK | 358 ± 4a | 369 ± 36b | 431 ± 26b | 302 ± 24b | 760 ± 56a |
| LG | 302 ± 1b | 381 ± 34ab | 464 ± 31b | 297 ± 5b | 763 ± 45a |
| HG | 245 ± 5c | 391 ± 31ab | 495 ± 13b | 315 ± 20ab | 785 ± 14a |
| HAP | 38.9 ± 3.1d | 403 ± 27ab | 632 ± 43a | 313 ± 18ab | 845 ± 64a |
| LGH | 33.6 ± 1.1d | 421 ± 24ab | 645 ± 27a | 332 ± 10ab | 824 ± 27a |
| HGH | 32.7 ± 0.8d | 463 ± 13a | 579 ± 2a | 349 ± 17a | 826 ± 65a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Cd (mg kg−1) | |||||
| CK | 12.4 ± 0.1a | 0.7 ± 0.04b | 0.64 ± 0.11b | 0.18 ± 0.01c | 3.53 ± 0.25a |
| LG | 12.3 ± 0.08a | 0.85 ± 0.04b | 0.8 ± 0.02b | 0.15 ± 0c | 3.27 ± 0.22a |
| HG | 12.2 ± 0.42a | 1 ± 0.07b | 0.87 ± 0.01b | 0.15 ± 0.02c | 3.29 ± 0.34a |
| HAP | 6.79 ± 0.06b | 3.48 ± 0.39a | 3.78 ± 0.07a | 0.53 ± 0.04a | 2.98 ± 0.2a |
| LGH | 6.46 ± 0.01bc | 3.57 ± 0.01a | 3.91 ± 0.3a | 0.44 ± 0.02b | 3.18 ± 0.18a |
| HGH | 6.14 ± 0.11c | 3.65 ± 0.07a | 4.15 ± 0.32a | 0.43 ± 0.02b | 3.23 ± 0.4a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Pb (mg kg−1) | |||||
| CK | 428 ± 4a | 170 ± 11a | 182 ± 8b | 29.9 ± 1.4b | 435 ± 29b |
| LG | 408 ± 1b | 171 ± 19a | 199 ± 12b | 36.5 ± 0.7b | 439 ± 46b |
| HG | 377 ± 2c | 175 ± 7a | 207 ± 10b | 40.9 ± 3.5b | 417 ± 31b |
| HAP | 20.4 ± 1.3d | 44 ± 3.2b | 401 ± 12a | 140 ± 5a | 630 ± 36a |
| LGH | 17.6 ± 0.2d | 40.5 ± 3.9b | 391 ± 26a | 143 ± 1a | 655 ± 15a |
| HGH | 15.4 ± 1.1d | 49.1 ± 1.8b | 397 ± 11a | 136 ± 10a | 658 ± 41a |
Compared with the control, single goethite addition did not change the distribution of Cu, Cd, and Pb bound to carbonate and their Fe–Mn oxides and organic matter fractions, but only HAP and composite additives enhanced the fraction of Cd bound to carbonate and decreased the fraction of Pb bound to carbonate. Moreover, HAP and composite additives both increased Cu, Cd, and Pb in fractions of Fe–Mn oxides and organic matter than the control. There were no significant differences in residual fractions of Cu and Cd among all the soils, and only HAP and composite additives increased the residual fractions of Pb with respect to the control soil.
3.3. Bioaccessibility of Cu, Cd and Pb
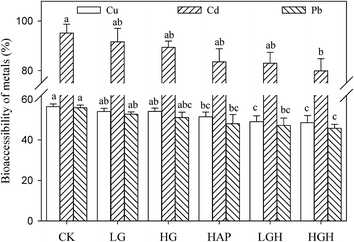
The simplified bioaccessibility extraction test (SBET) was applied to evaluate the bioaccessibility of metals in soil and calculate the amount of contaminants that could be absorbed by stomach via the ingestion of soils. The concentrations of Cu, Cd, and Pb extracted from the amended soils using the SBET method decreased slightly compared with the control (Fig. S6†). Compared with the control, goethite addition did not decrease the content of bioaccessible Cu, Cd, and Pb, and only composite additives decreased the content of bioaccessible Cu and Cd.The bioaccessibility of Cu, Cd, and Pb was calculated by dividing the extracted concentration of a metal in the gastric phase by the total concentration of metals in soil. The bioaccessibility of Cu (56.4%), Cd (95.2%), and Pb (55.9%) in the control was the highest among all the soils (Fig. 2). Similar to the concentrations of bioaccessible metals, HAP and composite additives decreased the bioaccessibility of Cu and Pb compared with the control, but only HAP plus 1% goethite treated soil showed decreased bioaccessibility of Cd. Moreover, the bioaccessibility of Cd was the highest with 79.9–95.2% in this experiment compared to that of Cu (48.6–56.4%) and Pb (45.7–55.9%).
3.4. Fractions of P
Table 3 shows the results of the sequential fractionation of P into resin-P, NaHCO3-Pi, NaHCO3-Po, NaOH-Pi, NaOH-Po, HCl-Pi, and residual-P speciation in soils. Total P in soil significantly increased from 600 mg kg−1 in control to 2360 mg kg−1 in HAP treated soil. The largest difference was that resin-P was higher in HAP amended soils (194–245 mg kg−1), which was 3.11 times than that of the control. Single goethite did not change resin-P compared with the control, and only 1% goethite plus HAP markedly decreased resin-P and NaHCO3-Pi than the single HAP treated soil. Moreover, single goethite decreased NaHCO3-Po, but composite additives increased NaHCO3-Po compared with the control.| Treatment | Total P | Labile P | Moderately labile P | Stable P | ||||
|---|---|---|---|---|---|---|---|---|
| Resin-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl–P | Residual-P | ||
| a CK = untreated soil, LG = 0.5% goethite plus soil, HG = 1% goethite plus soil, HAP = 1% HAP plus soil, LGH = 0.5% goethite and 1% HAP plus soil, HGH = 1% goethite and 1% HAP plus soil. Mean (n = 3) and standard error followed by different letters indicated significant differences (P < 0.05). | ||||||||
| CK | 600 ± 32b | 78.9 ± 4.7c | 45.9 ± 4.6bc | 10.3 ± 0.4b | 67.4 ± 6.7a | 24 ± 2.5bc | 106 ± 6c | 267 ± 26c |
| LG | 595 ± 25b | 74.9 ± 1.5c | 41.5 ± 2.1cd | 6.8 ± 0.2c | 74.5 ± 3.3a | 26.4 ± 0.4abc | 91 ± 4c | 280 ± 29c |
| HG | 602 ± 19b | 68.2 ± 3.3c | 35.2 ± 3.4d | 4.9 ± 0.2d | 81 ± 5.6a | 25.3 ± 2.3abc | 91 ± 2c | 296 ± 29c |
| HAP | 2360 ± 74a | 245 ± 25a | 61.0 ± 2a | 11.7 ± 1.2ab | 69.1 ± 6.6a | 21.8 ± 2c | 760 ± 27a | 1191 ± 82b |
| LGH | 2356 ± 81a | 226 ± 13ab | 54.9 ± 4.2ab | 12.3 ± 1.2a | 74.1 ± 4a | 29.9 ± 2.3ab | 693 ± 41b | 1266 ± 41ab |
| HGH | 2386 ± 28a | 194 ± 21b | 48.6 ± 4.6bc | 13.2 ± 0.1a | 80.7 ± 6.5a | 30.5 ± 2.1a | 669 ± 26b | 1351 ± 32a |
None of the treated soils showed any change in NaOH-Pi compared with the control, retaining NaOH-Pi at 67.4–81 mg kg−1. However, concentrations of NaOH-Po increased noticeably from 21.8 mg kg−1 in HAP soil to 29.9–30.5 mg kg−1 in composite additives. Furthermore, there were no significant differences in concentration of moderately labile P among all the soils. Single goethite addition did not change HCl–P compared with the control (106 mg kg−1, 17.6%), but HAP application increased HCl–P significantly to 760 mg kg−1 (32.2%), and composite additives decreased HCl–P markedly. Similar to HCl–P, residual-P increased significantly from 267 mg kg−1 (44.6%) in the control soil to 1191 mg kg−1 (50.5%) in HAP amended soil, and it also increased in composite additives. Usually, P in soils is classified as labile P (sum of P extracted with the anion-exchange resin and NaHCO3), moderately labile P (P extracted with NaOH), and stable P (the P extracted with HCl and residual P after digestion). Therefore, 1% goethite plus HAP amended soil showed significantly decreased labile P, but did not show any change in moderately labile P and stable P compared with the single HAP treated soil.
3.5. Soil enzyme activities
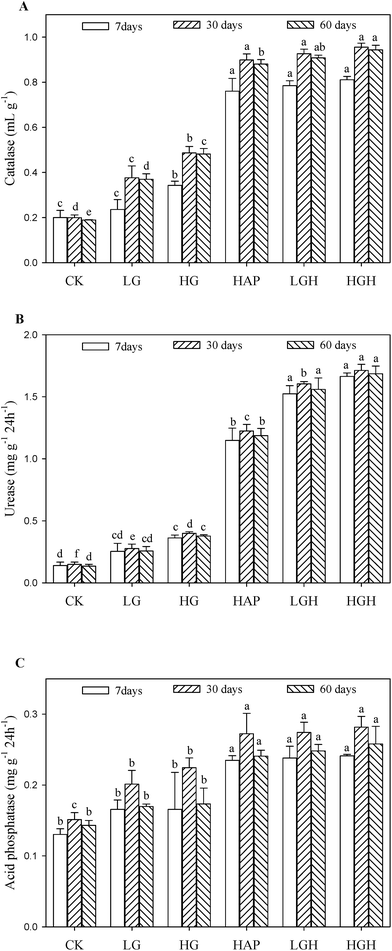
Catalase activities rose as the amount of goethite increased, and they were 2.54 times in 1% goethite treated soil and 3.65–3.98 times in HAP and composite additives than that in the control at 60 d (Fig. 3A). However, there were no significant changes for each soil over time. Similar to catalase, urease increased with increasing amount of goethite. For example, urease activity in 0.5% and 1% goethite soils was 1.9 and 2.81 times than that in the control at 60 d, respectively. Furthermore, urease activities in 0.5% goethite plus HAP and 1% goethite plus HAP soils were also significantly greater by 0.31 and 0.42 times than that in the HAP soils, respectively. In addition, acid phosphatase was the least (0.13–0.15 mg g−1 24 h−1) in the control. In contrast to catalase and urease, goethite addition did not increase acid phosphatase. Only HAP and composite additives increased acid phosphatase by 0.68–0.83 times than that in the control soil.4. Discussions
Herein, HAP and goethite applications increased soil pH, and the magnitude of pH increase for HAP was higher than that for goethite. Li et al.9 also reported that soil pH positively correlated with Fe oxide content in soils and the main anti-acidification mechanisms may be attributed to Fe oxide-induced double layer overlapping and coating. The increase of soil pH values with the application of HAP may be due to the dissolution of HA, which consumes H+ (eqn (1)).32 Similarly, Wei et al.18 also found that soil pH increased ∼1.5 units compared to the control after the application of HAP with 5 tha−1.| Ca10(PO4)6(OH)2 + 14H+ = 10Ca2+ + 6H2PO4− + 2H2O | (1) |
CaCl2-extractable Cu, Cd and Pb were decreased evidently in HAP and 1% goethite soils compared with the control. Moreover, in both HAP and composite additives, exchangeable fractions and bioaccessibility of Cu, Cd, and Pb decreased and transformed them from active to inactive fractions, but in single goethite, only exchangeable fraction of Cu and Pb decreased and the bioaccessibility of Cu, Cd, and Pb was not decreased. The results show higher immobilization efficiency for Cu and Pb than that of Cd. It may be attributed to the sorption maxima for metals on goethite and HAP. Both decreased in the order Cu > Pb > Cd,33–35 which resulted in the immobilization of least amount of Cd in soils.
Immobilization of Cu and Cd by HAP could be attributed to the increase of soil pH, which results in metal precipitation (hydroxide, carbonate, etc.) and increase of negative charges of variably charged colloids in soils, thus resulting in the high sorption of heavy metals by soils.36,37 Moreover, HAP could immobilize heavy metals by ion exchange (eqn (2)), surface complexation (eqn (3)), substitution of Ca in HA by other metals during recrystallization (coprecipitation) (eqn (4) and (5)) and precipitation of some amorphous to poorly crystalline, mixed metal phosphates.38–40 For Pb, the dominant process in the immobilization by HAP may be due to the dissolution (eqn (1)) and precipitation (eqn (6)).38
| Ca10(PO4)6(OH)2 + xCd2+ = Ca10−xCdx(PO4)6(OH)2 + xCa2+ | (2) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) POH + Cd2+ = POH + Cd2+ = ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) POCd+ + H+ POCd+ + H+
| (3) |
| xCd2+ + (5 − x)Ca2+ + 3H2PO4− + H2O = (Cdx,Ca5−x)(PO4)3OH + 7H+ | (4) |
| xCd2+ + (5 − x)Ca2+ + 3HPO42− + H2O = (Cdx,Ca5−x)(PO4)3OH + 4H+ | (5) |
| 5Pb2+ + 3H2PO4− + H2O = Pb5(PO4)3(OH) + 7H+ | (6) |
Metal fixation by goethite can be mainly attributed to the diffusion of metal into the structural lattice of goethite41 and the formation of metal precipitate on surface of goethite by the following reactions (eqn (7) and (8)).42,43 Moreover, a new iron-phosphate (vivianite) may be formed in the HAP and goethite composite additives, which could reduce the leachability and bioaccessibility of Pb by the following reactions (eqn (9) and (10)).44 Herein, the addition of goethite in the presence of HAP did not significantly decrease the availability of Cu, Cd, and Pb.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + Me2+ + H2O ↔ Fe–OH + Me2+ + H2O ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–O–MeOH2+ Fe–O–MeOH2+
| (7) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–O–MeOH2+ + Me2+ + 2H2O ↔ Fe–O–MeOH2+ + Me2+ + 2H2O ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–O–MeOH2+ + Me(OH)2(s) + 2H+ Fe–O–MeOH2+ + Me(OH)2(s) + 2H+
| (8) |
| Fe3(PO4)2·8H2O + 2H+ ⇔ 3Fe2+ + 2HPO42− + 8H2O | (9) |
| 5Pb2+ + 3HPO42− + X− ⇔ Pb5(PO4)3 + 3H+ | (10) |
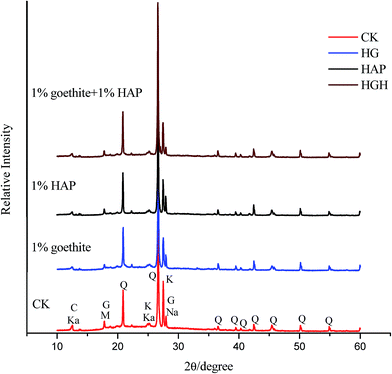
As shown in Fig. 4, XRD analysis indicate that the main mineral phases in the untreated soils included quartz, feldspar, and muscovite, etc. Hydroxyapatite and goethite were not found in the treated soils and it may be due to their low application rate (<2%, wt). Moreover, the XRD patterns of the HAP and composite additives were very similar to those of the control soils, suggesting that no new solid phases were found in the observations presented in Fig. 4. This was in agreement with the results of previous studies.45 This may be due to the fact that XRD cannot detect the precipitation of amorphous metal phosphate or less than 2 wt% of new crystalline minerals in the treated soils.46,47 Moreover, HAP and multi-metals may form unknown peaks, or peak broadening obscure the identification of peak positions.47 Therefore, more sensitive, extended X-ray absorption fine structure analysis should be applied for the identification of Cu, Cd and Pb minerals in the future.
Moreover, HAP addition evidently increased soil labile P (resin-P, NaHCO3-Pi, and NaHCO3-Po), moderately labile P (NaOH-Pi and NaOH-Po) and stable P (HCl–P and residual-P) (Table 3). The increase of labile inorganic P is likely to be derived from the dissolution of HAP. In order to immobilize heavy metals, the mole of P from HAP in the soil was 29.9 mmol kg−1, which is larger than the amount (27.0 mmol kg−1) required to form metal–phosphate precipitation including Cu3(PO4)2, Cd3(PO4)2 and Pb5(PO4)3OH. This is illustrated by the high concentrations of resin-P in HAP treated soil. Our results are supported by the significant increase of soil available P and biomass of soybean treated by HAP.48
Resin-P is freely exchangeable P and can be used as a good indicator of the short-term P loss potential in soil, which causes the eutrophication in aquatic ecosystem.49 In previous studies, application rates of HAP were 1–5%,3,17,18 and our recent study show that the phosphorus in effluents is higher than the Class Five limit (0.4 mg L−1) mandated by the Chinese National Quality Standards for Surface Waters (GB 3838-2002) in 1% HAP treated column.50 Fortunately, application of goethite decreased resin-P and NaHCO3-Pi in the presence of HAP. The results are well consistent with the reports of Liu and Zhao44 who reported that low phosphate concentration is found in iron phosphate nanoparticle amended soils compared to that in sodium phosphate treated soils due to the formation of vivianite under anaerobic conditions.13 Therefore, it could be concluded that the concentrations of phosphate may decrease significantly in some red soils with iron oxides present over 1% (wt%) due to the immobilization with hydroxyapatite, reducing water eutrophication risk. Nevertheless, more work is needed to investigate the P release risk in soils with different amounts of iron oxides accompanied with phytoremediation during immobilization of heavy metal-contaminated soils by HAP.
Soil enzyme activity is a direct indicator of soil microbial activity in response to metabolic requirements and available nutrients and thus it is useful for evaluating the impact of heavy metal pollution in soil.51,52 Soil catalase, urease, and acid phosphatase activities were selected due to their strong sensitivity to heavy metal in soil.51 Urease and acid phosphatase can be the indicators of soil organic N and P mineralization,53 respectively. Catalase level represents soil oxidation–reduction potential and is closely related to the soil biochemical processes.54 Results indicate that HAP and composite additives markedly increased soil catalase, urease, and acid phosphatase activities, and only 1% goethite treated soils show evident increase in catalase and urease activities. Similarly, Wei et al.18 also reported that soil urease and phosphatase activities were increased with the application of HAP in heavy metal-contaminated soils.
Pearson's correlation analysis indicate that soil pH values are positively correlated with catalase, urease, and acid phosphatase and negatively correlated with CaCl2-extractable and exchangeable fraction of metals. Moreover, significant negative correlations are found between CaCl2-extractable and exchangeable fraction of Cu, Cd, and Pb (Table S1†). The results indicate that HAP and goethite improved soil enzyme activities by decreasing available metals and increasing soil pH. Previous studies also report that there are negative correlations between available metals and soil enzyme activities.17,55 Generally, HAP and composite additives can effectively reduce the bioavailability of heavy metals to microorganisms and soil labile-P, but goethite exerts only little effects on the immobilization efficiency of heavy metals and soil biological characteristics in the absence of HAP.
5. Conclusions
Herein, it is indicated that single 1% goethite or 1% HAP application can increase soil pH and improve the immobilization of Cu, Cd, and Pb by transforming them from active to inactive fractions, but goethite did not influence the immobilization and bioaccessibility of heavy metals in the presence of HAP. The application of HAP increases soil resin-P, HCl–P, and residual-P and promotes the increase of soil catalase, urease, and acid phosphatase activities. Goethite plus HAP decreased soil labile-P, but they did not change soil enzyme activities compared with the single HAP treated soils. In conclusion, goethite did not affect the immobilization of heavy metals and soil biological characteristics, but decreased the soil labile-P in the presence of HAP.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (41601340), the Natural Science Foundation of Universities of Anhui Province (KJ2016A191, KJ2016A827) and the National Key Technology Research and Development Program of China (2015BAD05B01).We express our gratitude to the anonymous reviewers for their constructive comments and suggestions.References
- N. Bolan, A. Kunhikrishnan, R. Thangarajan, J. Kumpiene, J. Park, T. Makino, M. B. Kirkham and K. Scheckel, J. Hazard. Mater., 2014, 266, 141–166 CrossRef CAS PubMed.
- W. Mahar, L. Ping, I. Ronghua and Z. Zhang, Pedosphere, 2015, 25, 555–568 CrossRef.
- J. F. Xing, T. T. Hu, L. Cang and D. M. Zhou, SpringerPlus, 2016, 5, 1182 CrossRef PubMed.
- MLR, and MEP, The communique of national investigation of soil contamination. Ministry of Land and Resources of the People's Republic of China. Ministry of environmental protection of the People's Republic of China, 2014 Search PubMed.
- X. Zhang, Z. Li, G. Zeng, X. Xia, L. Yang and J. Wu, Environ. Earth Sci., 2012, 67, 1725–1734 CrossRef CAS.
- M. K. Zhang and J. M. Xu, Soil Tillage Res., 2005, 80, 13–21 CrossRef.
- D. Montalvo, M. J. McLaughlin and F. Degryse, Soil Sci. Soc. Am. J., 2015, 79, 551–558 CrossRef CAS.
- D. J. Wang, Y. Jin and D. P. Jaisi, Environ. Sci. Technol., 2015, 49, 8461–8470 CrossRef CAS PubMed.
- J. Y. Li, R. K. Xu and H. Zhang, J. Soils Sediments, 2012, 12, 876–887 CrossRef.
- J. X. Fan, Y. J. Wang, C. Liu, L. H. Wang, K. Yang, D. M. Zhou, W. Li and D. L. Sparks, J. Hazard. Mater., 2014, 279, 212–219 CrossRef CAS PubMed.
- H. Y. Yu, C. Liu, J. Zhu, F. Li, D. M. Deng, Q. Wang and C. Liu, Environ. Pollut., 2015, 209, 38–45 CrossRef PubMed.
- Z. Ioannou, A. Dimirkou and A. Ioannou, Water, Air, Soil Pollut., 2013, 224, 1–14 CrossRef CAS.
- J. O. Nriagu, Geochim. Cosmochim. Acta, 1972, 36, 459–470 CrossRef CAS.
- P. Brookes and S. McGrath, Eur. J. Soil Sci., 1984, 35, 341–346 CrossRef CAS.
- S. S. Staff, Keys to Soil Taxonomy, United States Department of Agriculture, 11th edn, 2010 Search PubMed.
- M. Brigante, G. Zanini and M. Avena, J. Hazard. Mater., 2010, 184, 241–247 CrossRef CAS PubMed.
- H. B. Cui, J. Zhou, Q. G. Zhao, Y. B. Si, J. D. Mao, G. D. Fang and J. N. Liang, J. Soils Sediments, 2013, 13, 742–752 CrossRef CAS.
- L. Wei, S. Wang, Q. Zuo, S. Liang, S. Shen and C. Zhao, Environ. Sci.: Processes Impacts, 2016, 18, 760–767 CAS.
- G. Qian, W. Chen, T. T. Lim and P. Chui, J. Hazard. Mater., 2009, 170, 1093–1100 CrossRef CAS PubMed.
- A. E. Walkley and I. A. Black, Soil Sci., 1934, 37, 29–38 CrossRef CAS.
- M. Pansu, and J. Gautheyrou, Handbook of Soil Analysis-Mineralogical, Organic and Inorganic Methods, Springer-Verlag, Berlin, Heidelberg, 2006 Search PubMed.
- R. K. Lu, Soil agricultural chemical analysis method, China Agricultural Science and Technology Press, Beijing, 2002 Search PubMed.
- S. R. Olsen, C. V. Cole, F. S. Watanabe, and L. A. Dean, Estimation of available phosphorus in soils by extraction with sodium bicarbonate, United States Department of Agriculture, Circular 939. United States Government Printing Office, Washington, DC, USA, 1954 Search PubMed.
- P. F. Pratt, Potassium, In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, ed. C. A. Black, American Society of Agronomy, Inc., Madison, 1965 Search PubMed.
- P. P. V. Veldhoven and G. P. Mannaerts, Anal. Biochem., 1987, 161, 45–48 CrossRef PubMed.
- M. B. Mcbride, B. K. Richards and T. Steenhuis, Plant Soil, 2004, 262, 71–84 CrossRef CAS.
- M. Ruby, R. Schoof, W. Brattin, M. Goldade, G. Post, M. Harnois, D. Mosby, S. Casteel, W. Berti and M. Carpenter, Environ. Sci. Technol., 1999, 33, 3697–3705 CrossRef CAS.
- A. Tessier, P. G. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844–851 CrossRef CAS.
- H. Tiessen, and J. O. Moir, Characterization of available P by sequential extraction, in Soil sampling and methods of analysis. Can J Soil Sci, ed. M. R Carter, Lewis Publishers, 1993 Search PubMed.
- J. L. Johnson and K. L. Temple, Soil Sci. Soc. Am. J., 1964, 28, 207–209 CrossRef CAS.
- A. Garzillo, L. Badalucco, F. De Cesare, S. Grego and V. Buonocore, Soil Biol. Biochem., 1996, 28, 1155–1161 CrossRef CAS.
- J. Boisson, M. Mench, J. Vangronsveld, A. Ruttens, P. Kopponen and T. De Koe, Commun. Soil Sci. Plant Anal., 1999, 30, 365–387 CrossRef CAS.
- E. A. Forbes, A. M. Posner and J. P. Quirk, Eur. J. Soil Sci., 1976, 272, 154–166 CrossRef.
- S. B. Chen, Y. B. Ma, L. Chen and K. Xian, Geochem. J., 2010, 44, 233–239 CrossRef CAS.
- H. Liu, T. Chen and R. L. Frost, Chemosphere, 2013, 103, 1–11 CrossRef PubMed.
- C. W. Gray, R. G. McLaren, A. H. C. Roberts and L. M. Condron, Aust. J. Soil Res., 1998, 36, 199–216 CrossRef CAS.
- P. Loganathan, S. Vigneswaran, J. Kandasamy and R. Naidu, Crit. Rev. Environ. Sci. Technol., 2012, 42, 489–533 CrossRef CAS.
- X. D. Cao, L. Q. Ma, D. R. Rhue and C. S. Appel, Environ. Pollut., 2004, 131, 435–444 CrossRef CAS PubMed.
- D. Marchat, D. Bernache-Assollant and E. Champion, J. Hazard. Mater., 2007, 139, 453–460 CrossRef CAS PubMed.
- A. Corami, S. Mignardi and V. Ferrini, J. Colloid Interface Sci., 2008, 317, 402–408 CrossRef CAS PubMed.
- N. Salami and F. A. Adekola, Bull. Chem. Soc. Ethiop., 2002, 16, 1–7 CAS.
- J. Lee and J. J. Doolittle, Soil Sci., 2002, 167, 390–400 CrossRef CAS.
- D. Buerge-Weirich, R. Hari, H. Xue, P. Behra and L. Sigg, Environ. Sci. Technol., 2002, 36, 328–336 CrossRef CAS PubMed.
- R. Liu and D. Zhao, Water Res., 2007, 41, 2491–2502 CrossRef CAS PubMed.
- X. D. Cao, A. Wahbi, L. Q. Ma, B. Li and Y. Yang, J. Hazard. Mater., 2009, 164, 555–564 CrossRef CAS PubMed.
- P. Zhang and J. A. Ryan, Environ. Sci. Technol., 1999, 33, 625–630 CrossRef CAS.
- G. M. Hettiarachchi, G. M. Pierzynski and M. D. Ransom, Environ. Sci. Technol., 2000, 34, 4614–4619 CrossRef CAS.
- R. Liu and R. Lal, Sci. Rep., 2014, 4, 5686 CrossRef CAS PubMed.
- B. Li, T. Ge, H. Xiao, Z. Zhu, Y. Li, O. Shibistova, S. Liu, J. Wu, K. Inubushi and G. Guggenberger, Environ. Sci. Pollut. Res., 2016, 23, 1494–1503 Search PubMed.
- H. B. Cui, S. W. Zhang, R. Y. Li, Q. T. Yi, X. B. Zheng, Y. B. Hu and J. Zhou, Environ. Sci. Pollut. Res., 2017, 24, 21128–21137 CrossRef CAS PubMed.
- G. Garau, P. Castaldi, L. Santona, P. Deiana and P. Melis, Geoderma, 2007, 142, 47–57 CrossRef CAS.
- F. P. Zhang, C. F. Li, L. G. Tong, L. X. Yue, P. Li, Y. J. Ciren and C. G. Cao, Applied Soil Ecology, 2010, 45, 144–151 CrossRef.
- G. Masciandaro, B. Ceccanti, S. Benedicto, H. C. Lee and H. F. Cook, Can. J. Soil Sci., 2004, 84, 19–30 CrossRef CAS.
- Y. L. Zhang and Y. S. Wang, Pedosphere., 2006, 16, 512–518 CrossRef CAS.
- X. X. Ye, S. H. Kang, H. M. Wang, H. Y. Li, Y. X. Zhang, G. Z. Wang and H. J. Zhao, J. Hazard. Mater., 2015, 289, 210–218 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08786a |
| This journal is © The Royal Society of Chemistry 2017 |