Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cav2.2 and Cav3.1 calcium channel inhibitors from Valeriana jatamansi Jones†
He-Hai Jiang‡
ac,
Fa-Wu Dong‡bd,
Jun Zhoub,
Jiang-Miao Hu*b,
Jian Yang*a and
Yin Nian *a
*a
aKey Laboratory of Bioactive Peptides of Yunnan Province/Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences, Ion Channel Research and Drug Development Center, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, P. R. China. E-mail: 13987692057@163.com
bState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, P. R. China
cFaculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, 650504, P. R. China
dFaculty of Pharmacy, Yunnan University of Traditional Chinese Medicine, Kunming 650500, P. R. China
First published on 26th September 2017
Abstract
In China, the roots and rhizomes of Valeriana jatamansi Jones are traditionally used to treat gastrointestinal and rheumatic pain. Small molecule inhibitors of N-type (Cav2.2) and T-type (Cav3.1–3.3) calcium channels have become attractive resources in analgesic drug development. Therefore, in the present study, the isolated compounds (1–13) from V. jatamansi, including three new valepotriates (1–3), were initially evaluated on Cav2.2 and Cav3.1. As a result, compounds 1–12 showed weak to potent inhibition on Cav2.2 peak currents at 30 μM. Among them, compounds 1, 6, 7, 11 and 12 exhibited significant antagonistic effects, with EC50 values of 4.33, 2.18, 1.13, 2.70 and 7.8 μM, respectively. Meanwhile, the aforementioned compounds exhibited 18.2 ± 2.5% to 49.2 ± 7.1% peak current inhibition on Cav3.1 at 30 μM. In addition, they also exhibited noticeable specificity against Cav1.2, Cav2.1, and KCNH2 (hERG) channels.
Introduction
In modern drug development, N-type (Cav2.2) and T-type (Cav3.1–3.3) calcium channels have been identified as potential analgesic targets. Ziconotide, a specific Cav2.2 blocker, was approved by the United States Food and Drug Administration to manage chronic pain through intrathecal administration.1 Furthermore, CNV2197944 and Z160, another two Cav2.2 antagonists, are also in Phase II trials to evaluate their therapeutic effects on chronic pain. In addition, T-type calcium channel inhibitors, such as ABT-639, TTA-P2, KYS-05090S, and Z944, have been proved as antinociceptive agents in different animal models.2–6 Specifically, Z944, an oral modulator, is in Phase II trials for treating inflammatory and neuropathic pain.6Valeriana jatamansi Jones (Caprifoliaceae), an annual herb, is mainly distributed in China and mainland India.7 The roots and rhizomes of this plant are recorded in the Chinese Pharmacopoeia to alleviate gastrointestinal and rheumatic pain.8 In addition, several clinically used medicines in Chinese market, such as “Xiaoshi Shunqi Pian” and “Xiangguo Jianxiao Pian”, are using the extracts of roots and rhizomes of V. jatamansi as a main component to treat gastrointestinal pain. Thus, it is of interest to explore whether the chemical constituents of V. jatamansi have inhibitory effects on N-type or T-type calcium channels.
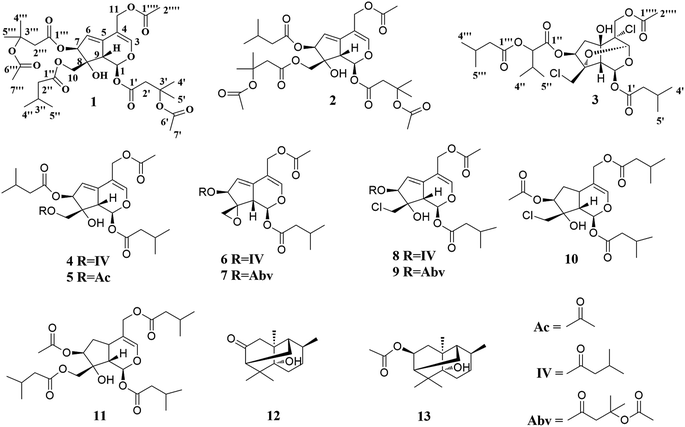
In the present study, three new valepotriates, velerivaltrates A (1), B (2) and C (3), together with ten known compounds (4–13) were isolated from roots and rhizomes of V. jatamansi (Fig. 1). Further biological evaluation revealed that all of compounds, except 13, indicated week to noticeable inhibitions on Cav2.2 peak currents at 30 μM. Of these compounds, 1, 6, 7, 11 and 12 are potent inhibitors with EC50 values ranging from 1.13 to 7.8 μM. In addition, these compounds also exhibited 18.2 ± 2.5% to 49.2 ± 7.1% peak current inhibitions on Cav3.1 at 30 μM. Finally, specificity study revealed that aforementioned compounds showed apparent selectivity against Cav1.2, Cav2.1, and KCNH2 (hERG) channels. Described herein are the isolation, structure elucidation, and biological activities of these compounds.
Results and discussion
Velerivaltrate A (1) was obtained as colorless oil. Its molecular formula C31H44O14 was determined by the HRESIMS ion peak at m/z 663.2626 [M + Na]+ (calcd for C31H44O14Na, 663.2623), indicating ten degrees of unsaturation. The IR (KBr, v) absorption bands at 3441 cm−1, 1738 cm−1, and 1635 cm−1, suggesting the presence of hydroxy, carbonyl and olefinic groups, respectively. The 1H and 13C spectroscopic data (Tables 1 and 2) displayed signals for a hemiketal methine [δH 6.21 (d, J = 10.1 Hz, H-1); δC 92.7 (d, C-1)], two trisubstituted olefinic bonds [δH 6.68 (s, H-3), δC 148.1 (d, C-3) and 108.7 (s, C-4); δH 5.78 (br s, H-6), δC 118.4 (d, C-6) and 139.1 (s, C-5)], and two oxymethylenes [δH 4.34 and 4.37 (each 1H, d, J = 11.7 Hz, H-10); δC 65.4 (t, C-10) and 4.62 and 4.70 (each 1H, d, J = 12.4 Hz, H-11); δC 60.8 (t, C-11)]. In addition, the resonances of six ester carbonyls at δC 167.9, 168.9, 170.3, 170.8, 170.9 and 173.2 were also observed in the 13C spectrum. Aforementioned data suggested that 1 was a valtrate hydrin-type iridoid.| Position | 1 | 2 | 3 |
|---|---|---|---|
| a Assignments may be interchangeable in each column. The 1H NMR data of the substituents at C-7 of 1 [(β-acetoxy)isovaleroxy group]: 1.96 (3H, s, H-7′′′); at C-10 of 2 [(β-acetoxy)isovaleroxy group]: 1.98 (3H, s, H-7′′). | |||
| 1 | 6.21, d (10.1) | 6.22, d (10.0) | 6.35, d (2.6) |
| 3 | 6.68, s | 6.67, s | 5.12, s |
| 6 | 5.78, br s | 5.79, br s | 2.83, dd (15.7, 7.3) 1.98, dd (13.4, 3.9) |
| 7 | 5.48, d (2.6) | 5.46, d (2.6) | 4.87, dd (4.4, 2.9) |
| 9 | 2.89, dd (10.1, 2.4) | 2.90, dd (10.0, 2.2) | 2.54, d (2.6) |
| 10 | 4.37, d (11.7) | 4.41, d (11.5) | 3.78, d (11.8) |
| 4.34, d (11.7) | 4.33, d (11.5) | 3.74, d (11.8) | |
| 11 | 4.70, d (12.4) | 4.69, d (12.4) | 4.51, d (11.9) |
| 4.62, d (12.4) | 4.63, d (12.4) | 4.38, d (11.9) | |
| 2′ | 3.03, d (14.7) | 3.07, d (14.7) | 2.26, m |
| 2.96, d (14.7) | 2.95, d (14.7) | ||
| 3′ | 2.14, m | ||
| 4′ | 1.49, sa | 1.51, sa | 0.98, d (6.5)a |
| 5′ | 1.55, sa | 1.52, sa | 0.98, d (6.5)a |
| 7′ | 2.00, s | 1.99, s | |
| 2′′ | 2.21, m | 2.92, d (14.6) | 4.93, m |
| 2.80, d (14.6) | |||
| 3′′ | 2.08, m | 2.28, m | |
| 4′′ | 0.95, d (6.7)a | 1.55, sa | 1.01, d (7.0)a |
| 5′′ | 0.95, d (6.7)a | 1.57, sa | 1.00, d (7.0)a |
| 2′′′ | 2.93, d (14.3) | 2.15, m | 2.29, m |
| 2.75, d (14.3) | |||
| 3′′′ | 2.05, m | 2.14, m | |
| 4′′′ | 1.57, sa | 0.92, d (6.5)a | 0.98, d (6.6)a |
| 5′′′ | 1.57, sa | 0.92, d (6.5)a | 0.98, d (6.6)a |
| 2′′′′ | 2.04, s | 2.03, s | 2.09, s |
| Position | 1 | 2 | 3 |
|---|---|---|---|
| a Assignments may be interchangeable in each column. The 13C NMR data of the substituents at C-7 of 1 [(3-acetoxy)isovaleroxy group]: 170.3 (C, C-6′′′), 22.3 (CH3, C-7′′′); and at C-10 of 2 [(3-acetoxy)isovaleroxy group]: 170.8 (C, C-6′′), 22.3 (CH3, C-7′′). | |||
| 1 | 92.7 CH | 92.7 CH | 87.6 CH |
| 3 | 148.1 CH | 147.9 CH | 93.5 CH |
| 4 | 108.7 C | 108.8 C | 73.8 C |
| 5 | 139.1 C | 138.8 C | 76.8 C |
| 6 | 117.5 CH2 | 117.8 CH2 | 40.8 CH2 |
| 7 | 83.4 CH | 83.2 CH | 76.3 CH |
| 8 | 80.2 C | 80.0 C | 83.6 C |
| 9 | 48.5 CH | 48.3 CH | 45.0 CH |
| 10 | 65.4 CH2 | 65.4 CH2 | 44.3 CH2 |
| 11 | 60.8 CH2 | 60.9 CH2 | 66.2 CH2 |
| 1′ | 167.9 C | 167.9 C | 170.9 C |
| 2′ | 43.9 CH2 | 43.8 CH2 | 43.1 CH2 |
| 3′ | 79.1 C | 79.2 C | 25.6 CH |
| 4′ | 26.5 CH3a | 26.9 CH3a | 22.3 CH3a |
| 5′ | 26.4 CH3a | 27.0 CH3a | 22.3 CH3a |
| 6′ | 170.8 C | 170.8 C | |
| 7′ | 22.3 CH3 | 22.3 CH3 | |
| 1′′ | 173.2 C | 170.0 C | 168.9 C |
| 2′′ | 43.0 CH2 | 44.1 CH2 | 72.8 CH |
| 3′′ | 25.6 CH | 79.3 C | 30.0 CH |
| 4′′ | 22.2 CH3a | 26.4 CH3a | 18.8 CH3a |
| 5′′ | 22.2 CH3a | 26.5 CH3a | 17.2 CH3a |
| 1′′′ | 168.9 C | 171.9 C | 172.7 C |
| 2′′′ | 44.0 CH2 | 43.4 CH2 | 43.0 CH2 |
| 3′′′ | 79.1 C | 25.7 CH | 26.7 CH |
| 4′′′ | 27.0 CH3a | 22.2 CH3a | 22.3 CH3a |
| 5′′′ | 26.5 CH3a | 22.2 CH3a | 22.3 CH3a |
| 1′′′′ | 170.9 C | 170.9 C | 169.5 C |
| 2′′′′ | 20.9 CH3 | 20.9 CH3 | 20.8 CH3 |
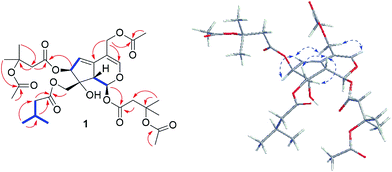
The 1H and 13C NMR spectra (Tables 1 and 2) of 1 were resemble to those of 10-acetoxy-1-acevaltrate hydrin (14)9 (Fig. S1†) with the major differences for the substituent groups at C-7 and C-10, respectively. The isovalerate group at C-7 and the acetate residue at C-10 in 14 were replaced by a β-OAc-isovalerate group and an isovalerate unit in 1, respectively. These deductions were supported by downfield shift of C-3′′′ by 53.4 ppm, the HMBC correlations of CH3-4′′′ (δH 1.57) and CH3-7′′′ (δH 1.96) to C-6′′′ (δC 170.3), H-2′′′ (δH 2.93 and 2.75, each 1H) to C-3′′′ (δC 79.1) and C-1′′′ (δC 168.9), and H-7 (δH 5.48) to C-1′′′, and the existence of spin system −2(CH3)CHCH2– (for C-4′′ and C-5′′ to C-3′′, and C-3′′ to C-2′′) and HMBC correlations of H-2′′ (δH 2.21) and H-10 (δH 4.37 and 4.34) to C-1′′ (δC 173.2), respectively. Further analyses of HMBC correlations (Fig. 2) from H-11 (δH 4.62, 4.70) and H-2′′′′ (δH 2.04, s, 3H) to C-1′′′′ (δC 170.9) located the acetoxyl group at C-11. Similarly, another β-OAc-isovalerate group was assigned at C-1 based on the HMBC correlations (Fig. 2).
The relative configuration of 1 was elucidated as follows. The anti-orientation of H-1/H-9 was suggested by a large 3JH,H (10.1 Hz), which consistent with that naturally occurring iridoids, H-1 is α-oriented and H-9 is β-oriented.9–18 In the ROESY spectrum (Fig. 2), correlations of H-10a and 10b to H-9, but not to H-1, were observed, which helped to establish the β-orientation of C-10. In contrast, correlation of H-7 and H-10 was absence in the ROESY spectrum, which revealed the orientation of H-7 as α. Comparison of the relative configurations, the experimental OR value, NMR data, as well as the biogenetic ground of 1 with those reported valepotriates9–18 indicated 1 be in agreement with the 1S, 7S, 8R and 9S configuration. Thus, the structure of 1 was determined as shown and named as velerivaltrate A.
Compound 2 appeared as colorless oil, possessing the same molecular formula C31H44O14 as that of 1 based on the positive HRESIMS (m/z 663.2626 [M + Na]+). The 1H and 13C spectroscopic data of 2 resembled to those of 1, except that the β-OAc-isovalerate group at C-7 and the isovalerate residue at C-10 in 1 were replaced by an isovalerate unit and a β-OAc-isovalerate group in 2, respectively. Study of the 1H–1H COSY spectrum of 2 revealed the existence of structure −2(CH3)CHCH2– (for C-4′′′ and C-5′′′ to C-3′′′, and C-3′′′ to C-2′′′). This evidence together with the HMBC correlations of H-2′′′ (δH 2.15) to C-3′′′ (δC 25.7) and C-1′′′ (δC 171.9), and H-7 (δH 5.46) to C-1′′′ confirmed an isovalerate located at C-7. Besides, a β-OAc-isovalerate group at C-10 was supported by downfield shift of C-3′′ by 53.7 ppm, and the HMBC correlations of CH3-4′′ and CH3-7′′/C-6′′, H-2′′/C-3′′ and C-1′′, and H-7/C-1′′. Based on the analyses of HMBC correlations an acetate unit and another β-OAc-isovalerate group were attached to C-11 and C-1, respectively, as those of 1. The configuration of 2 was demonstrated to be identical to 1 by comparison of the spectroscopic data and key ROESY correlations with those of reported valepotriates and 1.9–13 Therefore, the structure of 2 was established and named as velerivaltrate B.
Compound 3 had a molecular formula of C27H40Cl2O11 as established by HRESIMS peak at m/z 649.1579 [M + K]+ (calcd for C27H40Cl2O11K, 649.1579), with eight indices of hydrogen deficiency. Its IR spectrum exhibited the presence of OH group (3434 cm−1) and ester carbonyl unit (1744 cm−1), respectively. The 1H NMR spectrum (Table 1) displayed seven methyls [δH 2.09 (3H, s, H-2′′′′), 1.00 (6H, d, J = 7.0 Hz, H-4′′, 5′′) and 0.98 (12H, d, J = 6.6 Hz, H-4′, 4′′′, 5′, 5′′′)], four oxygenated methines [δH 6.35 (1H, d, J = 2.6 Hz, H-1), 5.12 (1H, s, H-3), 4.93 (1H, m, H-2′′) and 4.87 (1H, dd, J = 4.4, 2.9 Hz, H-7)], two characteristic isolated methylene groups [δH 3.78, 3.74 (each 1H, d, J = 11.8 Hz, H-10) and 4.51, 4.38 (each 1H, d, J = 11.9 Hz, H-11)]. The 13C NMR spectrum (Table 2) exhibited 27 carbon signals, including seven methyls, five methylenes (one oxygenated), and eight methines (four oxygenated), as well as seven quaternary carbons (four ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and three oxygenated ones). Taken together, these data indicated that, structurally, 3 was similar to chlorovaltrate A (15),11 (Fig. S1†) with the major differences for the substituent groups at C-4 and C-7. In 3, a chlorine atom substituted at C-4 (δC 73.8, s) and an acetylated methylene replaced the terminal olefinic bond at C-4 of 15. These elucidations were confirmed by its molecular formula and HMBC correlations of H-11 (4.51, 4.38, each 1H, d, J = 11.9 Hz) to C-4 (δC 73.8) and C-1′′′′ (δC 169.5), and CH3-2′′′′ (δH 2.09) to C-1′′′′ (δC 169.5), respectively. In the 1H–1H COSY spectrum, spin systems of −2(CH3)CHCH– (for C-2′′ to C-3′′ and C-4′′ and C-5′′ to C-3′′) and −2(CH3)CHCH2– (for C-2′′′ to C-3′′′ and C-4′′′ and C-5′′′ to C-3′′′) were observed, which together with HMBC correlations of H-2′′ (δH 4.93) to C-1′′ (δC 168.9) and C-1′′′ (δC 172.7), and H-2′′′ (δH 2.29) to C-1′′′ (δC 172.7) indicating the existence of an α-(isovaleroxy)isovaleroxy unit. In addition, this residue was attached to C-7 on the basis of the HMBC correlations from H-7 (δH 4.87) and H-2′′ (δH 4.93) to C-1′′ (δC 168.9).
O groups and three oxygenated ones). Taken together, these data indicated that, structurally, 3 was similar to chlorovaltrate A (15),11 (Fig. S1†) with the major differences for the substituent groups at C-4 and C-7. In 3, a chlorine atom substituted at C-4 (δC 73.8, s) and an acetylated methylene replaced the terminal olefinic bond at C-4 of 15. These elucidations were confirmed by its molecular formula and HMBC correlations of H-11 (4.51, 4.38, each 1H, d, J = 11.9 Hz) to C-4 (δC 73.8) and C-1′′′′ (δC 169.5), and CH3-2′′′′ (δH 2.09) to C-1′′′′ (δC 169.5), respectively. In the 1H–1H COSY spectrum, spin systems of −2(CH3)CHCH– (for C-2′′ to C-3′′ and C-4′′ and C-5′′ to C-3′′) and −2(CH3)CHCH2– (for C-2′′′ to C-3′′′ and C-4′′′ and C-5′′′ to C-3′′′) were observed, which together with HMBC correlations of H-2′′ (δH 4.93) to C-1′′ (δC 168.9) and C-1′′′ (δC 172.7), and H-2′′′ (δH 2.29) to C-1′′′ (δC 172.7) indicating the existence of an α-(isovaleroxy)isovaleroxy unit. In addition, this residue was attached to C-7 on the basis of the HMBC correlations from H-7 (δH 4.87) and H-2′′ (δH 4.93) to C-1′′ (δC 168.9).
The relative configuration of the core structure of 3 was established by the ROESY experiment. The cross-peaks of H-9 (biogenetically β-oriented) with H-10 and H-3 indicated the α-orientation of the oxo-bridge from C-3 to C-8. Similarly, the ROESY correlations of H-6α to H-7 indicated H-7 was α-oriented. Besides, the correlation between H-9/H-11 revealed the β-orientation of the acetylated methylene at C-4. The orientation of OH-5 was deduced as β on the basis of the comparison of the spectroscopic data of 3 with those reported chlorinated valepotriates and the molecular modeling with a rigid epoxy-bridge skeleton.10,11,13 The configuration of C-1, C-3, C-4, C-5, C-7, C-8 and C-9 were determine as S, R, S, R, S, S, and S, respectively, by the same way as that of 1. Consequently, the structure of 3 was elucidated and named as velerivaltrate C.
The known compounds were determined as valtrate hydrin B1 (4),19 valtrate hydrin B2 (5),20,21 valtrate (6),22 acevaltrate (7),22 chlorovaltrate (8),11 valeriandoid B (9),11 chlorovaltrate K (10),11 didrovaltratisovaleroyloxyhydrin (11),23 valeriananoid A (12),24 and valeriananoid C (13)24 by comparison their spectroscopic data with those reported in the literature.
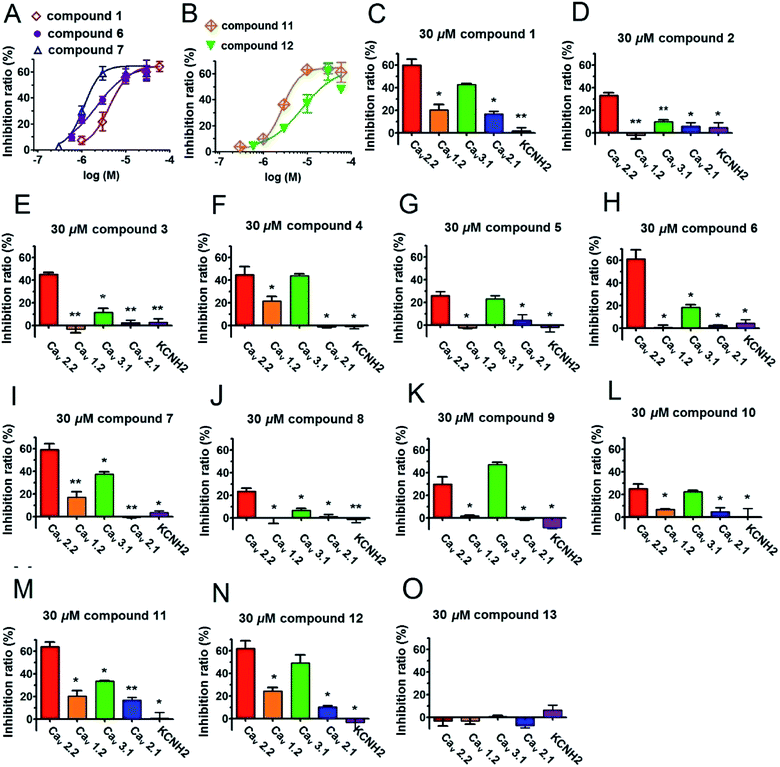
All of the isolated compounds (1–13) were evaluated their inhibitory effects on Cav2.2 and Cav3.1 calcium channels by two-electrode voltage clamp (the positive control ω-conotoxin MVIIA for Cav2.2 and mibefradil for Cav3.1 showed strong inhibitions at 200 nM and 30 μM, respectively Fig. S42†). As a result, compound 1–12 apparently inhibited Cav2.2 peak currents ranging from 23.6 ± 2.5% to 63.9 ± 3.9% at the concentration of 30 μM (Table 3). Of these, compounds 1, 6, 7, 11 and 12 were the most potent inhibitors, with EC50 values of 4.33, 2.18, 1.13, 2.70 and 7.8 μM, respectively. Interestingly, the inhibition by these compounds was incomplete, plateauing at ∼60% by a near saturation concentration of 30 μM (Fig. 3). This result suggests that compounds 1, 6, 7, 11 and 12 act allosterically to modulate CaV2.2 gating rather than block channel conduction. On the other hand, compounds 1, 4, 5, 6, 7, 9, 10, 11, and 12 obviously showed peak current inhibitions on Cav3.1 at 30 μM (inhibition ratio between 18.2 ± 2.5% to 49.2 ± 7.1%, Table 3). Among them, compounds 1, 4, 7, 9, 11 and 12 presented potent inhibitory effects on Cav3.1 with a roughly average value of 42% (Table 3). In addition, we also tested the antagonistic effects of compounds 1–12 on several other types of voltage-gated ion channels, including L-type (Cav1.2), P/Q-type (Cav2.1) and KCNH2 (hERG). Significantly, aforementioned compounds exhibited weaker or no effect on those channels (Table 3 and Fig. 3).
| Compounds (30 μM) | Inhibitory ratio (%) | ||||
|---|---|---|---|---|---|
| Cav1.2 | Cav2.1 | Cav2.2 | Cav3.1 | KCNH2 | |
| a As blank control. All the data were analyzed with two-tailed student T test and represented as mean ± SEM (n = 3), *P < 0.05, **P < 0.01, compared with blank control. | |||||
| 0.1% DMSOa | 1.3 ± 0.3 | 1.0 ± 0.2 | 0.5 ± 1.2 | 1.7 ± 0.4 | 1.8 ± 0.2 |
| 1 | 20.4 ± 4.6* | 16.0 ± 2.5* | 59.9 ± 2.4* | 43.5 ± 0.7** | 2.2 ± 6.7 |
| 2 | −2.1 ± 3.2* | 4.0 ± 3.2 | 33.1 ± 2.4* | 10.0 ± 1.2* | 2.7 ± 1.8 |
| 3 | −3.5 ± 3.2 | 2.2 ± 2.5 | 45.1 ± 1.7** | 11.5 ± 3.8* | 2.6 ± 3.2 |
| 4 | 21.6 ± 3.9* | −1.5 ± 0.7 | 41.4 ± 10.5* | 43.7 ± 2.0** | −1.4 ± 1.9 |
| 5 | −2.5 ± 1.0 | 4.4 ± 4.9 | 26.0 ± 3.5* | 22.9 ± 2.9* | −2.2 ± 3.8 |
| 6 | 0.6 ± 2.2 | 0.6 ± 2.3 | 61.2 ± 7.9* | 18.2 ± 2.5* | 4.4 ± 3.0 |
| 7 | 16.9 ± 4.9* | −0.7 ± 0.6 | 56.9 ± 7.2* | 37.5 ± 2.0** | 3.4 ± 1.5 |
| 8 | −0.1 ± 5.1 | 1.0 ± 2.2 | 23.6 ± 2.5* | 6.5 ± 1.8* | −1.3 ± 3.0 |
| 9 | 12.1 ± 1.7* | 2.0 ± 0.1 | 30.0 ± 6.4* | 47.0 ± 2.1** | −8.7 ± 1.2 |
| 10 | 6.5 ± 0.6* | 4.5 ± 3.4 | 25.1 ± 4.0* | 22.3 ± 1.3* | 0.2 ± 7.4 |
| 11 | 20.3 ± 4.6* | 16.7 ± 2.5* | 63.9 ± 3.9* | 33.3 ± 0.4** | 0.7 ± 5.3 |
| 12 | 24.2 ± 3.3* | 10.2 ± 1.1* | 61.8 ± 6.8* | 49.2 ± 7.1* | −3.5 ± 7.3 |
| 13 | −3.3 ± 2.7 | −9.5 ± 5.0* | −3.5 ± 4.1 | 0.6 ± 1.0 | 6.1 ± 4.4* |
Conclusions
In summary, our study shows that valepotriates, the main constituents of the V. Jatamansi, can selectively inhibit Cav2.2 and Cav3.1 calcium channels, which consistent with the analgesic effect of this herb medicine in alleviating gastrointestinal and rheumatic pain. This discovery not only gives the potential clue of the active constituents responsible for the traditional use of V. Jatamansi but also afford a new structural prototype of Cav2.2 and Cav3.1 dual-inhibitor. In future, bioassay-guided investigations on V. Jatamansi may yield more specific and potent compounds that target N-type and T-type calcium channels.Experimental section
General experimental procedures
Column chromatography (CC) was run on Silica gel (200–300 mesh, Qingdao Marine Chemical, Inc.), Lichroprep RP-18 (40–63 μm, Merck), MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Japan), and Sephadex LH-20 (Amersham Biosciences, Sweden). Fractions were monitored by TLC (silica gel 60 F254, Qingdao Marine Chemical, Inc.) using various solvent systems, and spots were visualized by UV light (254 nm) and sprayed with 5% sulfuric acid in EtOH, followed by heating. Semipreparative HPLC was carried out on an Agilent 1100 liquid chromatography system using an YMC-Pack 10 mm × 250 mm column (Pro C18 RS). All currents were recorded with an OC-725C Oocyte Clamp (Warner Instrument Corp.) and digitized by Digidata 1440A (Molecular Devices). Optical rotations were recorded on a JASCO model 1020 polarimeter (Horiba, Tokyo, Japan). IR (KBr) spectra were obtained using a Tenor 27 spectrophotometer (Bruker Optics GmbH, Ettlingen, Germany). UV spectra were measured on a Shimadzu UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan). 1D and 2D NMR spectra were performed on a Bruker Avance III 600 spectrometer (Bruker, Bremerhaven, Germany). Chemical shifts (δ) are expressed in ppm with reference to the solvent signals. ESIMS and HRESIMS were run on a Shimadzu LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan) or an Agilent G6230 TOF MS (Agilent Technologies, Palo Alto, USA) or an API QSTAR time-of-flight (AB-MDS Sciex, Concord, ON, Canada).Plant material
The roots of V. Jatamansi were purchased in July 2012 from Yunnan Hongxiang Yixintang Pharmaceutical Co., Ltd. and identified by Dr Zhi-Kun Wu. A voucher specimen (KUN no. 0864803) has been deposited at Herbarium of Kunming Institute of Botany, the Chinese Academy of Sciences, Kunming, China.Extraction and isolation
The air-dried roots of V. Jatamansi (450 g) were powdered and extracted with 95% EtOH (4 × 3 L, each 24 h) at room temperature. The combined EtOH extracts were evaporated in vacuo to afford a crude residue (75.5 g), part of which (43.62 g) was suspended in water (0.4 L) and fractionated successively into EtOAc soluble (13.83 g) and n-BuOH-soluble (5.39 g) fractions. The EtOAc layer was subjected to silica gel CC with a gradient elution of petroleum ether/Me2CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10 fractions (Fr.1–Fr.10). Fr.2 (160 mg) was separated by a repeated silica gel CC (petroleum ether/Me2CO, gradient from 50
1) to give 10 fractions (Fr.1–Fr.10). Fr.2 (160 mg) was separated by a repeated silica gel CC (petroleum ether/Me2CO, gradient from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and then purified by Sephadex LH-20 column (MeOH/CHCl3 = 1
1), and then purified by Sephadex LH-20 column (MeOH/CHCl3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 13 (2 mg). Similarly, Fr.3 (370 mg) was passed through silica gel CC (petroleum ether/Me2CO, gradient from 20
1) to give compound 13 (2 mg). Similarly, Fr.3 (370 mg) was passed through silica gel CC (petroleum ether/Me2CO, gradient from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then purified by Sephadex LH-20 column (MeOH), to afford compound 6 (82.0 mg). Fr.4 (59 mg) was subjected to RP-18 (MeOH/H2O, gradient from 60
1) and then purified by Sephadex LH-20 column (MeOH), to afford compound 6 (82.0 mg). Fr.4 (59 mg) was subjected to RP-18 (MeOH/H2O, gradient from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 90
40 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), then followed by a silica gel CC (petroleum ether/Me2CO, gradient from 15
10), then followed by a silica gel CC (petroleum ether/Me2CO, gradient from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and purified by Sephadex LH-20 column (MeOH) to give compounds 7 (2 mg), 10 (4 mg) and 4 (8 mg). Fr.5 (58 mg) was decolorized on a MCI gel (MeOH/H2O gradient, 60
1) and purified by Sephadex LH-20 column (MeOH) to give compounds 7 (2 mg), 10 (4 mg) and 4 (8 mg). Fr.5 (58 mg) was decolorized on a MCI gel (MeOH/H2O gradient, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–85
40–85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), then separated and purified by Sephadex LH-20 column (MeOH) to give 12 (6 mg). Fr.6 (289 mg) was successively separated by a silica gel CC (petroleum ether/Me2CO gradient, 10
15), then separated and purified by Sephadex LH-20 column (MeOH) to give 12 (6 mg). Fr.6 (289 mg) was successively separated by a silica gel CC (petroleum ether/Me2CO gradient, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Sephadex LH-20 column (MeOH) and semi-preparative HPLC (MeOH/H2O gradient, 80
1), Sephadex LH-20 column (MeOH) and semi-preparative HPLC (MeOH/H2O gradient, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–80
20–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 5 (2 mg), 8 (29 mg), 9 (41 mg) and 11 (25 mg). Fr.8 (594 mg) was subjected to RP-18 (MeOH/H2O gradient, 50
10) to obtain 5 (2 mg), 8 (29 mg), 9 (41 mg) and 11 (25 mg). Fr.8 (594 mg) was subjected to RP-18 (MeOH/H2O gradient, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50–80
50–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), then followed by a silica gel CC (petroleum ether/Me2CO gradient, 8
20), then followed by a silica gel CC (petroleum ether/Me2CO gradient, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and purified by Sephadex LH-20 column (MeOH) to give 3 (8 mg). Fr.9 (422 mg) was successively separated by a silica gel CC (petroleum ether/Me2CO gradient, 5
1) and purified by Sephadex LH-20 column (MeOH) to give 3 (8 mg). Fr.9 (422 mg) was successively separated by a silica gel CC (petroleum ether/Me2CO gradient, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–0
1–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), semi-preparative HPLC (MeOH/H2O, 75
1), semi-preparative HPLC (MeOH/H2O, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) and purified by Sephadex LH-20 column (MeOH) to yield 1 (3 mg) and 2 (2 mg).
25) and purified by Sephadex LH-20 column (MeOH) to yield 1 (3 mg) and 2 (2 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax): 255 (2.70), 201 (2.15) nm; IR (KBr): νmax 3441, 2961, 2928, 1738, 1635, 1618, 1466, 1441, 1371, 1258, 1166, 1101, 1066, 1049, 1023, 969 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 663; positive HRESIMS [M + Na]+ m/z 663.2626 (calcd for C31H44O14Na, 663.2623).
εmax): 255 (2.70), 201 (2.15) nm; IR (KBr): νmax 3441, 2961, 2928, 1738, 1635, 1618, 1466, 1441, 1371, 1258, 1166, 1101, 1066, 1049, 1023, 969 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 663; positive HRESIMS [M + Na]+ m/z 663.2626 (calcd for C31H44O14Na, 663.2623).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax): 255 (1.84), 203 (1.60) nm; IR (KBr): νmax 3439, 2961, 2928, 1737, 1629, 1613, 1383, 1371, 1260, 1166, 1102, 1065, 1045, 1024, 953, 803 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 663; positive HRESIMS [M + Na]+ m/z 663.2629 (calcd for C31H44O14Na, 663.2623).
εmax): 255 (1.84), 203 (1.60) nm; IR (KBr): νmax 3439, 2961, 2928, 1737, 1629, 1613, 1383, 1371, 1260, 1166, 1102, 1065, 1045, 1024, 953, 803 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 663; positive HRESIMS [M + Na]+ m/z 663.2629 (calcd for C31H44O14Na, 663.2623).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax): 201 (1.26), 216 (1.15) nm; IR (KBr): νmax 3434, 2963, 2933, 2874, 1744, 1467, 1448, 1371, 1335, 1295, 1243, 1185, 1166, 1123, 1032, 982, 956 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 633; positive HRESIMS [M + K]+ m/z 649.1579 (calcd for C27H40Cl2O11K, 649.1579).
εmax): 201 (1.26), 216 (1.15) nm; IR (KBr): νmax 3434, 2963, 2933, 2874, 1744, 1467, 1448, 1371, 1335, 1295, 1243, 1185, 1166, 1123, 1032, 982, 956 cm−1; see Tables 1 and 2 for 1H NMR (600 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3); positive ESIMS [M + Na]+ m/z 633; positive HRESIMS [M + K]+ m/z 649.1579 (calcd for C27H40Cl2O11K, 649.1579).Oocytes preparation and expression
Oocytes were got from adult Xenopus by digesting its ovarian lobes with collagenase A for 2–3 h under 180 rpm min−1 shaking in OR2 (NaCl 82.4 mM, KCl 2.5 mM, MgCl2 1 mM, HEPES 5 mM, pH adjusted to 7.6 with NaOH). The best Stages V–VI oocytes were selected, injected with RNA (50–100 ng), and were incubated at 18 °C for 3–6 days depending on RNA expression in ND96 (NaCl 96 mM, KCl 2.5 mM, MgCl2 1 mM, HEPES 5 mM, CaCl2 1.8 mM, penicillin 100 units per mL, streptomycin 100 μg mL−1, pH adjusted to 7.6 with NaOH).Electrophysiological assay
All experiments were performed at 20 °C. The whole-oocyte recordings were taken by two-electrode voltage clamp. The electrodes with a resistance of 0.3–1 MΩ were filled with KCl (3 mM). All the calcium channels were recorded in Ba2+ solution (NaOH 50 mM, KCl 2 mM, HEPES 5 mM, BaCl2 1.8 mM, Ba(OH)2 40 mM, pH adjusted to 7.4 with methanesulfonic acid). KCNH2 were recorded in DN96. Cav1.2, Cav2.1 and Cav2.2 current traces were evoked from a holding potential of −80 mV by 50 ms depolarizations ranging from −30 mV to +70 mV in 10 mV increment at 3 s interval. Cav3.1 current traces were evoked from a holding potential of −80 mV by 50 ms depolarizations ranging from −50 mV to +60 mV in 10 mV increment at 3 s interval. KCNH2 current traces were first depolarized at +40 mV for 1 s from a holding potential of −80 mV, then evoked by 3 s depolarization ranging from −120 mV to +40 mV in 10 mV increment at 30 s intervals. All the currents were sampled at 10 kHz and filtered at 10 kHz.All isolated compounds (1–13) were tested for their selectivity on CaV1.2, CaV2.1, CaV2.2, CaV3.1 and KCNH2 with the concentration of 30 μM. Moreover, five compounds (1, 6, 7, 11 and 12) were tested with different concentration (0.3, 0.6, 1, 3, 10, 30, 60, μM) on CaV2.2 to evaluate their dose–response relationships.
Data analysis and statistics
Data acquisition and analysis of the whole-oocyte recording was carried out by using pClamp 10 (Molecular Devices Corporation, California, United States of America). Data fitting and statistical analysis were performed using PRISM 5.0 (GraphPadSoftware Inc., San Diego, CA). EC50 values and Hill slopes were determined by fitting the data points from five different drug concentrations to a modified Hill equation with the form of % inhibition = max + (min − max)/[1 + (C/IC50)n], where max and min represent minimum and maximum inhibition, respectively, and C is the drug concentration, n the Hill coefficient. All the data were presented as mean ± SEM, and statistical analyses were performed using two-tailed Students t test. Different with P value < 0.05 were defined as significant and levels of significance were marked by asterisks (**P < 0.01, *P < 0.05).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from Yunnan province (2016FB138) and State Key Laboratory of Phytochemistry and Plant Resources in West China (P2015-KF03) to Y. Nian, by grant from the High-level Overseas Talents of Yunnan Province, and Yunnan Major Science and Technology Project (2015ZJ002) to J. Yang, and by grants from Yunnan province (2013IB021, 2015HB093 and provincial academician free exploration project) to J. M. Hu.Notes and references
- D. P. Wermeling, Pharmacotherapy, 2005, 25, 1084–1094 CrossRef CAS PubMed.
- W. J. Choe, V. M. Gadotti, F. X. Zhang, B. Park, J. H. Nam, V. Onnis, G. Balboni, J. Y. Lee and G. W. Zamponi, Pflugers Arch., 2016, 468, 193–199 CrossRef PubMed.
- W. Choe, R. B. Messinger, E. Leach, V. S. Eckle, A. Obradovic, R. Salajegheh, V. Jevtovic-Todorovic and S. M. Todorovic, Mol. Pharmacol., 2011, 80, 900–910 CrossRef CAS PubMed.
- G. W. Zamponi, Nat. Rev. Drug Discovery, 2016, 15, 19–34 CrossRef CAS PubMed.
- J. H. Kim, G. Keum, H. Chung and G. Nam, Eur. J. Med. Chem., 2016, 123, 665–672 CrossRef CAS PubMed.
- J. Serra, W. R. Duan, C. Locke, R. Solà, W. Liu and W. Nothaft, Pain, 2015, 156, 2175–2183 CrossRef CAS PubMed.
- C. S. Mathela, C. S. Chanotiya, S. S. Sammal, A. K. Pant and S. Pandey, Chem. Biodiversity, 2005, 2, 1174–1182 CAS.
- Chinese Pharmacopoeia Commission, The Pharmacopoeia of Chinese People's Republic, ed. L. M. Sao, The Chemical Industry Publishing House, Beijing, China, 2010, vol. 1, pp. 345–346 Search PubMed.
- Y. P. Tang, X. Liu and B. Yu, J. Nat. Prod., 2002, 65, 1949–1952 CrossRef CAS PubMed.
- S. Lin, Y. H. Sheng, Z. X. Zhang, L. Shen, H. L. Li, L. Shan, R. H. Liu, X. K. Xu and W. D. Zhang, J. Nat. Prod., 2010, 73, 1723–1726 CrossRef CAS PubMed.
- S. Lin, Z. X. Zhang, T. Chen, J. Ye, W. X. Dai, L. Shan, J. Su, Y. H. Shen, H. L. Li, R. H. Liu, X. K. Xu, H. Wang and W. D. Zhang, Phytochemistry, 2013, 85, 185–193 CrossRef CAS PubMed.
- R. Wang, D. Xiao, Y. H. Bian, X. Y. Zhang, B. J. Li, L. S. Ding and S. L. Peng, J. Nat. Prod., 2008, 71, 1254–1257 CrossRef CAS PubMed.
- S. J. Wang, X. Q. Qiu, J. Y. Zhu, X. Q. Ma, B. Lin, C. J. Zheng and L. P. Qin, Helv. Chim. Acta, 2014, 97, 722–726 CrossRef CAS.
- W. A. Catterall and A. P. Few, Neuron, 2008, 59, 882–901 CrossRef CAS PubMed.
- R. E. Westenbroek, J. W. Hell, C. Warner, S. J. Dubel, T. P. Snutch and W. A. Catterall, Neuron, 1992, 9, 1099–1115 CrossRef CAS PubMed.
- L. M. Kerr, F. Filloux, B. M. Olivera, H. Jackson and J. K. Wamsley, Eur. J. Pharmacol., 1988, 146, 181–183 CrossRef CAS PubMed.
- K. Gohil, J. R. Bell, J. Ramachandran and G. P. Miljanich, Brain Res., 1994, 653, 258–266 CrossRef CAS PubMed.
- A. Pertovaara, Prog. Neurobiol., 2006, 80, 53–83 CrossRef CAS PubMed.
- J. Hoelzl, V. M. Chari and O. Seligmann, Tetrahedron Lett., 1976, 15, 1171–1174 CrossRef.
- E. Finner, S. David and P. W. Thies, Planta Med., 1984, 50, 4–6 CrossRef CAS PubMed.
- S. Lin, Y. H. Shen, H. L. Li, X. W. Yang, T. Chen, L. H. Lu, Z. S. Huang, R. H. Liu, X. K. Xu, W. D. Zhang and H. Wang, J. Nat. Prod., 2009, 72, 650–655 CrossRef CAS PubMed.
- P. W. Thies and S. Funke, Tetrahedron Lett., 1966, 7, 1155–1162 CrossRef.
- P. W. Thies, S. David, I. Hell and K. U. Wolf, US Pat., 07191819, 1988.
- D. S. Ming, D. Q. Yu, Y. Y. Yang and C. H. He, Tetrahedron Lett., 1997, 38, 5205–5208 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: 1D and 2D NMR spectra, HRESIMS spectra, IR spectra, UV spectra of new compounds 1–3. See DOI: 10.1039/c7ra07327e |
| ‡ He-Hai Jiang and Fa-Wu Dong contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |