Open Access Article
Open Access ArticleFast screening of whole blood samples for early detection and monitoring of thyroid diseases
Raluca-Ioana Stefan-van Staden *ab and
Grigorina Mitrofana
*ab and
Grigorina Mitrofana
aFaculty of Applied Chemistry and Materials Science, Politehnica University of Bucharest, 1-7 Polizu St., Bucharest, 011061, Romania. E-mail: ralucavanstaden@gmail.com; Tel: +40751507779
bLaboratory of Electrochemistry and PATLAB Bucharest, National Institute of Research for Electrochemistry and Condensed Matter, 202 Splaiul Independentei St., Bucharest, 060021, Romania
First published on 8th September 2017
Abstract
This paper described a fast and reliable method for the screening of whole blood for TSH, L-T4, L-T3 and D-T4 using stochastic sensors. The main advantage of this method is the possibility of tracing the precise diagnosis of thyroid dysfunction from a single whole blood sample by multianalyte screening in one run, with low cost. Six stochastic microsensors based on mixtures between inulins: Frutafruit TEX (TEX) and inulin Inutec (IN), and ionic liquids: L-phenylalanine-tert-butyl-ester-lactate (L-PheC4-Lac), L-alanine-tert-butyl-ester-L-lactate (L-AlaC4-Lac) and L-alanine-tert-butyl-ester-nitrate (L-AlaC4-NO3), physically immobilized in a diamond paste matrix were designed and used for the screening tests. The tests were reliable for the assay of TSH, L-T4, L-T3 and D-T4 in whole blood samples, with recoveries higher than 98.00% with RSD values lower than 1.00% being recorded.
Introduction
The analysis of different biomolecules is of prime importance for life science research and medical diagnostics. Therefore, molecular medicine could represent an important step to personalized medicine, developing also the possibility of early diagnosis.1Stochastic sensors represent a new class of single-molecule detectors of increasing interest due to their capacity for assessing both qualitatively and quantitatively the analyte of interest, being able to detect simultaneously more than one biomarker.
The stochastic technique consists of measuring the electrical fluctuations of ions that pass through the nanopore at a fixed applied potential. The modulation of the ion current is induced by reversibly binding analytes of interest to the wall of the channel. From the diagrams associated with the individual binding events the signature of the analyte can be identified (the value of toff) and its concentration revealed by ton.
This type of analysis allows the usage of chemical modifiers in order to improve their potential to detect a wide range of molecules.2 Thus in this paper we demonstrated the ability of a mixture made by different types of ionic liquids and inulins to assess four thyroid hormones: thyroid stimulating hormone (TSH), levothyroxine (L-T4), dextrothyroxine (D-T4) and triiodothyronine (L-T3). The enantiorecognition ability of ionic liquids or inulins for the detection of thyroid hormones has been demonstrated elsewhere.3,4
One of the most frequent endocrine diseases are those of thyroid.5 The key assays that are used to detect thyroid dysfunction are serum thyroid stimulating hormone (TSH) and the main circulating thyroid hormones thyroxine (f-L-T4) and triiodothyronine (f-L-T3).1 The level of TSH is used by physicians as a screening test for thyroid diseases. Elevated concentrations of TSH usually represents a sign of a decreased L-T4 or L-T3 production, while suppressed levels can point an excessive activity of thyroid gland.1 The determination of TSH serves not only as a preliminary test for thyroid status, but the high level of TSH is associated with increased thyroid cancer incidence and advanced-stage disease.6
For L-T4 and L-T3 equilibrium dialysis and ultrafiltration represented the gold-standard methods7,8 but they are no longer used, being expensive and time consuming. Other methods such as MS9 LC-MS,10 HPLC,11 RIA12 and CLEIA13 have been developed. Also for the assessment of TSH the most used methods were developed based on immunoassay techniques such as ECLIA,14,15 ELISA,16 IRMA,17 EIA18 and RIA.19 Improved or newer technologies are proving to be viable alternatives, sometimes offering better sensitivity, increased throughput and lower matrix interference. Thus, L-T3, L-T4 and D-T4 have been analyzed with different electrochemical sensors,20–22 biosensors23 and immunosensors.24 For the assay of TSH, immunosensors,25 biosensors26 and optical sensors27 have been reported. Recently, reliable methods of analysis were proposed for assay of biomarkers specific to different illnesses.28–30
This paper proposed a fast and reliable method for the screening of whole blood for TSH, L-T4, L-T3 and D-T4 using stochastic sensors. The main advantage of this method is the possibility of tracing the precise diagnosis of thyroid dysfunction from a single whole blood sample by multianalyte assay in one run, with low cost. Six stochastic microsensors based on mixtures between inulins: Frutafruit TEX (TEX) and inulin Inutec (IN), and ionic liquids: L-phenylalanine-tert-butyl-ester-lactate (L-PheC4-Lac), L-alanine-tert-butyl-ester-L-lactate (L-AlaC4-Lac) and L-alanine-tert-butyl-ester-nitrate (L-AlaC4-NO3) physically immobilized in a diamond paste matrix were designed and used for the screening tests.
Experimental
Materials and methods
Standard solutions of different concentration were obtained by serial dilution. All solutions were fresh prepared before measurements. When not in use, all the solutions were stored in the freezer at 2–8 °C.
Each modified paste was places in a plastic tube with an internal diameter of the active surface of 300 μm. The electric contact was obtained using an Ag wire inserted into the modified paste. The surface of the microsensor was renewed by polishing with aluminum paper and wetted with deionised water before using. When not in use, the microelectrodes were stored in a dry state at room temperature.
Whole blood samples. Whole blood samples were obtained from 9 patients, who have been previously evaluated in the Elias Emergency University Hospital Bucharest. All experiments were performed in compliance with the EU guidelines, and approved by the medical ethics committee at University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania (Ethics committee approval no. 11/2013). Informed consents were obtained from human participants of this study. The apparatus cell was filled with the whole blood samples and the stochastic measurements were performed. The unknown concentrations were determined from the calibration graphs as described above in the Recommended procedure section.
Results and discussions
Response characteristics of the stochastic sensors
The response of stochastic sensors is based on channel conductivity, when a fixed potential is applied. Accordingly, for the assay of the thyroid hormones a potential of 125 mV vs. Ag/AgCl was applied. The measurement process of a single molecule is taking place in two stages: the first stage on which the molecule is entering the channel, blocking it – when the current is dropping to zero value (the time designed as toff, that the molecule needs to go into the channel is called signature of the analyte and depends on the size and geometry of the molecule, its capacity of unfolding and of velocity needed to enter the channel); the second stage is the one on which binding with the wall of the channel are taking place (the time spend for these processes is called ton – which is measured between two toff events):3,4| Ch(i) + f-L-T3(i) ⇔ Ch·f-L-T3(i) |
| Ch(i) + f-L-T4(i) ⇔ Ch·f-L-T4(i) |
| Ch(i) + f-D-T4(i) ⇔ Ch·f-D-T4(i) |
| Ch(i) + TSH(i) ⇔ Ch·TSH(i) |
Response characteristics are shown in Table 1. Different signatures of TSH, f-L-T4, f-L-T3 and f-D-T4 were obtained for the proposed sensors; there are only two sensors for which the differences between the signatures of TSH, f-L-T4, f-L-T3 and f-D-T4 are very small: the sensors based on IN-L-Ala-NO3, and TEX-L-Phe-C4-L-Lac and therefore difficult to assess in real samples. All sensors exhibited high sensitivity for the simultaneous assay of TSH, f-L-T4, f-L-T3 and f-D-T4. The lowest limit of determination for f-L-T3 was achieved using the sensor based on IN-L-Ala-C4-L-Lac, while for f-L-T4 was achieved using the sensor based on IN-L-Phe-C4-L-Lac, and for f-D-T4 the lowest limit of determination was given by the sensor based on TEX-L-Ala-C4-L-Lac. For TSH assay, no differences in limit of determination were recorded when the six sensors' response characteristics were determined. Regarding all response characteristics as well as the signatures of the hormones, the sensor of choice for simultaneous assay of thyroid hormones is the one based on TEX-L-Ala-C4-NO3-L-Lac.
| Microsensors based on: | Signature of the enantiomer (toff) | Sensibility | Linear concentration range | Quantification limit: | Equation of calibration; correlation coefficient |
|---|---|---|---|---|---|
| f-L-T3 (mol L−1 s−1) | |||||
| IN-L-Ala-C4-L-Lac | 1 | 3.08 × 1010 | 4 × 10−13 to 10−12 | 4 × 10−13 | 1/ton = 0.04 + 3.08 × 1010 × C; r = 0.9967 |
| IN-L-Phe-C4-L-Lac | 1 | 4.02 × 109 | 8 × 10−12 to 4 × 10−12 | 8 × 10−12 | 1/ton = 0.02 + 4.02 × 109 × C; r = 0.9977 |
| IN-L-Ala-C4-NO3-L-Lac | 1 | 3.00 × 1010 | 2 × 10−12 to 8 × 10−12 | 2 × 10−12 | 1/ton = 0.05+3 × 1010 × C; r = 0.9295 |
| TEX-L-Ala-C4-L-Lac | 1.7 | 2.63 × 109 | 6 × 10−12 to 10−11 | 6 × 10−12 | 1/ton = 0.03 + 2.63 × 109 × C; r = 0.9959 |
| TEX-L-Phe-C4-L-Lac | 0.7 | 7.21 × 1010 | 10−12 to 4 × 10−13 | 1 × 10−12 | 1/ton = 0.01 + 7.21 × 1010 × C; r = 0.9973 |
| TEX-L-Ala-C4-NO3-L-Lac | 0.7 | 2.28 × 107 | 10−9 to 10−11 | 1 × 10−9 | 1/ton = 0.05 + 2.28 × 107 × C; r = 0.0986 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| f-L-T4 (mol L−1 s−1) | |||||
| IN-L-Ala-C4-L-Lac | 0.7 | 5.44 × 108 | 8 × 10−12 to 10−10 | 8 × 10−12 | 1/ton = 0.03 + 5.44 × 108 × C; r = 0.9833 |
| IN-L-Phe-C4-L-Lac | 0.7 | 5.93 × 109 | 10−12 to 4 × 10−12 | 1 × 10−12 | 1/ton = 0.03 + 5.93 × 109 × C; r = 0.9833 |
| IN-L-Ala-C4-NO3-L-Lac | 0.7 | 5.41 × 109 | 8 × 10−13 to 2 × 10−12 | 8 × 10−13 | 1/ton = 0.01 + 5.41 × 109 × C; r = 0.9561 |
| TEX-L-Ala-C4-L-Lac | 1.3 | 5.62 × 106 | 10−10 to 10−8 | 1 × 10−10 | 1/ton = 0.01 + 5.62 × 106 × C; r = 0.9985 |
| TEX-L-Phe-C4-L-Lac | 0.5 | 1.21 × 108 | 8 × 10−12 to 10−10 | 8 × 10−12 | 1/ton = 0.03 + 1.21 × 108 × C; r = 0.9361 |
| TEX-L-Ala-C4-NO3-L-Lac | 1.1 | 5.97 × 108 | 8 × 10−12 to 10−10 | 8 × 10−12 | 1/ton = 0.01 + 5.97 × 108 × C; r = 0.9937 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| D-T4 (mol L−1 s−1) | |||||
| IN-L-Ala-C4-L-Lac | 2.1 | 2.63 × 104 | 10−6 to 10−8 | 1 × 10−6 | 1/ton = 0.03 + 2.63 × 104 × C; r = 0.9758 |
| IN-L-Phe-C4-L-Lac | 1.1 | 2.2 × 109 | 6 × 10−12 to 10−11 | 6 × 10−12 | 1/ton = 0.02 + 2.2 × 109 × C; r = 0.9991 |
| IN-L-Ala-C4-NO3-L-Lac | 0.4 | 3.56 × 109 | 4 × 10−12 to 8 × 10−12 | 4 × 10−12 | 1/ton = 0.02 + 3.56 × 109 × C; r = 0.9543 |
| TEX-L-Ala-C4-L-Lac | 2.4 | 4.8 × 1010 | 4 × 10−13 to 10−12 | 4 × 10−13 | 1/ton = −0.007 + 4.8 × 1010 × C; r = 0.995 |
| TEX-L-Phe-C4-L-Lac | 1.0 | 3.14 × 102 | 10−6 to 10−4 | 1 × 10−6 | 1/ton = 0.04 + 3.14 × 102 × C; r = 0.9411 |
| TEX-L-Ala-C4-NO3-L-Lac | 1.8 | 9.48 × 109 | 8 × 10−13 to 2 × 10−12 | 8 × 10−13 | 1/ton = 0.01 + 9.48 × 109 × C; r = 0.9981 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| TSH (UI L−1) | |||||
| IN-L-Ala-C4-L-Lac | 0.4 | 2.96 × 10−1 | 5.6 × 10−4 to 5.6 × 10−2 | 5.6 × 10−4 | 1/ton = 0.02 + 2.96 × 10−1 × C; r = 0.9994 |
| IN-L-Phe-C4-L-Lac | 1.2 | 5.89 × 10−4 | 5.6 × 10−1 to 5.6 × 101 | 5.6 × 10−1 | 1/ton = 0.03 + 5.89 × 10−4 × C; r = 0.9998 |
| IN-L-Ala-C4-NO3-L-Lac | 0.6 | 3.52 × 103 | 5.6 × 10−8 to 5.6 × 10−6 | 5.6 × 10−8 | 1/ton = 0.03 + 3.52 × 103 × C; r = 0.9994 |
| TEX-L-Ala-C4-L-Lac | 0.3 | 4.34 × 103 | 5.6 × 10−8 to 5.6 × 10−6 | 5.6 × 10−8 | 1/ton = 0.02 + 4.34 × 103 × C; r = 0.9996 |
| TEX-L-Phe-C4-L-Lac | 1.2 | 2.24 × 10−1 | 5.6 × 10−6 to 5.6 × 10−4 | 5.6 × 10−6 | 1/ton = 0.03 + 2.24 × 10−1 × C; r = 0.9993 |
| TEX-L-Ala-C4-NO3-L-Lac | 0.4 | 7.06 × 103 | 5.6 × 10−8 to 5.6 × 10−6 | 5.6 × 10−8 | 1/ton = 0.02 + 7.06 × 103 × C; r = 0.9788 |
The sensors were used for more than 3 months when their sensitivities' RSD (%) values did not exceed 1.00%, proving that the sensors are stable for this period, when accurate and precise determinations were performed for whole blood samples.
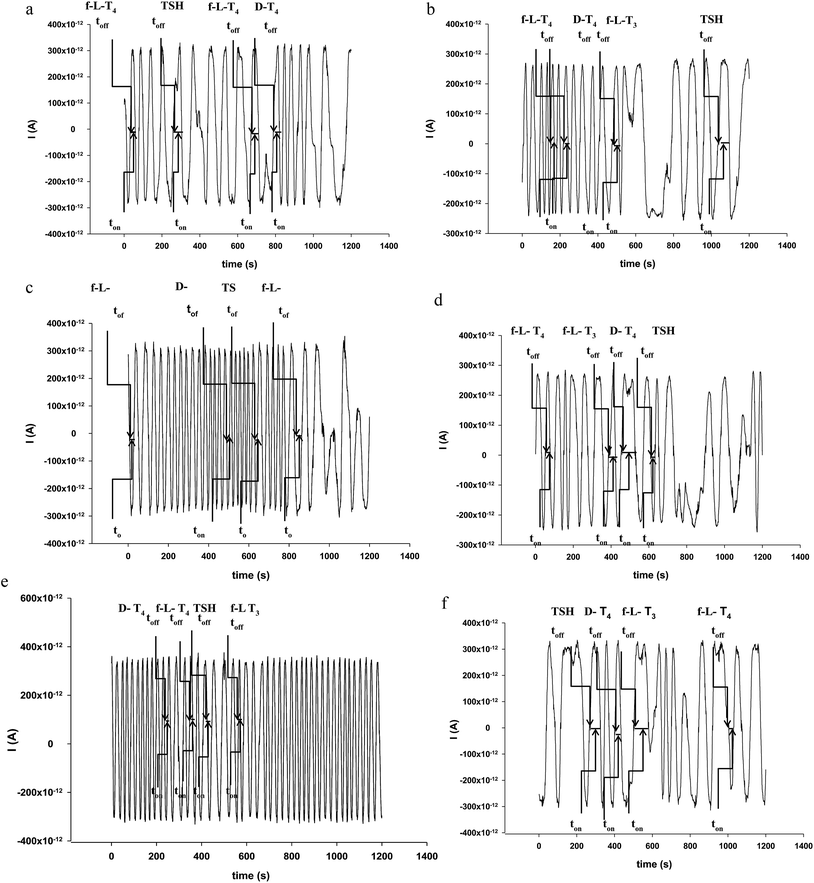
The selectivity of sensors is given for this type of sensors by the signatures recorded for each compound analysed with the same sensor. The sensors were tested for f-L-T3, f-L-T4, f-D-T4, TSH and thyroid hormone receptor; different values were obtained for each of the compounds when the same sensor was used. The different values obtained for the signatures made possible the correct identification of the signal of the hormones in the diagrams (Fig. 1), and accordingly reliable qualitative analysis followed by quantitative analysis.
Analytical applications
The response characteristics, selectivity, and reliability of the microsensors made possible the simultaneous assay of f-L-T3, f-L-T4, f-D-T4, and TSH in whole blood samples. The whole blood samples were analysed as collected from the nine patients, immediately after they were collected from patients. Diagrams were recorded (Fig. 1), and signatures (toff values) identified for f-L-T3, f-L-T4, f-D-T4 and TSH in each diagram. After identifying the signature for each biomarker (f-L-T3, f-L-T4, f-D-T4 and TSH), the corresponding ton value was read (see Fig. 1) and introduced in the equation of calibration of each sensors, to obtain the value of the concentration.Results presented in Table 2 shown a good correlations between the results obtained for the assay of f-L-T3, f-L-T4, f-D-T4, and TSH in whole blood samples. Recovery tests were performed by comparing the amounts of f-L-T3, f-L-T4, f-D-T4, and TSH determined in whole blood samples using ELISA (the standard method), and the proposed microsensors. The values of recoveries (Table 3) shown for each sensor, and each compound a very good correlation, given by the amount of compound found using the proposed microsensors reported to the amount of the same compound found in the sample blood sample using ELISA, the recovery being expressed as % recovery. All relative standard deviations recorded were less than 1.00%, proving a high precission and reliability of the measurements.
| Sample no. | Microsensor based on | f-L-T3 (ng dL−1) | f-L-T4 (ng dL−1) | f-D-T4 (ng dL−1) | TSH (μUI mL−1) |
|---|---|---|---|---|---|
| 1 | IN-L-Ala-C4-L-Lac | 53.99 ± 0.21 | 6.99 ± 0.01 | 0.16 ± 0.01 | 0.056 ± 0.003 |
| IN-L-Phe-C4-L-Lac | 54.05 ± 0.20 | 6.00 ± 0.05 | 0.14 ± 0.01 | 0.061 ± 0.002 | |
| IN-L-Ala-C4-NO3-L-Lac | 54.21 ± 0.19 | 6.81 ± 0.05 | 0.14 ± 0.02 | 0.060 ± 0.003 | |
| TEX-L-Ala-C4-L-Lac | 53.93 ± 0.20 | 7.08 ± 0.03 | 0.17 ± 0.02 | 0.067 ± 0.003 | |
| TEX-L-Phe-C4-L-Lac | 54.28 ± 0.18 | 6.06 ± 0.02 | 0.13 ± 0.03 | 0.049 ± 0.002 | |
| TEX-L-Ala-C4-NO3-L-Lac | 54.10 ± 0.10 | 6.78 ± 0.01 | 0.19 ± 0.02 | 0.046 ± 0.002 | |
| 2 | IN-L-Ala-C4-L-Lac | 108.06 ± 0.18 | 1.38 ± 0.05 | 0.09 ± 0.02 | 1.440 ± 0.009 |
| IN-L-Phe-C4-L-Lac | 107.95 ± 0.18 | 1.20 ± 0.02 | 0.06 ± 0.01 | 1.400 ± 0.004 | |
| IN-L-Ala-C4-NO3-L-Lac | 107.21 ± 0.21 | 1.80 ± 0.07 | 0.10 ± 0.01 | 1.600 ± 0.005 | |
| TEX-L-Ala-C4-L-Lac | 107.33 ± 0.19 | 1.28 ± 0.04 | 0.12 ± 0.02 | 1.610 ± 0.004 | |
| TEX-L-Phe-C4-L-Lac | 108.21 ± 0.20 | 1.30 ± 0.02 | 0.13 ± 0.02 | 1.609 ± 0.004 | |
| TEX-L-Ala-C4-NO3-L-Lac | 108.08 ± 0.11 | 1.27 ± 0.02 | 0.09 ± 0.01 | 1.590 ± 0.003 | |
| 3 | IN-L-Ala-C4-L-Lac | 143.00 ± 0.18 | 1.000 ± 0.005 | 0.011 ± 0.003 | 2.020 ± 0.005 |
| IN-L-Phe-C4-L-Lac | 143.02 ± 0.15 | 1.020 ± 0.007 | 0.010 ± 0.002 | 1.920 ± 0.005 | |
| IN-L-Ala-C4-NO3-L-Lac | 145.60 ± 0.13 | 0.991 ± 0.003 | 0.015 ± 0.002 | 2.190 ± 0.003 | |
| TEX-L-Ala-C4-L-Lac | 145.69 ± 0.13 | 0.978 ± 0.005 | 0.015 ± 0.005 | 2.000 ± 0.004 | |
| TEX-L-Phe-C4-L-Lac | 143.78 ± 0.12 | 0.956 ± 0.006 | 0.017 ± 0.002 | 1.940 ± 0.003 | |
| TEX-L-Ala-C4-NO3-L-Lac | 142.56 ± 0.10 | 0.966 ± 0.003 | 0.016 ± 0.001 | 1.720 ± 0.002 | |
| 4 | IN-L-Ala-C4-L-Lac | 11.72 ± 0.17 | 3.90 ± 0.03 | 0.12 ± 0.02 | 0.028 ± 0.005 |
| IN-L-Phe-C4-L-Lac | 11.22 ± 0.15 | 4.10 ± 0.03 | 0.11 ± 0.02 | 0.023 ± 0.005 | |
| IN-L-Ala-C4-NO3-L-Lac | 11.36 ± 0.17 | 4.66 ± 0.07 | 0.15 ± 0.01 | 0.030 ± 0.002 | |
| TEX-L-Ala-C4-L-Lac | 11.98 ± 0.12 | 4.25 ± 0.02 | 0.12 ± 0.01 | 0.024 ± 0.002 | |
| TEX-L-Phe-C4-L-Lac | 11.24 ± 0.12 | 4.52 ± 0.05 | 0.17 ± 0.03 | 0.029 ± 0.007 | |
| TEX-L-Ala-C4-NO3-L-Lac | 12.08 ± 0.11 | 4.88 ± 0.03 | 0.11 ± 0.01 | 0.023 ± 0.001 | |
| 5 | IN-L-Ala-C4-L-Lac | 106.21 ± 0.20 | 1.38 ± 0.02 | 0.05 ± 0.01 | 0.330 ± 0.012 |
| IN-L-Phe-C4-L-Lac | 104.96 ± 0.18 | 1.37 ± 0.02 | 0.05 ± 0.02 | 0.390 ± 0.012 | |
| IN-L-Ala-C4-NO3-L-Lac | 106.00 ± 0.18 | 1.37 ± 0.03 | 0.06 ± 0.01 | 0.330 ± 0.015 | |
| TEX-L-Ala-C4-L-Lac | 105.92 ± 0.22 | 1.27 ± 0.05 | 0.06 ± 0.01 | 0.360 ± 0.013 | |
| TEX-L-Phe-C4-L-Lac | 105.33 ± 0.15 | 1.39 ± 0.01 | 0.07 ± 0.02 | 0.330 ± 0.017 | |
| TEX-L-Ala-C4-NO3-L-Lac | 106.02 ± 0.11 | 1.39 ± 0.03 | 0.05 ± 0.01 | 0.308 ± 0.011 | |
| 6 | IN-L-Ala-C4-L-Lac | 170.02 ± 0.22 | 1.08 ± 0.02 | 0.08 ± 0.01 | 0.451 ± 0.011 |
| IN-L-Phe-C4-L-Lac | 179.21 ± 0.15 | 1.31 ± 0.04 | 0.07 ± 0.02 | 0.414 ± 0.012 | |
| IN-L-Ala-C4-NO3-L-Lac | 178.24 ± 0.15 | 1.13 ± 0.03 | 0.08 ± 0.01 | 0.482 ± 0.012 | |
| TEX-L-Ala-C4-L-Lac | 176.66 ± 0.18 | 1.20 ± 0.02 | 0.08 ± 0.01 | 0.445 ± 0.021 | |
| TEX-L-Phe-C4-L-Lac | 177.22 ± 0.10 | 1.24 ± 0.03 | 0.09 ± 0.02 | 0.443 ± 0.010 | |
| TEX-L-Ala-C4-NO3-L-Lac | 173.23 ± 0.12 | 1.27 ± 0.02 | 0.08 ± 0.01 | 0.453 ± 0.010 | |
| 7 | IN-L-Ala-C4-L-Lac | 163.34 ± 0.18 | 1.25 ± 0.05 | 0.09 ± 0.02 | 1.410 ± 0.008 |
| IN-L-Phe-C4-L-Lac | 164.00 ± 0.18 | 1.22 ± 0.03 | 0.10 ± 0.01 | 1.370 ± 0.008 | |
| IN-L-Ala-C4-NO3-L-Lac | 161.40 ± 0.15 | 1.34 ± 0.03 | 0.08 ± 0.01 | 1.390 ± 0.009 | |
| TEX-L-Ala-C4-L-Lac | 162.50 ± 0.17 | 1.21 ± 0.05 | 0.08 ± 0.01 | 1.430 ± 0.007 | |
| TEX-L-Phe-C4-L-Lac | 164.06 ± 0.11 | 1.30 ± 0.02 | 0.09 ± 0.02 | 1.360 ± 0.007 | |
| TEX-L-Ala-C4-NO3-L-Lac | 164.03 ± 0.12 | 1.30 ± 0.02 | 0.10 ± 0.01 | 1.359 ± 0.005 | |
| 8 | IN-L-Ala-C4-L-Lac | 55.48 ± 0.11 | 0.776 ± 0.009 | 0.017 ± 0.002 | 7.200 ± 0.009 |
| IN-L-Phe-C4-L-Lac | 55.59 ± 0.12 | 0.771 ± 0.012 | 0.015 ± 0.002 | 7.150 ± 0.009 | |
| IN-L-Ala-C4-NO3-L-Lac | 55.62 ± 0.12 | 0.771 ± 0.011 | 0.014 ± 0.003 | 7.610 ± 0.011 | |
| TEX-L-Ala-C4-L-Lac | 55.27 ± 0.13 | 0.729 ± 0.009 | 0.017 ± 0.003 | 7.470 ± 0.012 | |
| TEX-L-Phe-C4-L-Lac | 55.53 ± 0.11 | 0.731 ± 0.011 | 0.015 ± 0.002 | 7.000 ± 0.009 | |
| TEX-L-Ala-C4-NO3-L-Lac | 54.09 ± 0.11 | 0.729 ± 0.013 | 0.010 ± 0.002 | 7.240 ± 0.009 | |
| 9 | IN-L-Ala-C4-L-Lac | 59.93 ± 0.11 | 1.12 ± 0.03 | 0.011 ± 0.003 | 2.149 ± 0.011 |
| IN-L-Phe-C4-L-Lac | 59.98 ± 0.11 | 1.15 ± 0.02 | 0.012 ± 0.003 | 2.180 ± 0.012 | |
| IN-L-Ala-C4-NO3-L-Lac | 60.60 ± 0.12 | 1.11 ± 0.01 | 0.011 ± 0.023 | 2.200 ± 0.012 | |
| TEX-L-Ala-C4-L-Lac | 60.67 ± 0.13 | 1.13 ± 0.01 | 0.010 ± 0.003 | 2.198 ± 0.015 | |
| TEX-L-Phe-C4-L-Lac | 60.22 ± 0.10 | 1.16 ± 0.02 | 0.010 ± 0.001 | 2.230 ± 0.013 | |
| TEX-L-Ala-C4-NO3-L-Lac | 60.63 ± 0.08 | 1.17 ± 0.01 | 0.009 ± 0.001 | 2.159 ± 0.013 |
| Microsensor based on | f-L-T3%, recovery | f-L-T4%, recovery | f-D-T4%, recovery | TSH%, recovery |
|---|---|---|---|---|
| IN-L-Ala-C4-L-Lac | 98.92 ± 0.09 | 98.70 ± 0.05 | 99.22 ± 0.04 | 99.21 ± 0.02 |
| IN-L-Phe-C4-L-Lac | 98.72 ± 0.09 | 98.99 ± 0.05 | 99.05 ± 0.04 | 99.20 ± 0.02 |
| IN-L-Ala-C4-NO3-L-Lac | 98.90 ± 0.08 | 98.01 ± 0.06 | 98.98 ± 0.05 | 99.43 ± 0.03 |
| TEX-L-Ala-C4-L-Lac | 98.50 ± 0.07 | 98.20 ± 0.07 | 98.32 ± 0.05 | 99.32 ± 0.02 |
| TEX-L-Phe-C4-L-Lac | 99.01 ± 0.07 | 99.03 ± 0.07 | 99.99 ± 0.02 | 99.99 ± 0.02 |
| TEX-L-Ala-C4-NO3-L-Lac | 99.24 ± 0.08 | 99.56 ± 0.04 | 99.93 ± 0.04 | 99.98 ± 0.02 |
Conclusion
Six stochastic microsensors based on diamond paste modified with inulins and ionic liquids were proposed for the assay of f-L-T4, f-D-T4, f-L-T3 and TSH in whole blood samples. The microsensors were selective, and enantioselective and presented high sensitivity and reliability for the assay of thyroid hormones in whole blood samples. Regarding all response characteristics as well as the signatures of the hormones, the sensor of choice for simultaneous assay of thyroid hormones is the one based on TEX-L-Ala-C4-NO3-L-Lac.Compared with ELISA and chemiluminescence methods used in clinical laboratories for their determination, the main advantages of the proposed method are: there is no need for sample pretreatment before assay, samples being used as taken from the patient, all four hormones can be determined in one run, low cost, decreased time of determination, high sensitivities and lower limits of determination.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by UEFISCDI PNIII Program PCE Contract nr. 46/2017.References
- G. S. Ginsburg and H. F. Willard, Genomic and personalized medicine: foundations and applications, Transl. Res., 2009, 154, 277–287 CrossRef PubMed.
- J. Schmidt, Stochastic sensors, J. Mater. Chem., 2005, 15, 831–840 RSC.
- R. I. Stefan-van Staden, G. Mitrofan, I. R. Comnea-Stancu, J. K. van Staden, C. Kapnissi-Christodoulou and H. Y. Aboul-Enein, Ionic liquids for the molecular enantiorecognition of free L-T3, L-T4 and D-T4, RSC Adv., 2015, 5, 75451–75457 RSC.
- G. Mitrofan, R. I. Stefan-van Staden, I. R. Comnea-Stancu, J. K. van Staden, G. Bazylak, C. Christodoulou and H. Y. Aboul-Enein, Fast screening of whole blood samples and pharmaceutical compounds for Enantiorecognition of free L-T3, L-T4 and D-T4, Chirality, 2015, 27, 973–978 CrossRef CAS PubMed.
- L. E. Braveman and D. S. Cooper, The Thyroid: A Fundamental and Clinical Text, Lippincott Williams and Wilkins, Philadelphia, US, 2013 Search PubMed.
- M. R. Haymart, S. L. Glinberg, J. Liu, R. S. Sippel, J. C. Jaume and H. Chen, Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension, Clin. Endocrinol., 2009, 71, 434–439 CrossRef CAS PubMed.
- J. E. Midgley, Direct and indirect free thyroxine assay methods: theory and practice, Clin. Chem., 2001, 47, 1353–1363 CAS.
- Clinical and Laboratory Standards Institute (CLSI), Measurement of free thyroid hormones; approved guideline, CLSI, Wayne, PA, ISBN 1-56238-548-8, 2004 Search PubMed.
- O. P. Soldin and S. J. Soldin, Thyroid hormone testing by tandem mass spectrometry, Clin. Biochem., 2011, 44, 89–94 CrossRef CAS PubMed.
- D. Wang and H. M. Srapleton, Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem., 2011, 397, 1831–1839 CrossRef PubMed.
- V. Samanidou, H. Gika and I. N. Papadoyannis, Rapid HPLC analysis of thyroid gland hormones tri-iodothyronine (T-3) and thyroxine (T-4) in human biological fluids after SPE, J. Liq. Chromatogr. Relat. Technol., 2000, 23, 681–692 CrossRef CAS.
- S. Georgiou and I. Christofidis, Radioimmunoassay of free thyroxine (T4) using 125I-labeled T4-IgG complex with very large molecular weight, Clin. Chim. Acta, 1996, 244, 209–220 CrossRef CAS.
- X. Wang, H. Chen, J.-M. Lin and X. Ying, Development of a highly sensitive and selective microplate chemiluminescence enzyme immunoassay for the determination of free thyroxine in human serum, Int. J. Biol. Sci., 2007, 3, 274–280 CrossRef CAS PubMed.
- M. S. Cabayo, M. Mauri, R. Alfayate and F. Soria, Analytical and clinical evaluation of TSH and thyroid hormones by electrochemiluminescent immunoassays, Clin. Biochem., 1999, 32, 395–403 CrossRef.
- A. Abdulaziz and A. A. El-Reweny, The diagnostic utility of third generation TSH electro-chemiluminescence immunoassay in detecting the incidence of thyroid dysfunctions in Saudi Arabia, Med. J. Cairo Univ., 2011, 79, 497–507 Search PubMed.
- F. Kazerouni and H. Amirrasouli, Performance characteristics of three automated immunoassays for thyroid hormones, Caspian J. Intern. Med., 2012, 3, 400–404 Search PubMed.
- L. Shi, Z. Shi, J. Zhang, Q. Ma, D. Kong, L. Yang and Y. Tan, The measurement and application of TSH-IRMA levels among different age groups in areas with iodine deficiency disorders, Chin. Med. Sci. J., 1995, 10, 30–33 CAS.
- L. Grasso, L. Bartalena, C. Mammoli, E. Martino, A. C. Kessler and A. Pinchera, Serum TSH measurements by a sensitive enzyme immunoassay discriminate euthyroid from hyperthyroid subjects and avoid the need for TRH test during suppressive therapy with L-thyroxine, Clin. Biochem., 1987, 20, 197–200 CrossRef CAS PubMed.
- C. Seidel, D. Ziegelitz, A. Weber, T. Dittmer, H. Gerl, G. Knappe and H. J. Correns, Clinical value of a sensitive TSH-RIA, Endokrinologie, 1982, 80, 181–193 CAS.
- A. Das and M. V. Sangaranarayanan, Electroanalytical sensor based on unmodified screen-printed carbon electrode for the determination of levo-thyroxine, Electroanalysis, 2014, 27, 360–367 CrossRef.
- R. I. Stefan, J. F. van Staden and H. Y. Aboul-Enein, Determination of (+)-3,3′,5-triiodo-L-thyronine (L-T3) from serum using a sequential injection analysis/immunosensor system, J. Immunoassay Immunochem., 2003, 25, 348–355 Search PubMed.
- R. I. Stefan, J. F. van Staden, H. Y. Aboul-Enein, M. C. Mirica, I. Balcu and N. Mirica, Determination of (+)-3,3′,5,5′-tetraiodo-L-thyronine (L-T4) in serum and pharmaceutical formulations using a sequential injection analysis/immunosensor system, J. Immunoassay Immunochem., 2008, 29, 348–355 CrossRef PubMed.
- H. Wang, X. Wu, P. Dong, C. Wang, J. Wang, Y. Liu and J. Chen, Electrochemical biosensor based on interdigitated electrodes for determination of thyroid stimulating hormone, Int. J. Electrochem. Sci., 2014, 9, 12–21 Search PubMed.
- R. I. Stefan and H. Y. Aboul-Enein, The construction and characterization of an amperometric immunosensor for the thyroid hormone (+)-3,3′,5,5′-tetraiodo-L-thyronine (L-T4), J. Immunoassay Immunochem., 2002, 23, 429–437 CrossRef CAS.
- B. Zhang, D. Tang, B. Liu, Y. Cui, H. Chen and G. Chen, Nanogold-functionalized magnetic beads with redox activity for sensitive electrochemical immunoassay of thyroid-stimulating hormone, Anal. Chim. Acta, 2012, 711, 17–23 CrossRef CAS PubMed.
- H. Wang, P. Dong, D. Di and X. Wu, Interdigitated microelectrodes biosensor with nanodot arrays for thyroid stimulating hormone detection, Micro Nano Lett., 2013, 8, 11–14 CAS.
- S. Kubitschko, J. Spinke, T. Bruckner, S. Pohl and N. Oranth, Festivity Enhancement of optical immunosensors with nanoparticles, Anal. Biochem., 1997, 253, 112–122 CrossRef CAS PubMed.
- M. Gao, F. Yu, L. Changjun, J. Choo and L. Chen, Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy, Chem. Soc. Rev., 2017, 46, 2237–2271 RSC.
- X. Han, X. Song, F. Yu and L. Chen, A ratiometric near-infrared fluorescent probe for quantification and evaluation of selenocysteine-protective effects in acute inflammation, Adv. Funct. Mater., 2017, 27, 1700769 CrossRef.
- X. Han, X. Song, F. Yu and L. Chen, A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress, Chem. Sci. 10.1039/C7SC02888A.
- L. R. Mandoc, I. Moldoveanu, R. I. Stefan-van Staden and E. M. Ungureanu, Pattern recognition of Cu(II), Pb(II), Hg(II), and Cd(II) in waste waters, Microsys. Technol., 2017, 23, 1141–1145 CrossRef.
- R. I. Stefan-van Staden, I. R. Comnea-Stancu and C. C. Surdu-Bob, Molecular screening of blood samples for the simultaneous detection of CEA, HER-1, NSE, CYFRA 21-1 using stochastic sensors, J. Electrochem. Soc., 2017, 164, B267–B273 CrossRef CAS.
- L. A. Gugoasa, R. I. Stefan-van Staden, A. J. M. Al-Ogaidi and C. Stanciu-Gavan, Molecular recognition of colon cancer biomarkers: P53, KRAS and CEA in whole blood samples, J. Electrochem. Soc., 2017, 164, B443–B447 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |