Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controllable wettability and adhesion of superhydrophobic self-assembled surfaces based on a novel azobenzene derivative†
Qiongqiong Gao,
Liu He,
Yajie Li,
Xia Ran* and
Lijun Guo *
*
Institute of Micro/Nano Photonic Materials and Application, Henan University, Kaifeng 475004, China. E-mail: ranxia@henu.edu.cn; juneguo@henu.edu.cn
First published on 30th October 2017
Abstract
Superhydrophobic surfaces with controllable adhesion have received considerable attention due to their potential in numerous applications. Here, we report a controllable adhesion of superhydrophobic surfaces through tuning the self-assembly structure based on a novel Y-shaped molecule AOB-Y8, consisting of azobenzene groups, 1,3,4-oxadiazole moieties, and three octyl chains. The surface hydrophobicity of AOB-Y8 films was successfully regulated by tuning the assembly morphologies through changing the composition of CHCl3/CH3CN mixed solvents, concentration and temperature. Morphological studies of the film prepared from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN solvents revealed that the self-assembled hierarchical, flower-like structure constructs a lot of grooves to trap the surrounding air, generating the superhydrophobic effect at room temperature. Particularly, the surface adhesion of this superhydrophobic film was further regulated from a low level to a very high level by simply changing the concentration of AOB-Y8 in mixed solvents. Meanwhile, the AOB-Y8 self-assembled surfaces showed an excellent chemical resistance to acid and alkali, which is suitable for applications in many environmental conditions. As examples, the tunable adhesive superhydrophobic AOB-Y8 surfaces demonstrated good features to be used in selective transportation of droplets, self-cleaning and droplet-based microreactors to quantitatively detect NaOH and FeCl3.
50 CHCl3/CH3CN solvents revealed that the self-assembled hierarchical, flower-like structure constructs a lot of grooves to trap the surrounding air, generating the superhydrophobic effect at room temperature. Particularly, the surface adhesion of this superhydrophobic film was further regulated from a low level to a very high level by simply changing the concentration of AOB-Y8 in mixed solvents. Meanwhile, the AOB-Y8 self-assembled surfaces showed an excellent chemical resistance to acid and alkali, which is suitable for applications in many environmental conditions. As examples, the tunable adhesive superhydrophobic AOB-Y8 surfaces demonstrated good features to be used in selective transportation of droplets, self-cleaning and droplet-based microreactors to quantitatively detect NaOH and FeCl3.
Introduction
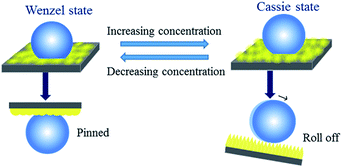
Hydrophobic surfaces with tunable wettability and adhesion have received considerable attention due to their great potential for applications in many fields, such as self-cleaning,1 anti-snow/fog,2 antisticking,3 anticorrosion,4 oil/water separation,5 and microdroplet transportation.6 Besides biomimetic methods, the physicochemical wetting concept is in part feasible for fabricating superhydrophobic surfaces by combining a low surface energy material with surface roughness/morphology at the micro/nano scale.7–9 Generally, superhydrophobic surfaces can be normally classified into two types: low adhesion and high adhesion to water. Low-adhesion superhydrophobic surfaces are usually inspired by biological organisms, the self-cleaning lotus leaf is the typical example.10 On the other hand, the high-adhesion superhydrophobic surface is inspired by the gecko's attachment system and rose petals.11 On these surfaces, water drop shows a static water contact angles (CA) larger than 150°, but the drop is pinned on the surfaces at any titled angles.12 It has been previously demonstrated that liquid adhesion to solid surface can be regulated by either tuning the surface chemical composition or tailoring the morphology.13–18 For example, Guittard and coworkers obtained highly hydrophobic surfaces with various sticking behaviors from electro-deposition of poly(3,4-bis(alkoxy)thiophene)s.19 Li group successfully fabricated tunable adhesive superhydrophobic ZnO surfaces by spraying ZnO nanoparticle suspensions onto desired substrates.13 Jiang group reported on a versatile strategy to reversibly control the mobility of spherical microdroplets of water on the same surface from rollable to pinned by temperature.20Whether water droplets are pinned on (high adhesion) or rolled off (low adhesion) a particular superhydrophobic surface depends on the distinct contact modes: the Wenzel state, the metastable state, and the Cassie state.21,22 In the Wenzel state,23 the water droplet completely fills the grooves of the rough surface at the point of contact. Thus, the Wenzel state exhibits an extremely high adhesive property. Conversely, in the Cassie state,24 the water droplet contacts the top of asperities with air trapped under the liquid. In this state, the water droplet can easily roll off the surface with extremely low adhesion. Eclectically, in the metastable state, a water droplet partially wets the roughness features with air trapped in the rest of valleys, thus the adhesion of the surface is between that of the above two states and the water droplet rolls off with the surface significantly tilted.25 So far, there is still a practical interest to produce highly hydrophobic surfaces with various adhesive properties.
However, previous reports mostly focused on the construction of superhydrophobic and tunable adhesive surface with polymer molecules or inorganic materials,26–39 in spite of the fact that small organic molecules have well-defined molecular structures and are easy to synthesize and modify. When assembling surfaces from organic molecule, molecular interactions such as hydrogen bonding, π–π stacking, and van der Waals interaction play a key role in determining the ultimate structures and surface properties. Solvent mediated balancing of these interactions is an effective way to construct various film morphologies with different surface wettability and adhesion. To regulate surface wettability and adhesion through solvent and concentration tuning, we designed and synthesized a novel Y-shaped compound AOB-Y8 containing azobenzene groups, 1,3,4-oxadiazole moieties, and three octyl chains (Fig. 1). We then demonstrated the potential applications of this novel azobenzene derivative by fabricating tunable adhesive superhydrophobic surfaces that could be used as a droplet transporter, self-cleaner and droplet-based microreactor.
Results and discussion
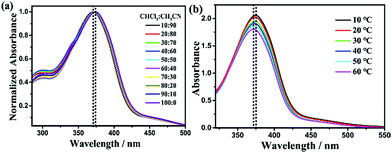
AOB-Y8 is soluble in non-polar and moderate polar solvents but insoluble in strong polar solvents. A facile and versatile approach of regulating self-assembly structure is to introduce a second liquid, and the introduced liquid component is usually miscible with the original solvent. In order to regulate the self-assembly structure with different surface wettability and adhesion of AOB-Y8, we first investigated the self-assembly behavior of AOB-Y8 in chloroform-acetonitrile mixed solvents. As shown in Fig. 2a, AOB-Y8 in dilute solution (1 × 10−5 M) shows an intense absorption around 374 nm in chloroform, as well as a weak band around 450 nm. The large absorption band at shorter wavelength belongs to a π–π* type absorption of the trans-azobenzene moieties. The position of the absorbance maxima is slightly dependent on the compositions of CHCl3/CH3CN mixed solvents, with a total red-shift from 371 nm to 374 nm when changing from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 CHCl3/CH3CN to 90
90 CHCl3/CH3CN to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CHCl3/CH3CN. The temperature and concentration dependence of absorption spectroscopies were also employed to investigate the effects of molecular arrangements on self-assembly behaviours. For the 5 × 10−5 M of AOB-Y8 in 50
10 CHCl3/CH3CN. The temperature and concentration dependence of absorption spectroscopies were also employed to investigate the effects of molecular arrangements on self-assembly behaviours. For the 5 × 10−5 M of AOB-Y8 in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN, the π–π* absorption maximum of azobenzene group locates around 372 nm at 60 °C and almost stays at the same position at 10 °C in the diluted AOB-Y8 solution (Fig. S1†). Fig. 2b shows the temperature-dependent UV-Vis absorption spectra of 5 × 10−4 M of AOB-Y8 in 50
50 CHCl3/CH3CN, the π–π* absorption maximum of azobenzene group locates around 372 nm at 60 °C and almost stays at the same position at 10 °C in the diluted AOB-Y8 solution (Fig. S1†). Fig. 2b shows the temperature-dependent UV-Vis absorption spectra of 5 × 10−4 M of AOB-Y8 in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN mixed solvents, which clearly demonstrates the existence of aggregation state at higher concentration. Specifically, the π–π* absorption maximum of azobenzene group locates around 372 nm at 60 °C and red-shifts to 375 nm at 10 °C, indicating the role of J-type molecular arrangement through π–π interactions in regulating the self-assembly behavior of AOB-Y8 molecule in mixed solvent.40,41
50 CHCl3/CH3CN mixed solvents, which clearly demonstrates the existence of aggregation state at higher concentration. Specifically, the π–π* absorption maximum of azobenzene group locates around 372 nm at 60 °C and red-shifts to 375 nm at 10 °C, indicating the role of J-type molecular arrangement through π–π interactions in regulating the self-assembly behavior of AOB-Y8 molecule in mixed solvent.40,41
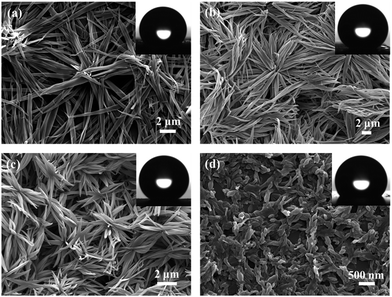
The AOB-Y8 surfaces were simply fabricated by casting a small drop of AOB-Y8 solution in CHCl3/CH3CN mixed solvents with different composition onto silicon plates at room temperature. Fig. 3 shows the scanning electron microscopy (SEM) images and water contact angle (CA) photographs of drop-cast AOB-Y8 surfaces, clearly demonstrating a strong dependence on the nature of solvents. With a composition of 10% CH3CN (Fig. 3a), the morphology of assembled surface consists of flexible root-like fibres with an average width of 540 nm and length of a few micrometers. With the CH3CN component increased to 50% and 60%, we observed a more flexible and dense flower-like nanowire array with an average width of 360 nm (Fig. 3b and c). These self-assembled hierarchical flower-like structures can generate numerous grooves to trap the surrounding air, thus leading to a superhydrophobic effect.42–44 However, when the CH3CN was increased to 90% (Fig. 3d), the floral structures disappeared thoroughly and transformed into short sticks with the average diameter of 100 nm and length of several hundred nanometers. These regulated morphologies of AOB-Y8 surfaces by CHCl3/CH3CN mixed solvents are expected to be closely associated with the surface wettability. As shown in Fig. S2,† with the increase of CH3CN composition, the corresponding CA of water droplets on the as-prepared surfaces first increases and then decreases, and reaches a superhydrophobic CA of 151.2° for CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 50
CH3CN = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Obviously, solvent is responsible for the changes in structure and wettability of these self-assembled AOB-Y8 surfaces.
50. Obviously, solvent is responsible for the changes in structure and wettability of these self-assembled AOB-Y8 surfaces.
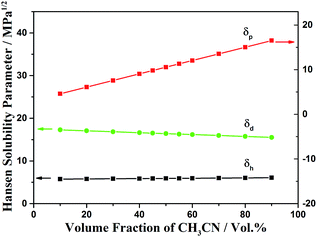
In an attempt to dissect the factors related to the changes in the morphologies and wettability, we have calculated the Hansen solubility parameters (HSPs) of the solvent mixtures and discussed the correlation between the HSPs and the morphology of AOB-Y8. HSPs have been successfully used for several decades to select solvents for coating materials in the field of polymer science.45 In this approach, the cohesive energy density is decomposed into three contributions: dispersive interactions (δd), polar interactions (δp) and hydrogen bonds (δh). Fig. 4 gives the HSPs of different CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN compositions, and the values for the HSPs of single solvents can be found in the literature.45,46 It can be seen that the value of δp increases with the increasing of CH3CN composition, but the value of δd and δh remain almost invariant. When the value of δp is 4.59, the morphology of assembled surface consists of flexible root-like fibers with the corresponding CA of 133.4° (Fig. S2†). With the value of δp increases to 10.55 and 12.04, we observed the flower-like nanowire array with the superhydrophobic CA (Fig. S2†). However, when the value of δp is increased to 16.51, the floral structures disappears thoroughly with the corresponding CA of 129.8°. Therefore, the major contributor to the morphology and wettability changes can be attributed to the polar interactions parameter, δp. Meanwhile, considering the fact that the boing point of CH3CN is higher than that of CHCl3, it can be deduced that the increasing proportion of CH3CN slows down the evaporation rate of mixed solvent and there is more time to grow the crystallites of AOB-Y8, thus resulting in the obtained different morphologies of AOB-Y8 from different compositions of CHCl3
CH3CN compositions, and the values for the HSPs of single solvents can be found in the literature.45,46 It can be seen that the value of δp increases with the increasing of CH3CN composition, but the value of δd and δh remain almost invariant. When the value of δp is 4.59, the morphology of assembled surface consists of flexible root-like fibers with the corresponding CA of 133.4° (Fig. S2†). With the value of δp increases to 10.55 and 12.04, we observed the flower-like nanowire array with the superhydrophobic CA (Fig. S2†). However, when the value of δp is increased to 16.51, the floral structures disappears thoroughly with the corresponding CA of 129.8°. Therefore, the major contributor to the morphology and wettability changes can be attributed to the polar interactions parameter, δp. Meanwhile, considering the fact that the boing point of CH3CN is higher than that of CHCl3, it can be deduced that the increasing proportion of CH3CN slows down the evaporation rate of mixed solvent and there is more time to grow the crystallites of AOB-Y8, thus resulting in the obtained different morphologies of AOB-Y8 from different compositions of CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN mixed solvent.
CH3CN mixed solvent.
The XRD patterns of AOB-Y8 surfaces prepared from 3 mg mL−1 in different CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN compositions are compared in Fig. S3,† in which the corresponding d values of diffraction peaks were calculated from the Bragg equation. The patterns of AOB-Y8 surfaces prepared from CHCl3
CH3CN compositions are compared in Fig. S3,† in which the corresponding d values of diffraction peaks were calculated from the Bragg equation. The patterns of AOB-Y8 surfaces prepared from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 50
CH3CN = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and CHCl3
50 and CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 40
CH3CN = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 display a series of sharper diffractions than that of AOB-Y8 surfaces prepared from CHCl3
60 display a series of sharper diffractions than that of AOB-Y8 surfaces prepared from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 90
CH3CN = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and CHCl3
10 and CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 10
CH3CN = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, indicating the existence of a crystal-like ordered structure in the superhydrophobic surfaces. Specifically, the XRD data of AOB-Y8 surfaces prepared from CHCl3
90, indicating the existence of a crystal-like ordered structure in the superhydrophobic surfaces. Specifically, the XRD data of AOB-Y8 surfaces prepared from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 50
CH3CN = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 demonstrate a series of sharp diffractions with the main peaks centred at 2θ = 2.03° (d = 43.56 Å), 2θ = 4.07° (d = 21.71 Å), 2θ = 6.26° (d = 14.11 Å) and 2θ = 8.67° (d = 10.12 Å) in the low-angle region. The d-spacing ratio of 1
50 demonstrate a series of sharp diffractions with the main peaks centred at 2θ = 2.03° (d = 43.56 Å), 2θ = 4.07° (d = 21.71 Å), 2θ = 6.26° (d = 14.11 Å) and 2θ = 8.67° (d = 10.12 Å) in the low-angle region. The d-spacing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3
1/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/4 in the low-angle regions suggests a lamellar structure and an interlayer distance of 43.56 Å.47,48 This distance is smaller than the calculated molecular length of AOB-Y8 (52.13 Å, estimated by the DFT molecular modelling), implying a possible tilt or interdigitated packing arrangement of AOB-Y8 molecule in layers. The existence of other three peaks centred at 2θ = 5.01, 9.95 and 14.93° corresponding to d-spacings of 17.62, 8.88 and 5.93 Å, respectively, indicates that other types of aggregates also exist in the superhydrophobic surfaces.49 The AOB-Y8 surfaces prepared from CHCl3
1/4 in the low-angle regions suggests a lamellar structure and an interlayer distance of 43.56 Å.47,48 This distance is smaller than the calculated molecular length of AOB-Y8 (52.13 Å, estimated by the DFT molecular modelling), implying a possible tilt or interdigitated packing arrangement of AOB-Y8 molecule in layers. The existence of other three peaks centred at 2θ = 5.01, 9.95 and 14.93° corresponding to d-spacings of 17.62, 8.88 and 5.93 Å, respectively, indicates that other types of aggregates also exist in the superhydrophobic surfaces.49 The AOB-Y8 surfaces prepared from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 40
CH3CN = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 shows the similar spectral features, and thus indicates a similar packing mode of AOB-Y8.
60 shows the similar spectral features, and thus indicates a similar packing mode of AOB-Y8.
Furthermore, the temperature control during the casting process might also affect the evaporation, condensation, surface tension, viscosity of the solution and solubility. By adjusting the initial temperature of substrates, we found the temperature imposes an important effect on the morphologies of the AOB-Y8 surfaces. For comparison of the well-developed flat nanowires structure prepared from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 50
CH3CN = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 on silicon substrates at 25 °C and 1 mg mL−1 (Fig. 3b), the SEM images from the solution of AOB-Y8 at other two different temperatures (5 °C and 60 °C) are presented in Fig. 5. At 5 °C, the surface is composed of more flexible nanowires with the average width of 340 nm (Fig. 5a), the corresponding CA of 148.1°. However, when the substrate temperature was set to 60 °C, we observed a surface composed of an acute and flower-like sheet array with an average thickness of 220 nm (Fig. 5b), and the CA reaches a superhydrophobic value of 152.7°. These observations confirm that temperature is a critical determinant for forming the superhydrophobic surfaces and their assembly morphology.
50 on silicon substrates at 25 °C and 1 mg mL−1 (Fig. 3b), the SEM images from the solution of AOB-Y8 at other two different temperatures (5 °C and 60 °C) are presented in Fig. 5. At 5 °C, the surface is composed of more flexible nanowires with the average width of 340 nm (Fig. 5a), the corresponding CA of 148.1°. However, when the substrate temperature was set to 60 °C, we observed a surface composed of an acute and flower-like sheet array with an average thickness of 220 nm (Fig. 5b), and the CA reaches a superhydrophobic value of 152.7°. These observations confirm that temperature is a critical determinant for forming the superhydrophobic surfaces and their assembly morphology.
 | ||
Fig. 5 SEM images of AOB-Y8 surfaces prepared from 1 mg mL−1 in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3 50 CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN solution on silicon substrates at (a) 5 °C and (b) 60 °C. CH3CN solution on silicon substrates at (a) 5 °C and (b) 60 °C. | ||
The surface adhesivity is a vitally important parameter among various surface properties, reflecting the ability to deliver liquid on surface. Therefore, superhydrophobic surfaces with tunable water adhesion have become a new focus due to their potential applications in liquid transportation, biochemical separation, and microfluid systems.8–12 Besides being superhydrophobic, the self-assembly surface of AOB-Y8 from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 50
CH3CN = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixed solvents also demonstrates a strong adhesive force to hold water droplet even when the surface is tuned upside down (Fig. S4†). Interestingly, we regulated the water adhesion of these superhydrophobic surfaces by simply changing the concentration of AOB-Y8 in 50
50 mixed solvents also demonstrates a strong adhesive force to hold water droplet even when the surface is tuned upside down (Fig. S4†). Interestingly, we regulated the water adhesion of these superhydrophobic surfaces by simply changing the concentration of AOB-Y8 in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
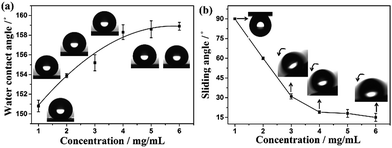
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN mixed solvents. With the concentration increased from 1 mg mL−1 to 6 mg mL−1, as shown in Fig. 6, the water CAs increased from 151 ± 0.6° to 159 ± 0.4°, and the corresponding water sliding angle decreased from 90° to 62 ± 1°, 31 ± 2°, 19 ± 1°, 18 ± 3°, and 15 ± 3°, respectively. Concomitantly, the contact angle hysteresis (CAH) of water droplet demonstrates a dependence on the concentration of AOB-Y8 as well (Table S1†). Quantitatively, the adhesive force of these as-prepared superhydrophobic surfaces was further characterized by using a high-sensitive microelectromechanical balance system. Fig. 7a exhibits a typical adhesive force measuring process, and Fig. 7b shows the adhesive force being dependent on AOB-Y8 concentration in 50
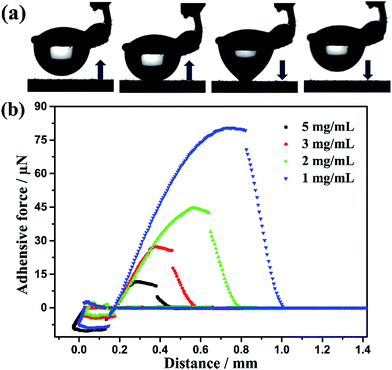
50 CHCl3/CH3CN mixed solvents. With the concentration increased from 1 mg mL−1 to 6 mg mL−1, as shown in Fig. 6, the water CAs increased from 151 ± 0.6° to 159 ± 0.4°, and the corresponding water sliding angle decreased from 90° to 62 ± 1°, 31 ± 2°, 19 ± 1°, 18 ± 3°, and 15 ± 3°, respectively. Concomitantly, the contact angle hysteresis (CAH) of water droplet demonstrates a dependence on the concentration of AOB-Y8 as well (Table S1†). Quantitatively, the adhesive force of these as-prepared superhydrophobic surfaces was further characterized by using a high-sensitive microelectromechanical balance system. Fig. 7a exhibits a typical adhesive force measuring process, and Fig. 7b shows the adhesive force being dependent on AOB-Y8 concentration in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN mixed solvents. As a result, the adhesive force of superhydrophobic surfaces can be tuned from a low level of 13 μN to a very high level of 85 μN by decreasing the concentration of AOB-Y8 from 5 mg mL−1 to 1 mg mL−1 in mixed solvents.
50 CHCl3/CH3CN mixed solvents. As a result, the adhesive force of superhydrophobic surfaces can be tuned from a low level of 13 μN to a very high level of 85 μN by decreasing the concentration of AOB-Y8 from 5 mg mL−1 to 1 mg mL−1 in mixed solvents.
To describe the correlation between surface adhesion and self-assembly structure, we carried out morphology measurements of the AOB-Y8 surfaces from different concentrations in mixed solvent. Fig. 8 and S5† show the SEM cross-sectional and planar view of AOB-Y8 surfaces from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN solutions with the concentration ranging from 1 mg mL−1 to 5 mg mL−1. In the case of 1 mg mL−1, the surface morphology is microscopically composed of well-developed flat nanowires with the average width of 360 nm and length of a few micrometers (Fig. S5a†). Macroscopically, these nanowires are constructed into a hill-like morphology consisting of 18 ± 0.1 μm tower peaks and 10 ± 0.1 μm deep valleys in the film (Fig. 8a). With the increase of AOB-Y8 concentration to 5 mg mL−1, a flower-like morphology with a diameter of 10–18 μm and the petals upright like thorns of 6–10 μm in length is formed (Fig. S5d† and 8c). This observed difference in adhesion of superhydrophobic surfaces can be well described using Cassie's theory and Wenzel's theory,7 respectively. In the Cassie state, the contact angle on the pincushion-like surface can be described by the Cassie's equation:
50 CHCl3/CH3CN solutions with the concentration ranging from 1 mg mL−1 to 5 mg mL−1. In the case of 1 mg mL−1, the surface morphology is microscopically composed of well-developed flat nanowires with the average width of 360 nm and length of a few micrometers (Fig. S5a†). Macroscopically, these nanowires are constructed into a hill-like morphology consisting of 18 ± 0.1 μm tower peaks and 10 ± 0.1 μm deep valleys in the film (Fig. 8a). With the increase of AOB-Y8 concentration to 5 mg mL−1, a flower-like morphology with a diameter of 10–18 μm and the petals upright like thorns of 6–10 μm in length is formed (Fig. S5d† and 8c). This observed difference in adhesion of superhydrophobic surfaces can be well described using Cassie's theory and Wenzel's theory,7 respectively. In the Cassie state, the contact angle on the pincushion-like surface can be described by the Cassie's equation:
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϑ = f1 ϑ = f1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϑflat − f2 ϑflat − f2
| (1) |
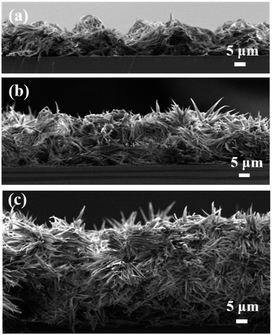 | ||
Fig. 8 Dependence of cross-sectional view of AOB-Y8 surfaces prepared from different concentrations in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN solvents at 25 °C: (a) 1 mg mL−1, (b) 3 mg mL−1 and (c) 5 mg mL−1. 50 CHCl3/CH3CN solvents at 25 °C: (a) 1 mg mL−1, (b) 3 mg mL−1 and (c) 5 mg mL−1. | ||
The environmental adaptability of superhydrophobic surface is an important factor for its practical application. The AOB-Y8 superhydrophobic surfaces with tuneable adhesion exhibit a good acid/base-resisting property. The contact angle or super-hydrophobicity changes little when the water pH varies from 1 to 14, indicating chemical stability (Fig. S6†). Furthermore, the as-prepared AOB-Y8 surfaces can retain such wettability even after 3 months without special protection, demonstrating a good durability as well.
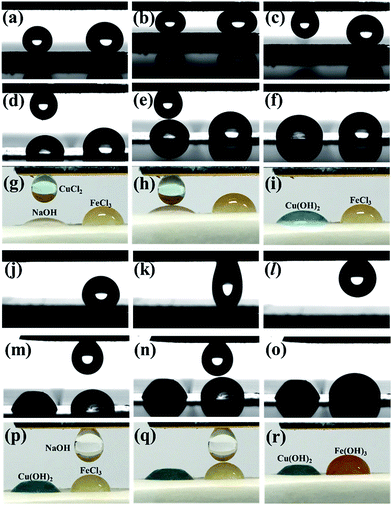
The tuneable surface adhesion and selective microdroplet transportation of AOB-Y8 superhydrophobic surfaces can be applied as droplet-based microreactor.50–52 As an example, we performed the selective and step-by-step transportation of microdroplets towards the quantitative detection of NaOH and FeCl3 in a microreactor. In Fig. 10a, two droplets were placed on the AOB-Y8 superhydrophobic surface with low adhesion to water: left, 4 μL of 0.3 mol L−1 CuCl2; right, 8 μL of 0.6 mol L−1 NaOH. These two water droplets were contacted with the medium adhesive AOB-Y8 superhydrophobic surface, and only the CuCl2 droplet was captured by the surface and the NaOH droplet stayed in situ (Fig. 10b and c). Subsequently, the CuCl2 droplet was transported to contact with another 4 μL of 0.6 mol L−1 NaOH droplet on a silicon paper (Fig. 10d, e, g and h). These two droplets coalesced and produced the blue Cu(OH)2 after reaction (Fig. 10f and i). Similarly, the 8 μL of 0.6 mol L−1 NaOH droplet was transported to the high adhesion superhydrophobic surface (Fig. 10j–l), and reacted with 8 μL of 0.2 mol L−1 FeCl3 droplet to produce reddish-brown Fe(OH)3 in a microreactor (Fig. 10m–r). Meanwhile, these superhydrophobic surfaces with low water adhesion also demonstrate a good self-cleaning effect or antifouling ability (Fig. S7†), although the dusts in our experiments are far more than those in natural environments.
Experimental
Synthesis
The compound AOB-Y8 was synthesized according to the routes shown in Scheme S1.† 4-(4′-Octanoxyphenyl) azobenzoic acid was prepared according to the literature.53,54 4-(4′-Octanoxyphenyl) azobenzoic acid (3.2 g, 0.009 mol) and thionyl chloride (50 mL) were refluxed for 10 h, and the product of (4′-octanoxyphenyl) azobenzoic acid chloride was collected after removing unreacted thionyl chloride. The purified (4′-octanoxyphenyl) azobenzoic acid chloride was dissolved in tetrahydrofuran, and then 5-octanoxyphenyl-1,3-benzenedicarboxylic acid dihydrazide (A)55 was added slowly. The resulting mixture was stirred for 10 h, and then the reaction mixture was poured into an excess of ice water. The precipitant was recrystallized from 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 tetrahydrofuran/ethanol mixed solvents to obtain BNB-Y8.
20 tetrahydrofuran/ethanol mixed solvents to obtain BNB-Y8.
1H NMR (300 MHz, DMSO-d6), (ppm, from TMS): 10.75 (s, 4H), 8.19–8.07 (m, 5H), 8.03–7.87 (m, 8H), 7.69 (s, 2H), 7.21–7.11 (d, 4H, J = 8.04), 4.32–3.92 (m, 6H), 1.91–1.65 (m, 6H), 1.57–1.15 (m, 30H), 0.98–0.76 (m, 9H).
FT-IR (KBr, pellet, cm−1): 3206, 2926, 2856, 1669, 1644, 1598, 1502, 1468, 1394, 1303, 1252, 1184, 1141, 1048, 860, 837, 768, 729, 679.
Elemental analysis: calculated for C58H74N8O7 (%): C, 69.99; H, 7.49; N, 11.26. Found: C, 68.75; H, 7.56; N, 11.18.
The purified BNB-Y8 was dissolved in phosphorous oxychloride (POCl3) and refluxed for approximately 30 h. The excess POCl3 was removed through distillation and the residue was slowly added to ice–water. After removal of the solvent under reduced pressure, the final product AOB-Y8 was purified by recrystallization from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 tetrahydrofuran/ethanol mixed solvents for further NMR, FT-IR spectroscopy, and elemental analysis.
80 tetrahydrofuran/ethanol mixed solvents for further NMR, FT-IR spectroscopy, and elemental analysis.
1H NMR (300 MHz, CDCl3-d6), (ppm, from TMS): 8.47 (s, 1H), 8.37–8.28 (d, 4H, J = 7.89), 8.1–8.01 (d, 4H, J = 7.26), 8–7.92 (d, 4H, J = 7.06), 7.88 (s, 2H), 7.08–6.97 (d, 4H, J = 7.7), 4.24–4.01 (m, 6H), 1.95–1.76 (m, 6H), 1.69–1.2 (m, 30H), 0.97–0.84 (m, 9H).
FT-IR (KBr, pellet, cm−1): 3068, 2925, 2858, 1598, 1545, 1495, 1471, 1404, 1365, 1303, 1251, 1138, 1076, 1038, 1013, 968, 887, 847, 783, 750, 723, 686, 634, 586, 548, 507, 478.
Elemental analysis: calculated for C58H70N8O5 (%): C, 72.62; H, 7.36; N, 11.68. Found: C, 72.51; H, 7.43; N, 11.54.
Preparation of AOB-Y8 surfaces
The weighed AOB-Y8 was mixed in a cap-sealed test vial with the specified ratio of CHCl3/CH3CN solvents. The mixture was heated until the solid dissolved, and then the sample vial was cooled to 35 °C. The AOB-Y8 films were fabricated by casting a small drop of AOB-Y8 solution in CHCl3/CH3CN mixed solvents with different composition onto silicon plates. The silicon plates (10 mm × 10 mm) were cleaned by chloroform (corresponding water contact angle of 52.9°, Fig. S8†). For preparing the superhydrophobic AOB-Y8 surfaces with tuneable water adhesion, a certain volume (100 μL) of AOB-Y8 solution with different concentrations in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CHCl3/CH3CN mixed solvents was casted onto the silicon plates. The evaporation rate of AOB-Y8 solution with 1 mg mL−1, 2 mg mL−1, 3 mg mL−1, 4 mg mL−1 and 5 mg mL−1 is 7.69 μL min−1, 8.45 μL min−1, 9.52 μL min−1, 10.52 μL min−1 and 11.11 μL min−1 at room temperature, respectively.
50 CHCl3/CH3CN mixed solvents was casted onto the silicon plates. The evaporation rate of AOB-Y8 solution with 1 mg mL−1, 2 mg mL−1, 3 mg mL−1, 4 mg mL−1 and 5 mg mL−1 is 7.69 μL min−1, 8.45 μL min−1, 9.52 μL min−1, 10.52 μL min−1 and 11.11 μL min−1 at room temperature, respectively.
Characterization
All the reagents for the synthesis and spectral measurements were of spectroscopic grade and used as received.1 H NMR spectra were recorded with a Bruker AVANCE III-300 MHz spectrometer, using tetramethylsilane (TMS) as an internal standard. Field emission scanning electron microscopy (FE-SEM) images were taken with a JSM-7001F apparatus for investigating the surface morphology of the as-prepared surfaces. The UV/Vis absorption spectra were carried out on a Cary 5000 UV-Vis-NIR Spectrophotometer. XRD experiments of samples were performed using a Bruker D8 advance diffractometer with CuKα radiation (λ = 1.54 Å, 40 kV, and 40 mA). Data processing and analysis were carried out with Materials Data JADE (version 6) XRD pattern processing software.The representative contact angle and sliding angle were measured using an optical contact angle meter DSA25S (KRUSS ADVANCE, Germany) with a water drop volume of 4 μL at ambient temperature. The water contact angle of each surface was an average value calculated through at least four different points by means of sessile-drop method. A similar approach was explored to detect dynamic sliding angles (SAs), by means of slowly rotating the substrate with a 4 μL water droplet until the water droplet began to slide. The contact angle hysteresis (CAH) was calculated from the difference between the values of advancing contact angle (θadv) and receding contact angle (θrec),56 which were obtained by approaching and departing sample surface from settled sessile droplet until the contact line shifted, respectively.57,58
The corrosive water of different pH ranging from 1 to 14 was prepared by adding hydrochloric acid and/or sodium hydroxide. The adhesive forces of superhydrophobic surfaces were assessed through a high-sensitivity micro-electromechanical balance system (Dataphysics DCAT11, Germany). First, a water droplet of 5 μL was suspended on a metal ring and the superhydrophobic surface was placed on the balance table. Then, the superhydrophobic surface was moved upward at a constant speed of 0.02 mm s−1 in an ambient environment with a relative humidity of about 35%. Finally, the superhydrophobic surface was moved downward once in contact with the droplet while the balance force gradually increased until reached the maximum. The adhesive force was defined as the maximum force when the surface was just leaving the water droplet.
Conclusions
In summary, we have designed and synthesized a novel Y-shaped compound AOB-Y8 consisting of azobenzene groups, 1,3,4-oxadiazole moieties, and three octyl chains, in order to regulate self-assembly structure in mixed solvents, and thus surface wettability and adhesion through solvent and concentration tuning. The surface hydrophobicity of AOB-Y8 films was successfully regulated by tuning the assembly morphologies through changing the polar interactions parameter of CHCl3/CH3CN mixed solvents. By simply changing the concentration of AOB-Y8 in CHCl3/CH3CN solution, we have tuned the adhesion of superhydrophobic surface from a low level to a very high level. In the meantime, the self-assembled superhydrophobic surfaces of AOB-Y8 demonstrated an excellent chemical resistance to acid and alkali, suitable for applications in various environmental conditions. As examples, we have presented that the tuneable adhesive superhydrophobic AOB-Y8 surfaces can be effectively used in selective transportation of droplets, self-cleaning and drop-based microdetection or microreaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the National Science Foundation Committee of China (projects No. U1504510, U1604129, U1404619 and 21173068) for their financial support of this work.Notes and references
- K. Liu, X. Yao and L. Jiang, Chem. Soc. Rev., 2010, 39, 3240 RSC.
- J. A. Howarter and J. P. Youngblood, Adv. Mater., 2007, 19, 3838 CrossRef CAS.
- J. Li, L. Yan, Q. Ouyang, F. Zha, Z. Jing, X. Li and Z. Lei, Chem. Eng. J., 2014, 246, 238 CrossRef CAS.
- J. Ou, W. Hu, M. Xue, F. Wang and W. Li, ACS Appl. Mater. Interfaces, 2013, 5, 3101 CAS.
- S. Zhang, F. Lu, L. Tao, N. Liu, C. Gao, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 11971 CAS.
- J. Li, X. Liu, Y. Ye, H. Zhou and J. Chen, Colloids Surf., A, 2011, 384, 109 CrossRef CAS.
- M. Liu and L. Jiang, Adv. Funct. Mater., 2010, 20, 3753 CrossRef CAS.
- J. Long, P. Fan, D. Gong, D. Jiang, H. Zhang, L. Li and M. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 9858 CAS.
- J. Ju, K. Xiao, X. Yao, H. Bai and L. Jiang, Adv. Mater., 2013, 25, 5937 CrossRef CAS PubMed.
- R. Blossey, Nat. Mater., 2003, 2, 301 CrossRef CAS PubMed.
- M. H. Jin, X. J. Feng, L. Feng, T. L. Sun, J. Zhai, T. J. Li and L. Jiang, Adv. Mater., 2005, 17, 1977 CrossRef CAS.
- L. Feng, Y. A. Zhang, J. M. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Langmuir, 2008, 24, 4114 CrossRef CAS PubMed.
- J. Li, Z. Jing, F. Zha, Y. Yang, Q. Wang and Z. Lei, ACS Appl. Mater. Interfaces, 2014, 6, 8868 CAS.
- E. Zhang, Y. Wang, T. Lv, L. Li, Z. Cheng and Y. Liu, Nanoscale, 2015, 7, 6151 RSC.
- X. Mo, Y. Wu, J. Zhang, T. Hang and M. Li, Langmuir, 2015, 31, 10850 CrossRef CAS PubMed.
- D. Wu, S. Wu, Q. Chen, Y. Zhang, J. Yao, X. Yao, L. Niu, J. Wang, L. Jiang and H. Sun, Adv. Mater., 2011, 23, 545 CrossRef CAS PubMed.
- S. Boduroglu, M. Cetinkaya, W. J. Dressick, A. Singh and M. C. Demirel, Langmuir, 2007, 23, 11391 CrossRef CAS PubMed.
- Y. Lai, C. Lin, J. Huang, H. Zhuang, L. Sun and T. Nguyen, Langmuir, 2008, 24, 3867 CrossRef CAS PubMed.
- T. Darmanin and F. Guittard, Soft Matter, 2013, 9, 1500 RSC.
- C. Li, R. Guo, X. Jiang, S. Hu, L. Li, X. Cao, H. Yang, Y. Song, Y. Ma and L. Jiang, Adv. Mater., 2009, 21, 4254 CrossRef CAS.
- M. Liu, Y. Zheng, J. Zhai and L. Jiang, Acc. Chem. Res., 2010, 43, 368 CrossRef CAS PubMed.
- E. Bormashenko, R. Pogreb, T. Stein, G. Whyman, M. Erlich, A. Musin, V. Machavariani and D. Aurbach, Phys. Chem. Chem. Phys., 2008, 10, 4056 RSC.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
- E. Bormashenko, R. Pogreb, G. Whyman and M. Erlich, Langmuir, 2007, 23, 12217 CrossRef CAS PubMed.
- X. Huang, D. Kim, M. Im, J. Lee, J. Yoon and Y. Choi, Small, 2009, 5, 90 CrossRef CAS PubMed.
- J. Yong, F. Chen, Q. Yang, D. Zhang, H. Bian, G. Du, J. Si, X. Meng and X. Hou, Langmuir, 2013, 29, 3274 CrossRef CAS PubMed.
- J. Ou, W. Hu, C. Li, Y. Wang, M. Xue, F. Wang and W. Li, ACS Appl. Mater. Interfaces, 2012, 4, 5737 CAS.
- Z. Cheng, M. Du, H. Lai, N. Zhang and K. Sun, Nanoscale, 2013, 5, 2776 RSC.
- B. A. Kakade, Nanoscale, 2013, 5, 7011 RSC.
- X. Yu, Q. Zhong, H. Yang, L. Wan and Z. Xu, J. Phys. Chem. C, 2015, 119, 3667 CAS.
- B. Zhou, J. Tian, C. Wang, Y. Gao and W. Wen, Appl. Surf. Sci., 2016, 389, 679 CrossRef CAS.
- H. Wang, Y. Zhu, Z. Hu, X. Zhang, S. Wu, R. Wang and Y. Zhu, Chem. Eng. J., 2016, 303, 37 CrossRef CAS.
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132 CrossRef CAS PubMed.
- W. Zhang, T. Xiang, F. Liu, M. Zhang, W. Gan, X. Zhai, X. Di, Y. Wang, G. Liu and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15776 CAS.
- H. Wu, K. Zhu, B. Cao, Z. Zhang, B. Wu, L. Liang, G. Chai and A. Liu, Soft Matter, 2017, 13, 2995 RSC.
- Y. Liu, X. Li, J. Jin, J. Liu, Y. Yan, Z. Han and L. Ren, Appl. Surf. Sci., 2017, 400, 498 CrossRef CAS.
- J. Lv, Y. Song, L. Jiang and J. Wang, ACS Nano, 2014, 8, 3152 CrossRef CAS PubMed.
- S. B. Subramanyam, V. Kondrashov, J. Rühe and K. K. Varanasi, ACS Appl. Mater. Interfaces, 2016, 8, 12583 Search PubMed.
- M. Shimomura and T. Kunitake, J. Am. Chem. Soc., 1987, 109, 5175 CrossRef CAS.
- H. Kobayashi, K. Koumoto, J. H. Jung and S. Shinkai, J. Chem. Soc., Perkin Trans. 1, 2002, 2, 1930 RSC.
- S. Wang, L. Feng and L. Jiang, Adv. Mater., 2006, 18, 767 CrossRef CAS.
- Q. Zhang and J. Wang, Mater. Lett., 2012, 67, 334 CrossRef CAS.
- J. Li, X. Liu, Y. Ye, H. Zhou and J. Chen, J. Phys. Chem. C, 2011, 115, 4726 CAS.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press LLC, Boca Raton, FL, 2nd edn, 2007 CrossRef CAS; C. M. Hansen, Prog. Org. Coat., 2004, 51, 77 CrossRef CAS.
- A. A. Abdala, K. Olesen and S. A. Khan, J. Rheol., 2003, 47, 497 CrossRef CAS.
- H. Xu, J. Song, T. Tian and R. Feng, Soft Matter, 2012, 8, 3478 RSC.
- J. X. Cui, J. Zheng, W. Q. Qiao and X. H. Wan, J. Colloid Interface Sci., 2008, 326, 267 CrossRef CAS PubMed.
- L. B. Niu, J. Song, J. J. Li, N. M. Tao, M. Lu and K. Q. Fan, Soft Matter, 2013, 9, 7780 RSC.
- X. Yao, J. Gao, Y. Song and L. Jiang, Adv. Funct. Mater., 2011, 21, 4270 CrossRef CAS.
- Z. Cheng, R. Hou, Y. Du, H. Lai, K. Fu, N. Zhang and K. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 8753 CAS.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336 CrossRef CAS PubMed.
- J. Liu and Y. Chiu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1142 CrossRef CAS.
- Y. Zhang, K. G. Jespersen, M. Kempe, J. A. Kornfield, S. Barlow, B. Kippelen and S. R. Marder, Langmuir, 2003, 19, 6534 CrossRef CAS.
- D. Zha and L. You, ACS Appl. Mater. Interfaces, 2016, 8, 2399 CAS.
- D. Jeong, S. Kim, J. Park, S. Kim, D. Lee and J. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 2770 CAS.
- Y. Xue, Y. Wu, X. Pei, H. Duan, Q. Xue and F. Zhou, Langmuir, 2015, 31, 226 CrossRef CAS PubMed.
- Y. Wu, Y. Xue, X. Pei, M. Cai, H. Duan, W. T. S. Huck, F. Zhou and Q. Xue, J. Phys. Chem. C, 2014, 118, 2564 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08465j |
| This journal is © The Royal Society of Chemistry 2017 |