 Open Access Article
Open Access ArticleAg2S quantum dots in situ coupled to hexagonal SnS2 with enhanced photocatalytic activity for MO and Cr(VI) removal†
Jie Liua,
Liquan Jinga,
Guofang Gaoa,
Yuanguo Xu *a,
Meng Xieb,
Liying Huanga,
HaiYan Jia,
Jimin Xie
*a,
Meng Xieb,
Liying Huanga,
HaiYan Jia,
Jimin Xie *a and
Huaming Li
*a and
Huaming Li a
a
aSchool of Chemistry and Chemical Engineering, Institute for Energy Research, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: xuyg@ujs.edu.cn; xiejm391@sohu.com
bSchool of Pharmacy, Jiangsu University, Zhenjiang 212013, P. R. China
First published on 5th October 2017
Abstract
A novel visible-light-driven Ag2S/SnS2 composite photocatalyst was successfully fabricated via a simple in situ hydrothermal-ion-exchange method. The materials were systematically characterized by various techniques. XRD, XPS, and TEM analysis demonstrated the successful formation of Ag2S quantum dots on the surface of SnS2 nanoplates. The Ag2S/SnS2 composite materials exhibited increased photocatalytic activity compared to pure SnS2 for the removal of methyl orange (MO) and Cr(VI), and the 1% Ag2S/SnS2 composite material showed the best activity under visible light irradiation. Holes (h+) and superoxide radicals (˙O2−) were the major active species during the photocatalytic process. The in situ formation of Ag2S quantum dots provided an effective way to facilitate carrier transfer and separation, which is believed to be responsible for the enhanced photocatalytic performance.
1. Introduction
Nowadays, environmental pollution and energy issues have hindered the sustainable development of modern society. Among them, the excessive discharge of industrial wastewater containing both organic pollutants and heavy metal irons at the same time is a major problem. Worse still, both organic pollutants such as methyl orange (MO) and heavy metal ions like Cr(VI) produce serious harm to the environment.1,2 Therefore, how to remove these two types of pollutant efficiently and economically becomes an urgent issue. Semiconductor photocatalysis, as an efficient and promising technology directly utilizing solar energy to solve the environmental pollution and energy demand problems, has aroused considerable attention in the past decades.3,4 Hitherto, various kinds of semiconductor materials (such as TiO2,5 Fe2O3,6 AgVO3,7 CdS8) have been developed for the photodegradation of organic pollutants or the photoreduction of heavy metal ions. However, some semiconductors like TiO2 only can be excited under UV light irradiation due to its wide band gap (∼3.2 eV), which restricts its practical applications.5,9 Therefore, to explore visible-light response and high-performance photocatalysts is a key objective for researchers.Among a large variety of semiconductor photocatalysts, tin disulfide (SnS2), a peculiar CdI2-structure metal-sulfide semiconductor, depend on its excellent oxidation resistance, splendid thermal stability in the air and non-toxicity, low cost,10–15 has been in-depth investigated and widely applied in the photocatalysis field involving reduction of CO2,16 reduction of Cr(VI),17 degradation of organic compounds,18 and production of H2 from water.19 As the research goes on, different morphologies of SnS2 nanomaterials have been obtained (such as nanoflowers,20 nanotubes,21 nanorods,22 and nanoplates23) to produce higher specific area or control the band gap energies. Even so, the photocatalytic ability of SnS2 still has been limited by the high recombination rate of photo-generated carriers.24 Numerous strategies have been carried out to overcome this limitation, particularly, constructing suitable semiconductor composites is a powerful and effective method and various different composites with oxides (SnO2,25 Al2O3,26 TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27), sulfides (CdS,28 SnS2,29 In2S3
27), sulfides (CdS,28 SnS2,29 In2S3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), and others (ZnFe2O4,31 g-C3N4,32 rGO33), have been successful synthesized.
30), and others (ZnFe2O4,31 g-C3N4,32 rGO33), have been successful synthesized.
Recent years, silver sulfide (Ag2S), as a direct semiconductor material with narrow band gap, possesses a high absorption coefficient and excellent conductivity properties, attracts substantial research interest and has been widely applied in sustainable photocatalysis field.34–36 Up to now, a series of researches have been employed to use nanosized Ag2S or Ag2S quantum dots to modify some materials in order to obtain higher photocatalytic properties such as Ag2S–SnO2,37 ZnS–Ag2S,38,39 Ag2S/TiO2,40 CuS/Ag2S41 and so on. However, according to our knowledge, there is still no research compositing Ag2S quantum dot with hexagonal SnS2 to minimize the recombination rate of photo-generated carriers and further promote the photocatalytic property of SnS2. Therefore, it is highly desirable to synthesis Ag2S/SnS2 composite materials for removing the organic pollutants and heavy metal ions efficiently.
Inspired by the above ideas, in this study, a new type of Ag2S/SnS2 composite photocatalysts was synthesized via a simple in situ strategy, and determined by XRD, XPS, TEM, DRS, and some other techniques in detail. The experimental results showed that, Ag2S/SnS2 composite can achieve significantly enhanced photocatalytic performance for the removal of MO and Cr(VI), indicating that the excellent electron conductivity property of Ag2S can be used sufficiently. The reusability of Ag2S/SnS2 composite was examined. Finally, combining with the ESR and active species trapping results, a possible photocatalytic mechanism for the enhanced photocatalytic performance was presented.
2. Experimental
2.1 Materials
All chemical reagents and solvents were of analytical reagent grade and used as received.2.2 Catalysts synthesis
2.3 Characterization
The crystal structure of samples was determined by a Shimadzu XRD-6000 X-ray diffractometer (Cu Kα radiation). Transmission electron microscopy (TEM, JEOL JEM 2010, 200 kV) were used to observe the morphologies and structures of Ag2S/SnS2 composites. Energy dispersive X-ray spectroscope (EDS) was used to analyze the elementary composition. X-ray photoelectron spectroscopy (XPS, VG-MultiLab 2000 system, Mg Kα) was applied to the surface analysis of samples. The optical properties of samples were analyzed by a UV-2450 Shimadzu UV-vis spectrophotometer, where BaSO4 powder played the role of internal reflectance standard. The photoluminescence (PL) spectra of the as-prepared samples were investigated on a Varian Cary Eclipse Fluorescence spectrometer with an excitation wavelength of 360 nm. To investigate the transfer rate of the photo-induced carriers for the as-prepared samples, the electrochemical impedance spectroscopy (EIS) were performed in an electrochemical workstation, in which Ag/AgCl and a platinum wire and were used as reference and counter electrode, respectively. Meanwhile, the working electrode was photocatalyst (0.1 mg) films coated on ITO (0.5 × 1 cm2). The ESR spectra were taken on an ESR spectrometer (JES-FA200) with DMPO (Sigma Chemical Co.) as the spin-trap reagent dispersed in water and methanol, respectively.2.4 Photocatalytic activity
The photocatalytic activities of the SnS2 and Ag2S/SnS2 composites were performed by the degradation of MO and reduction of Cr(VI) irradiated by a 300 W Xe lamp with a 420 nm cutoff filter. The photocatalytic experiment was taken with a circulating water system controlling the temperature in 30 °C to avoid the thermal catalytic effects. Specific experimental details are as follows, 70 mg as-prepared samples were totally dispersed in 70 mL MO (10 mg L−1) and K2Cr2O7 (50 mg L−1) aqueous solution, respectively, and then the mixed solution was magnetically stirred for 30 min in the dark to obtain the absorption–desorption equilibrium, after which the reaction solution was irradiated for 60 min and about 4 mL solution was taken out in each 10 min and centrifuged. The concentrations of MO were determined on a UV-vis spectrophotometer (Shimadzu, UV-2450). The concentrations of Cr(VI) were analyzed by the following steps: 1 mL of the supernatant and 9 mL of sulfuric acid solution (0.2 mol L−1) diluted in the volumetric flask, then added 0.2 mL of chromogenic agent (0.25% DPC dissolved in acetone) followed by shaking and putting it aside for 15 min, to ensure complete coloration. Finally, the absorbance was measured at a wavelength of 540 nm using a UV-vis spectrophotometer.3. Results and discussion
3.1 Characterization of samples
The crystal structures of Ag2S, SnS2 and Ag2S/SnS2 composite materials with different Ag2S contents were investigated by XRD analysis, as shown in Fig. 1. The diffraction peaks of pure SnS2 can well be indexed to hexagonal SnS2 (JCPDS 23-0677) with 2θ = 15.03°, 28.20°, 32.12°, 41.89° and 49.96°, which are consistent with (001), (100), (101), (102) and (110) diffraction planes.42 The diffraction peaks of pure Ag2S are indexed to the standard card of pure monoclinic Ag2S (JCPDS 14-0072).38 For Ag2S/SnS2 composite materials, no Ag2S phase can be observed, which may be due to the low amount of Ag2S, and no obvious change is observed compared to pure SnS2, indicating that the introduction of Ag2S doesn't affect the crystal structure of SnS2.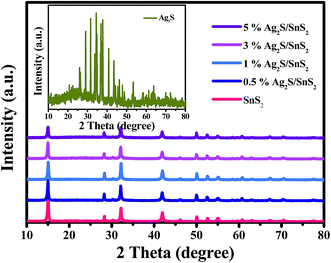 | ||
| Fig. 1 XRD patterns of pure SnS2 and Ag2S/SnS2 composites with different Ag2S contents (0.5%; 1%; 3%; 5%). Inset: XRD patterns of pure Ag2S. | ||
The microscopic morphologies and surface microstructures of the Ag2S/SnS2 composite materials were determined by the transmission electron microscopy (TEM). The pure SnS2 nanoplate (Fig. 2a) presents a smooth hexagonal structure with a diameter of about 400 nm, which has a large surface area for the in situ formation of Ag2S. The morphology of 1% Ag2S/SnS2 composite material (Fig. 2b) is similar to pure SnS2, which can be further seen in Fig. 2c, the Ag2S quantum dots have intimately attached on the surface of SnS2 nanoplates.43 The EDS analysis (Fig. 2d) of Ag2S/SnS2 composite samples displayed only Ag, Si, S and Sn elements with no other elements, indicating that Ag2S/SnS2 composite materials were successfully performed with only composed of both Ag2S quantum dots and SnS2 nanoplates.
 | ||
| Fig. 2 TEM images of (a) SnS2; (b and c) 1% Ag2S/SnS2 composite; (d) EDS analysis of 5% Ag2S/SnS2 composite sample. | ||
Fig. 3 is the XPS analysis for 1% Ag2S/SnS2 composite. Fig. 3a reveals Sn, S and Ag elements are three main elements in Ag2S/SnS2 composites. Two obvious peaks at 487.0 and 495.3 eV could be attributed to the Sn 3d5/2 and Sn 3d3/2, respectively, in the Sn 3d high-resolution spectra (Fig. 3b).44 Meanwhile, the typical peaks in Fig. 3c located at 161.7 and 162.9 eV could be assigned to S 2p3/2 and S 2p1/2 orbits of S2−, respectively.45 While the peaks at binding energy 368.3 and 374.5 eV could be ascribed to Ag 3d3/2 and Ag 3d5/2 of Ag+ in Ag2S quantum dots, respectively.46 Above all, from the analysis of XRD, EDS and XPS, it can be confirmed that SnS2 and Ag2S exist in the Ag2S/SnS2 composites.
The light absorption abilities of pure SnS2 and Ag2S/SnS2 composites were investigated via UV-vis spectroscopy. From Fig. 4a, we can see that SnS2 has strong and broad absorption in the region of visible light, and Ag2S quantum dot has broad absorption in both UV and visible light. Besides, the light absorption abilities of Ag2S/SnS2 composite materials show enhancement with the addition of Ag2S quantum dots. Hence the combination both SnS2 and Ag2S improves the absorption abilities, which may result in higher photocatalytic abilities for Ag2S/SnS2 composite materials. According to the equation: ahv = A (hv − Eg)n/2, where a, h, v, A, Eg is absorption coefficient, Planck constant, light frequency, a constant and band gap energy, respectively.47 The value of n is equal to 1 and 4 for direct and indirect transition semiconductors, respectively.48 Thus, the values of n are 4 for SnS2 and 1 for Ag2S. The band gap energy of SnS2 and Ag2S were calculated to be 2.05 eV and 1.1 eV, respectively (as shown in Fig. 4b and c).
 | ||
| Fig. 4 (a) The optical absorption spectroscopy of SnS2 and Ag2S/SnS2 composite materials; plots of (ahv)2 and (ahv)1/2 versus energy for the band gap energy of (b) SnS2 and (c) Ag2S, respectively. | ||
3.2 Photocatalytic activity
The photocatalytic properties of samples were evaluated by the degradation of MO (Fig. 5a) and the reduction of Cr(VI) (Fig. 5b) under visible light irradiation. We can see from Fig. 5a, under visible light irradiation, MO is too stable to degrade without any photocatalysts, but about 68% MO is degraded with the addition of pure SnS2. Meanwhile, in the case of Ag2S/SnS2 composite materials, MO degradation rates are enhanced for the same time period (60 min), and 1% Ag2S/SnS2 composite material shows the best photocatalytic activity with about 88% MO degraded. Besides, the removal rate of Cr(VI) can quickly increase from 42% for pure SnS2 to 53%, 57%, 51% and 45% for 0.5%, 1%, 3%, and 5% Ag2S/SnS2 composite materials, and the 1% Ag2S/SnS2 composite also shows the best performance, just as shown in Fig. 5b. Above all, the in situ formation of Ag2S makes good effect on the photocatalytic process.The reaction kinetics of MO photocatalytic degradation over different samples were investigated by using a first-order kinetics model with the formula: −ln(Ct/C0) = kt. Where Ct and C0 are the MO concentration at times t and 0, k (min−1) is the rate constant of apparent first-order and t (min) is the irradiation time. According to the Fig. 5c, we can see the k for SnS2, 0.5% Ag2S/SnS2, 1% Ag2S/SnS2, 3% Ag2S/SnS2 and 5% Ag2S/SnS2 are calculated to be 0.01822, 0.02787, 0.03228, 0.02352 and 0.02083 min−1, respectively. The rate constant of 1% Ag2S/SnS2 composite is 1.77 times as high as the pure SnS2 under the same conditions, indicating there has synergistic effect between Ag2S and SnS2, which improves the MO photocatalytic degradation. Moreover, the stability and recycling of the Ag2S/SnS2 materials were tested and shown in Fig. 5d, the photocatalytic performance has no significant decrease (only 3.2% decreased) after three cycles. The XRD and SEM analysis of 1% Ag2S/SnS2 composite after cycle runs were performed and the results are shown in Fig. S1 and S2.† For 1% Ag2S/SnS2 composite, the phase structure has no obvious changes before and after the experiment and the hexagonal structure still maintain with Ag2S quantum dots intimately attached on the surface. The results confirm that the Ag2S/SnS2 has good stability.
Photoluminescence (PL) spectroscopy is usually used to investigate the transfer and recombination ability of photo-generated carriers in photocatalysts. Generally, the lower the PL emission intensity, the higher the efficiency of charge recombination, and the more charge can join in photocatalytic reaction, and finally, the higher the photocatalytic performance. The PL spectra of Fig. 6 show that the emission peaks positions of SnS2 and 1% Ag2S/SnS2 are uniform, but the emission intensities of 1% Ag2S/SnS2 are lower than pure SnS2, indicating Ag2S/SnS2 composite materials have lower recombination rate of carriers than pure SnS2. The results show that the synergistic effect between Ag2S and SnS2 promotes the photo-induced carriers separation, and eventually, enhances the photocatalytic performance of Ag2S/SnS2 composite materials. EIS are performed on 1% Ag2S/SnS2 composite and pure SnS2 to further determine the charge separation efficiency over different samples and the results are shown in Fig. 6b. We can see that the diameter of Ag2S/SnS2 composite is smaller than that of pure SnS2, indicating the Ag2S/SnS2 composite has a relatively lower resistance than pure SnS2. Therefore, the coupling of Ag2S to SnS2 can enhance the separation and transfer efficiency of charge, and realize enhanced photocatalytic performance.
 | ||
| Fig. 6 (a) PL spectroscopy of SnS2 and 1% Ag2S/SnS2 composite; (b) electrochemical impedance spectroscopy plots of 1% Ag2S/SnS2 composite and pure SnS2. | ||
It was generally accepted that holes (h+), hydroxyl radical (˙OH), and superoxide radical (˙O2−) are three active species during photocatalytic oxidation processes.49 The electron spin resonance (ESR) was executed with DMPO spin-trapping in methanol and water, respectively, to determine the major active species in the photocatalytic process, and the results are shown in Fig. 7. There is no ESR signal for Ag2S/SnS2 composites at dark condition, but the DMPO˙O2− adducts signals can be observed under the visible light irradiation even the signal is not strong (Fig. 7a) and the DMPO˙OH is still negligible (Fig. 7b), indicating that a handful of ˙O2− can be generated while ˙OH cannot be generated.50 To further ascertain the active species over Ag2S/SnS2 photocatalyst, various active species trapping experiments were performed, just as shown in Fig. 8. After adding isopropanol (IPA) to trap ˙OH, no obvious variation of photocatalytic efficiency can be observed, revealing the ˙OH was not the major active species.51 However, the photocatalytic performance decreased in Ar atmosphere trapping ˙O2−, and instantly decreased after the TEOA was added to trap the h+.52,53 Combining with ESR results, the ˙O2− have a littler effect on the photodegradation process and the h+ is the major active species in the photocatalysis reaction.
 | ||
| Fig. 7 ESR spectra of radical adducts trapped by DMPO in 1% Ag2S/SnS2 composite in (a) methanol dispersion (for DMPO˙O2−) and (b) aqueous dispersion (for DMPO˙OH) under visible light irradiation. | ||
 | ||
| Fig. 8 Effects of various scavengers on the visible light photocatalytic activity of the 1% Ag2S/SnS2 composite material. | ||
The value of valence band (VB) can be confirmed by the XPS analysis. Fig. 9 was the VB spectra of the pure SnS2 sample and the VB maximum edge can be estimated to be 1.30 eV. According to the DRS results, the band gap energy (Eg) of pure SnS2 is 2.05 eV. Based on the equations EVB = ECB + Eg, the CB potential of SnS2 can be calculated to be −0.75 eV. Fig. 10 illustrates the possible mechanisms for the increased photocatalytic activity of Ag2S/SnS2 sample towards the pollutant degradation. After the visible light irradiated on the Ag2S/SnS2 composites, the electrons in the VB of SnS2 and Ag2S could be inspired and transfer from VB to the CB and the photo-generated holes would leaving in the VB. Generally, most of electrons and holes would recombine rapidly for pure SnS2, which results in pure SnS2 having relatively poor photocatalytic activity. However, when Ag2S quantum dots was in situ formed on the surface of the SnS2, the most electrons would transfer from the CB of SnS2 to the more positive CB of Ag2S due to the excellent electron transfer ability of Ag2S, leading to effective separation of charge carriers.54–56 But there still have a handful of electrons don't transferred, which would reduce the adsorbed molecule oxygen to yield ˙O2−, and because the remaining electrons are little, the content of ˙O2− is few. Then the produced few ˙O2− and vast photo-generated holes would worked as the reactive oxygen species for the subsequently photocatalytic degradation reaction. The excellent photocatalytic performance of Ag2S/SnS2 composite photocatalyst material can be attributed to the positive effect of Ag2S quantum dots, which provides an effective way to prevent the recombination of photo-generated carriers.
 | ||
| Fig. 10 The possible reaction mechanism for photocatalytic process with Ag2S/SnS2 composite material. | ||
4. Conclusions
In summary, high efficient Ag2S/SnS2 composite photocatalysts were successfully fabricated via a facile in situ hydrothermal-ion-exchange method. The photocatalytic ability of Ag2S/SnS2 composite materials toward MO and Cr(VI) under visible light irradiation is considerably enhanced compared to pure SnS2. The introduction of Ag2S quantum dots in the composites resulted in enhanced and stability photocatalytic performance, which provides an effective way for the separation of photo-generated carriers. The major active species were determined to be holes (h+) and superoxide radical (˙O2−) in photocatalytic reaction. Moreover, this work provided meaningful information on the preparation of other metal sulfide composite materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China for Youths (No. 21407065, 21506079), Natural Science Foundation of Jiangsu Province for Youths (BK20140533), China Postdoctoral Science Foundation (2015T80514). Jiangsu University Scientific Research Funding (No. 14JDG052). A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- Z. Yu, B. S. Yin, F. Y. Qu and X. Wu, Chem. Eng. J., 2014, 258, 203–209 CrossRef CAS.
- S. C. Xu, S. S. Pan, Y. Xu, Y. Y. Luo, Y. X. Zhang and G. H. Li, J. Hazard. Mater., 2015, 283, 7–13 CrossRef CAS PubMed.
- Z. Pei, M. Zhu, Y. Huang, Y. Huang, Q. Xue, H. Geng and C. Zhi, Nano Energy, 2016, 20, 254–263 CrossRef CAS.
- S. Ma, J. Xie, J. Wen, K. He, X. Li, W. Liu and X. Zhang, Appl. Surf. Sci., 2017, 391, 580–591 CrossRef CAS.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- M. Barroso, A. J. Cowan, S. R. Pendlebury, M. Grätzel, D. R. Klug and J. R. Durrant, J. Am. Chem. Soc., 2011, 133, 14868–14871 CrossRef CAS PubMed.
- S. W. Zhang, J. X. Li, X. K. Wang, Y. S. Huang, M. Y. Zeng and J. Z. Xu, J. Mater. Chem. A, 2015, 3, 10119–10126 CAS.
- W. M. Wu, G. D. Liu, Q. H. Xie, S. J. Liang, H. R. Zheng, R. S. Yuan, W. Y. Su and L. Wu, Green Chem., 2012, 14, 1705–1709 RSC.
- Y. J. Yuan, Z. J. Ye, H. W. Lu, B. Hu, Y. H. Li, D. Q. Chen, J. S. Zhong, Z. T. Yu and Z. G. Zou, ACS Catal., 2016, 6, 532–541 CrossRef CAS.
- L. C. A. Oliveira, H. S. Oliveira, G. Mayrink, H. S. Mansur, A. A. P. Mansur and R. L. Moreir, Appl. Catal., B, 2014, 152–153, 403–412 CrossRef CAS.
- X. Q. An, J. C. Yu and J. W. Tang, J. Mater. Chem. A, 2013, 2, 1000–1005 Search PubMed.
- Y. D. Zhang, P. Y. Zhu, L. L. Huang, J. Xie, S. C. Zhang, G. S. Cao and X. B. Zhao, Adv. Funct. Mater., 2015, 25, 481–489 CrossRef CAS.
- O. Brontvein, A. Albu-Yaron, M. Levy, D. Feuerman, R. Popovitz-Biro, R. T. A. Enyashin and J. M. Gordon, ACS Nano, 2015, 9, 7831–7839 CrossRef CAS PubMed.
- C. Wang, L. Wang, J. Jin, J. Liu, Y. Li, M. Wu, L. H. Chen, B. J. Wang, X. Y. Yang and B. L. Su, Appl. Catal., B, 2016, 188, 351–359 CrossRef CAS.
- S. Liang, Z. Zhou, X. Wu, S. Zhu, J. Bi, L. Zhou, M. Liu and L. Wu, Molecules, 2016, 21, 213–226 CrossRef PubMed.
- F. W. Li, L. Chen, M. Q. Xue, T. Williams, Y. Zhang, D. R. MacFarlane and J. Zhang, Nano Energy, 2017, 31, 270–277 CrossRef CAS.
- X. Zhang, P. Zhang, L. J. Wang, H. Q. Gao, J. T. Zhao, C. H. Liang, J. H. Hu and G. S. Shao, Appl. Catal., B, 2016, 192, 17–25 CrossRef CAS.
- F. Deng, L. N. Zhao, X. L. Pei, X. B. Luo and S. L. Luo, Mater. Chem. Phys., 2017, 189, 169–175 CrossRef CAS.
- G. W. Li, R. Su, J. C. Rao, J. Q. Wu, P. Rudolf, G. R. Blake, R. A. de Groot, F. Besenbacher and T. T. M. Palstra, J. Mater. Chem. A, 2016, 4, 209–216 CAS.
- M. He, L. X. Yuan and Y. H. Huang, RSC Adv., 2013, 3, 3374–3383 RSC.
- A. Yella, E. Mugnaioli, M. Panthöfer, H. A. Therese, U. Kolb and W. Tremel, Angew. Chem., Int. Ed., 2009, 48, 6426–6430 CrossRef CAS PubMed.
- P. Wu, N. Du, H. Zhang, J. Liu, L. Chang, L. Wang, D. Yang and J. Z. Jiang, Nanoscale, 2012, 4, 4002–4006 RSC.
- C. X. Zhai, N. Du, H. Zhang and D. Yang, Chem. Commun., 2011, 47, 1270–1272 RSC.
- F. Deng, X. Y. Lu, X. L. Pei, X. B. Luo, S. L. Luo and D. D. Dionysiou, J. Hazard. Mater., 2017, 332, 149–161 CrossRef CAS PubMed.
- J. F. Qu, D. Y. Chen, N. J. Li, Q. F. Xu, H. Li, J. H. He and J. M. Lu, Appl. Catal., B, 2017, 207, 404–411 CrossRef CAS.
- J. L. Mu, H. Miao, E. Z. Liu, L. D. Chen, J. Feng, T. X. Han, Y. Gao, J. Fan and X. Y. Hua, Ceram. Int., 2017, 43, 4992–5001 CrossRef CAS.
- H. Liu, D. Lu, Z. Zhang, G. Jie, Y. Yang and Z. Zhu, J. Mater. Sci., 2015, 50, 3207–3211 CrossRef CAS.
- X. Li, J. Zhu and H. Li, Appl. Catal., B, 2012, 123–124, 174–181 CrossRef CAS.
- S. Y. Hong, R. Popovitz-Biro, Y. Prior and R. Tenne, J. Am. Chem. Soc., 2003, 125, 10470 CrossRef CAS PubMed.
- Z. Yang, X. Huang, J. Li, Y. Zhang, S. Yu, Q. Xu and X. Y. Hu, Mikrochim. Acta, 2012, 177, 381–387 CrossRef CAS.
- F. Deng, X. Y. Lu, X. L. Pei, X. B. Luo, S. L. Luo and D. D. Dionysiou, J. Hazard. Mater., 2017, 332, 149–161 CrossRef CAS PubMed.
- Z. Zhang, J. Huang, M. Zhang, Q. Yuan and B. Dong, Appl. Catal., B, 2015, 163, 298–305 CrossRef CAS.
- H. Liu, L. Deng, Z. F. Zhang, J. Guan, Y. Ying and Z. F. Zhu, J. Mater. Sci., 2015, 50, 3207–3211 CrossRef CAS.
- Y. Xie, S. H. Heo, Y. N. Kim, S. H. Yoo and S. O. Cho, Nanotechnology, 2010, 21, 015703 CrossRef PubMed.
- H. M. Jia, W. W. He, W. G. Wamer, X. N. Han, B. B. Zhang, S. Zhang, Z. Zheng, Y. Xiang and J. J. Yin, J. Phys. Chem. C, 2016, 118, 21447–21456 Search PubMed.
- K. Ikeue, Y. Shinmura and M. Machida, Appl. Catal., B, 2012, 123–124, 84–88 CrossRef CAS.
- B. Subash, B. Krishnakumar, M. Swaminathan and M. Shanthi, Spectrochim. Acta, Part A, 2013, 105, 314–319 CrossRef CAS PubMed.
- D. A. Reddy, R. Ma, M. Y. Choi and T. K. Kim, Appl. Surf. Sci., 2015, 324, 725–735 CrossRef.
- X. Yang, H. T. Xue, J. Xu, X. Huang, J. Zhang, Y. B. Tang, T. W. Ng, H. L. Kwong, X. M. Meng and C. S. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 9078–9084 CAS.
- Y. Xie, S. H. Heo, Y. N. Kim, S. H. Yoo and S. O. Cho, Nanotechnology, 2010, 21, 015703 CrossRef PubMed.
- B. Zeng and W. Zeng, Nano, 2016, 12, 1750005 CrossRef.
- L. Y. Mao, J. J. Li, Y. L. Xie, Y. J. Zhong and Y. Hu, RSC Adv., 2014, 4, 29698–29701 RSC.
- X. L. Hu, Y. Y. Li, J. Tian, H. R. Yang and H. Z. Cui, J. Ind. Eng. Chem., 2017, 45, 189–196 CrossRef CAS.
- L. L. Chen, M. Chen, D. L. Jiang and J. M. Xie, J. Mol. Catal. A: Chem., 2016, 425, 174–182 CrossRef CAS.
- D. Jiang, L. Chen, J. Xie and M. Chen, Dalton Trans., 2014, 43, 4878–4885 RSC.
- H. L. Zhang, B. Wei, L. Zhu, J. H. Yu, W. J. Sun and L. L. Xu, Appl. Surf. Sci., 2013, 270, 133–138 CrossRef CAS.
- M. Y. Xu, H. L. Niu, J. J. Huang, J. M. Song, C. J. Mao, S. Y. Zhang, C. F. Zhu and C. L. Chen, Appl. Surf. Sci., 2015, 351, 374–381 CrossRef CAS.
- J. Tian, T. J. Yan, Z. Qiao, L. L. Wang, W. J. Li, J. M. You and B. B. Huang, Appl. Catal., B, 2017, 209, 566–578 CrossRef CAS.
- Y. F. Liu, W. Q. Yao, L. Di, R. L. Zong, M. Zhang, X. G. Ma and Y. F. Zhu, Appl. Catal., B, 2015, 163, 547–553 CrossRef CAS.
- Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed.
- R. Qiao, M. M. Mao, E. L. Hu, Y. J. Zhong, J. Q. Ning and Y. Hu, Inorg. Chem., 2015, 54, 9033–9039 CrossRef CAS PubMed.
- A. Etogo, E. L. Hu, C. M. Zhou, Y. J. Zhong, Y. Hu and Z. L. Hong, J. Mater. Chem. A, 2015, 3, 22413–22420 CAS.
- X. H. Gao, H. B. Wu, L. X. Zheng, Y. J. Zhong, Y. Hu and X. W. Lou, Angew. Chem., 2014, 126, 6027–6031 CrossRef.
- H. L. Zhang, B. Wei, L. Zhu, J. H. Yu, W. J. Sun and L. L. Xu, Appl. Surf. Sci., 2013, 270, 133–138 CrossRef CAS.
- D. Jiang, L. Chen, J. Xie and M. Chen, Dalton Trans., 2014, 43, 4878–4885 RSC.
- Y. Wang, M. X. Sun, Y. L. Fang, S. F. Sun and J. He, J. Mater. Sci., 2016, 51, 779–787 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08369f |
| This journal is © The Royal Society of Chemistry 2017 |




