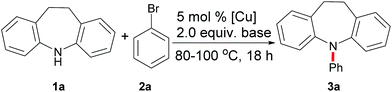Open Access Article
Open Access ArticleA general and mild Cu-catalytic N-arylation of iminodibenzyls and iminostilbenes using unactivated aryl halides†‡
Wubing Yao *,
Bin Zhang,
Rongrong Li,
Huajiang Jiang*,
Xiaoying Chen and
Fang Li
*,
Bin Zhang,
Rongrong Li,
Huajiang Jiang*,
Xiaoying Chen and
Fang Li
Taizhou Univ, Dept Chem, Jiaojiang 318000, Zhejiang, PR China. E-mail: yaowb@tzc.edu.cn; jhj@tzc.edu.cn
First published on 25th October 2017
Abstract
A ligand-free, highly efficient Cu-catalytic N-arylation of iminodibenzyl and iminostilbene derivatives with a broad scope of unactivated aryl halides under mild conditions has been developed for the first time. Moreover, the first Ni-based catalytic system was also applied to the N-arylation of pre-existing derivatives. These novel protocols provide facile and convenient access to the construction of dibenzazepines and offer promising alternatives to the widely used palladium catalysts.
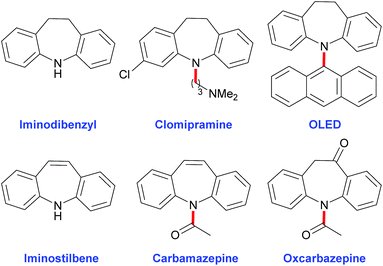
Iminodibenzyl and iminostilbene analogues are value-adding building blocks for the construction of drug molecules1 and notably the synthesis of organic functional materials, including organic light emitting diodes (OLEDs) (Scheme 1).2 The applications have generated tremendous attention and demands to develop efficient, practical and general processes for the preparation of these functionalized ring derivatives.
In recent years, a series of synthetic methods, mainly catalyzed by noble-metal catalytic systems, for the construction of iminodibenzyls and iminostilbenes have been reported, such as the cyclization of amines3 and anilines,4 or multi-step syntheses.5 Recently, classic copper-catalysed Friedel–Crafts cycliacylations have also been applied to the synthesis of these functionalized rings.6 However, the above approaches still have obvious limitations: (1) the cyclization reactions are often limited by the availability of manufacturable substrates; (2) the multi-step processes always suffer from drawbacks such as narrow functional group compatibility and low catalytic efficiency; and (3) the Friedel–Crafts cycliacylations typically require harsh reaction conditions (excessive reagents and super-lewis acid catalysts).6a
An alternative, but less developed synthetic strategy for iminodibenzyl and iminostilbene analogues is to use aryl halides as the arylating agents. Industrially, aryl halides are the preferred reagents because they are widely available and really low-cost. However, the N-arylation of these dibenzazepines with aryl halides is still rare, and mainly occupied by the precious metal Pd.2a,e,i,7–10 Very recently, Buchwald reported that a Ruphos-based palladacycle complex catalyses the N-arylation of iminodibenzyls and iminostilbenes with aryl halides.11 The reaction occurred at 80–100 °C in 1,4-dioxane with 0.1–1 mol% Pd in the presence of 1.1–2.2 equivalents of Li(NSiMe3)2. This work represents a breakthrough in the catalytic N-arylation of iminodibenzyls and iminostilbenes. However, to the best of our knowledge, the earth-abundant, environmentally benign, and low-cost base-metal catalyzed N-arylation of these functionalized rings has remained undisclosed, although a stoichiometric arylating process with copper under high temperature (180–200 °C) has been reported.2a So, for dibenzazepine derivatives, it is still necessary to develop more efficient and practical methods.
Herein, we firstly demonstrate that copper oxide is remarkably active for the N-arylation of iminodibenzyls and iminostilbenes with unactivated aryl iodides, bromides and even aryl chlorides under mild conditions, providing the desired products in moderate to excellent yields. Furthermore, the first Ni-catalyzed arylation of dibenzazepines was also developed.
Our initial studies focused on the direct N-arylation of iminodibenzyl (1a) with bromobenzene (2a) as a coupling partner by testing the catalytic activities of commercially available copper catalysts (Table 1, entries 1–5). Gratifyingly, the reaction with copper oxide showed the topmost efficiency and gave the desired product 3a in 71% yield (entry 1). More importantly, after evaluating a variety of bases, the catalytic efficiency was improved by the substitution of the base from KOH to KOtBu (95%, entry 9), while the processes using KOMe, KOAc and K2CO3 showed unsatisfactory yields (entries 6–8). Further optimization revealed that the solvents also have enormous impact on this arylation reaction. DMSO proved to be the best medium among the solvents studied, giving 95% of 3a in the presence of 5 mol% CuO at 100 °C (entry 9). However, when using other solvents, including toluene (PhMe), THF, DMF, 1,4-dioxane, CH3CN or CH3OH, the reactions were not suitable for arylation (entries 10–15). To our delight, the high activity of CuO allowed this transformation to proceed at 80 °C, furnishing 3a in 95% yield (entry 16). On the other hand, it should be noted that this process will not proceed at 25 °C or in the absence of copper catalysts (entries 17–18).
| Entry | [Cu] | Solvent | Base | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: iminodibenzyl (1.0 mmol), bromobenzene (2.0 mmol), [Cu] (50 μmol), base (2.0 mmol) and solvent (2.0 mL) under Ar atmosphere at 80–100 °C for 18 h.b The GC yield based on iminodibenzyl (1a) with mesitylene as an internal standard, and the isolated yield is in parentheses. | |||||
| 1 | CuO | DMSO | KOH | 100 | 71 |
| 2 | CuCl | DMSO | KOH | 100 | 42 |
| 3 | CuCN | DMSO | KOH | 100 | 35 |
| 4 | Cu(OAc)2 | DMSO | KOH | 100 | 47 |
| 5 | CuI | DMSO | KOH | 100 | 42 |
| 6 | CuO | DMSO | KOMe | 100 | 40 |
| 7 | CuO | DMSO | KOAc | 100 | 0 |
| 8 | CuO | DMSO | K2CO3 | 100 | 0 |
| 9 | CuO | DMSO | KOtBu | 100 | 95 |
| 10 | CuO | PhMe | KOtBu | 100 | 7 |
| 11 | CuO | THF | KOtBu | 100 | 16 |
| 12 | CuO | DMF | KOtBu | 100 | 77 |
| 13 | CuO | 1,4-Dioxane | KOtBu | 100 | 19 |
| 14 | CuO | CH3CN | KOtBu | 100 | 0 |
| 15 | CuO | CH3OH | KOtBu | 100 | 0 |
| 16 | CuO | DMSO | KOtBu | 80 | 95(90) |
| 17 | CuO | DMSO | KOtBu | 25 | 0 |
| 18 | — | DMSO | KOtBu | 25 | 0 |
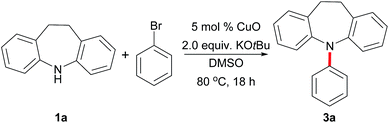
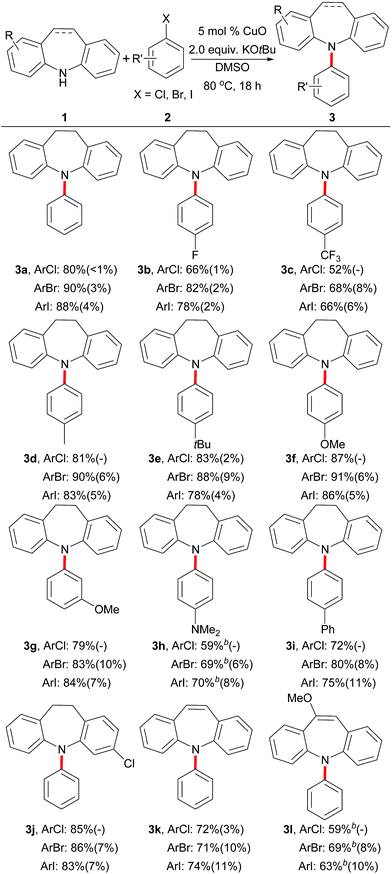
Using the highly active copper catalyst and optimal reaction conditions (Table 1, entry 16), the substrate scope of the reaction was explored by altering the dibenzazepines and aryl halides (Table 2). Importantly, the aryl bromides, iodides, or even aryl chlorides containing electron-deficient groups (2b and 2c) or electron-rich groups (2d–2h) were all well-tolerated in this transformation, offering the products in moderate to excellent yields. Furthermore, the amine- or phenyl-substituted substrates (2h and 2i) also proceeded smoothly with iminodibenzyl and produced chloroiminodibenzyl, which also underwent arylation smoothly using the standard conditions. Moreover, the yields of the arylating products in most cases indicate that the reaction occurred more smoothly when using an aryl bromide as the arylating partner. However, the introduction of a stronger electron-withdrawing group (–CF3) at the para-position of the aryl halides gave reduced yields of the corresponding products (3c). In addition, iminostilbenes are suitable substrates in the same arylation reaction, as demonstrated by the coupling with bromo-, iodo- or even chloro-benzene to form 3k and 3l in moderate to useful yields.
| a Reaction conditions: 1 (1.0 mmol), 2 (2.0 mmol), CuO (50 μmol, 5 mol%), KOtBu (2.0 mmol) and THF (2.0 mL) under Ar atmosphere at 80 °C for 18 h; the yields of the isolated products are shown; the approximate ArH yields in the parentheses were estimated by GC analysis.b CuO (0.1 mmol, 10 mol%), at 100 °C. |
|---|
 |
Similarly, nickel-based catalysts are also recognized in C–N bond-formation reactions, constituting an essential part of the amination methodologies.12 Moreover, the catalytic N-arylation of iminodibenzyls and iminostilbenes has not been disclosed so far. Therefore, we focused our attention on developing the N-arylation of dibenzazepines catalyzed by the low-cost and abundant metal Ni.
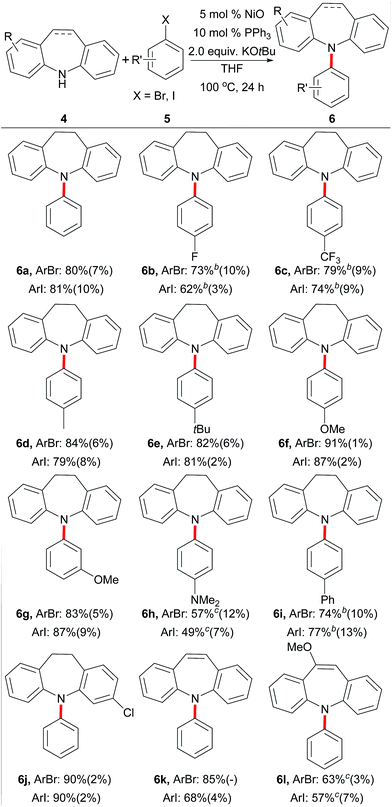
After the optimization of the reaction conditions,13 we evaluated dibenzazepines and various aryl halides (Table 3). The unactivated aryl bromides or iodides were also smoothly converted into the corresponding products in moderate to excellent yields at 100 °C after 24 h with 5 mol% NiO and 10 mol% PPh3. Many synthetically important functional groups, including fluorine (5b), trifluoromethyl (5c), alkyl (5d and 5e), methoxyl (5f and 5g), N,N-dimethyl (5h) and phenyl (5i) groups, are all well tolerated with the yields of iminodibenzyl ranging from 49% to 91%. On the other hand, chlorine-containing iminodibenzyl (4j) also gave the desired dibenzazepine in excellent yield (90%). Furthermore, the coupling of iminostilbenes (4k and 4l) with aryl halides also formed the desired products 6k–6l in useful yields.
| a Reaction conditions: 4 (1.0 mmol), 5 (2.0 mmol), NiO (50 μmol, 5 mol%), PPh3 (0.1 mmol, 10 mol%), KOtBu (2.0 mmol) and THF (2.0 mL) under Ar atmosphere at 100 °C for 24 h; the yields of the isolated products are shown; the approximate ArH yields in the parentheses were estimated by GC analysis.b NiO (0.1 mmol, 10 mol%) and PPh3 (0.2 mmol, 20 mol%).c NiO (0.1 mmol, 10 mol%) and PPh3 (0.2 mmol, 20 mol%) at 120 °C for 24 h. |
|---|
 |
To investigate the possibility of a radical-mediated pathway, we explored the Cu-catalyzed arylation of dibenzazepines (Table 4). Consequently, the reactions of 1a with bromobenzene in standard conditions using commonly available radical scavengers, including 9,10-dihydroanthracene (entry 2), BHT (butylated hydroxytoluene) (entry 3), or TEMPO (2,2,6,6-tetramethylpiperidinooxy) (entry 4), were conducted. However, these reactions indicated slightly decreased yields of 3a compared with the reaction in the absence of radical scavengers.
| Entry | Additive | Equivalent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), ArBr (2.0 mmol), CuO (50 μmol, 5 mol%), KOtBu (2.0 mmol), additive (0.5 or 1.0 equiv.), and THF (2.0 mL), under Ar atmosphere at 80 °C for 18 h.b The yields were determined by GC, and the isolated yield is in parentheses. | |||
| 1 | — | — | 95(90) |
| 2 | 9,10-Dihydroanthracene | 0.5 | 77 |
| 1.0 | 56 | ||
| 3 | BHT | 0.5 | 74 |
| 1.0 | 49 | ||
| 4 | TEMPO | 0.5 | 76 |
| 1.0 | 58 | ||
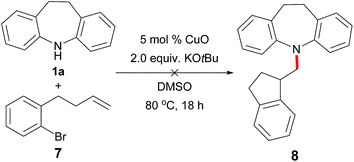
Meanwhile, in order to gain insight into the reaction pathway, we also carried out a radical-clock experiment using substrate 7, which is a radical well-known for 5-exo-trig cyclization to form 1-methylindane after H-atom abstraction.14 Under the current conditions, the reaction of 7 with iminodibenzyl 1a failed to produce the cyclization product 8 (Scheme 2). In summary, these results all indicated that this Cu-catalytic reaction might not appear to go through a radical process.
To distinguish homogeneous from heterogeneous catalysis, we conducted the reactions of iminodibenzyl with bromobenzene in the presence of the commonly used heterogeneous catalyst poisons liquid Hg15 and PMe3 (ref. 15d) (Table 5). The addition of Hg or PMe3 resulted in a slight decrease of the yield of 3a in comparison with that without the heterogeneous catalyst poison in the Cu-based catalytic system. However, the reactions catalysed by the metal Ni showed that the yield of the N-arylation process decreased significantly in the presence of 100 equivalents of Hg or 1.0 equivalent of PMe3 (relative to Ni). Therefore, these results suggest that the Cu catalyst is likely to be homogeneous under the standard conditions, whereas the reactions catalyzed by nickel are possible with metal particles.
| Entry | Additive | Equivalent | Yieldc (%) |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), ArBr (2.0 mmol), CuO (50 μmol, 5 mol%), KOtBu (2.0 mmol) and THF (2.0 mL) under Ar atmosphere at 80 °C for 18 h.b 4a (1.0 mmol), ArBr (2.0 mmol), NiO (50 μmol, 5 mol%), PPh3 (0.1 mmol, 10 mol%), KOtBu (2.0 mmol) and THF (2.0 mL) under Ar atmosphere at 100 °C for 24 h.c The yields were determined by GC, and the isolated yields are in parentheses. | |||
| [Cu]a | — | — | 95(90) |
| Hg | 100 | 88 | |
| PMe3 | 1.0 | 89 | |
| [Ni]b | — | — | 86(80) |
| Hg | 100 | 11 | |
| PMe3 | 1.0 | 16 | |
Conclusions
We have developed the first and efficient approach for the N-arylation of iminodibenzyl and iminostilbene derivatives with aryl bromides, iodides and even aryl chlorides, using the really simple copper reagent CuO in the absence of any ligand. Moreover, a Ni-based catalyst was also applied to the N-arylation of these derivatives for the first time. Featuring broad substrate scopes, low-cost metal-based catalysts, and mild conditions, these novel methods are practical and general processes for the N-arylation of dibenzazepines, which are useful for a variety of value-adding applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21542010), Science and Technology Plan Project of the Zhejiang Province (LY14B020012, 2016C37040), and Taizhou Science and Technology Project (No. 131KY08).Notes and references
- For examples, see: (a) F. Albani, R. Riva and A. Baruzzi, Pharmacopsychiatry, 1995, 28, 235 CrossRef CAS PubMed; (b) N. M. Tsankova, O. Berton, W. Renthal, A. Kumar, R. L. Neve and E. J. Nestler, Nat. Neurosci., 2006, 9, 519 CrossRef CAS PubMed; (c) P. K. Gillman, Br. J. Pharmacol., 2007, 151, 737 CrossRef CAS PubMed; (d) J. M. Gomez-Arguelles, R. Dorado, J. M. Sepulveda, R. Huet, F. G. Arrojo, E. Aragon, A. Herrera, C. Trron and B. Anciones, J. Clin. Neurosci., 2008, 15, 516 CrossRef CAS PubMed; (e) V. Krishnan and E. J. Nestler, Nature, 2008, 455, 894 CrossRef CAS PubMed; (f) M. Tian, A. Abdelrahman, S. Weinhausen, S. Hinz, S. Weyer, S. Dosa, A. El-Tayeb and C. E. Müller, Bioorg. Med. Chem., 2014, 22, 1077 CrossRef CAS PubMed; (g) P. Christian, C. Röthel, M. Tazreiter, A. Zimmer, I. Salzmann, R. Resel and O. Werzer, Cryst. Growth Des., 2016, 16, 2771 CrossRef CAS PubMed; (h) A. Brinkø, M. T. Larsen, H. Koldsø, L. Besenbacher, A. Kolind, B. Schiøtt, S. Sinning and H. H. Jensen, Bioorg. Med. Chem., 2016, 24, 2725 CrossRef PubMed; (i) A. Arndt, C. W. Liria, J. K. U. Yokoyama-Yasunaka, M. T. Machini, S. R. B. Uliana and B. P. Espósito, J. Inorg. Biochem., 2017, 172, 9 CrossRef CAS PubMed.
- For examples, see: (a) B. E. Koene, D. E. Loy and M. E. Thompson, Chem. Mater., 1998, 10, 2235 CrossRef CAS; (b) M.-X. Yu, J.-P. Duan, C.-H. Lin, C.-H. Cheng and Y.-T. Tao, Chem. Mater., 2002, 14, 3958 CrossRef CAS; (c) C.-T. Chen, J.-S. Lin, M. V. R. K. Moturu, Y.-W. Lin, W. Yi, Y.-T. Tao and C.-H. Chien, Chem. Commun., 2005, 51, 3980 RSC; (d) Y.-H. Yu, C.-H. Huang, J.-M. Yeh and P.-T. Huang, Org. Electron., 2011, 12, 694 CrossRef CAS; (e) Y. Suzuki, N. Fukui, K. Murakami, H. Yorimitsu and A. Osuka, Asian J. Org. Chem., 2013, 2, 1066 CrossRef CAS; (f) S. Sasaki, K. Hattori, K. Igawa and G.-i. Konishi, J. Phys. Chem. A, 2015, 119, 4898 CrossRef CAS PubMed; (g) S. Sasaki, G. P. C. Drummen and G.-i. Konishi, J. Mater. Chem. C, 2016, 4, 2731 RSC; (h) M. Bayda, G. Angulo, G. L. Hug, M. Ludwiczak, J. Karolczak, J. Koput, J. Dobkowski and B. Marciniak, Phys. Chem. Chem. Phys., 2017, 19, 11404 RSC; (i) G. Sathiyan and P. Sakthivel, Dyes Pigm., 2017, 143, 444 CrossRef CAS.
- For examples, see: (a) N. Božinović, I. Opsenica and B. A. Šolaja, Synlett, 2013, 24, 49 Search PubMed; (b) H. Christensen, C. Schjøth-Eskesen, M. Jensen, S. Sinning and H. H. Jensen, Chem.–Eur. J., 2011, 17, 10618 CrossRef CAS PubMed; (c) J. Tsoung, J. Panteleev, M. Tesch and M. Lautens, Org. Lett., 2014, 16, 110 CrossRef CAS PubMed; (d) D. A. Petrones, I. Franzoni, J. Ye, J. F. Rodríguez, A. I. Poblador-Bahamonde and M. Lautens, J. Am. Chem. Soc., 2017, 139, 3546 CrossRef PubMed.
- For examples, see: (a) M. Carril, R. SanMartin, F. Churruca, I. Tellitu and E. Domínguez, Org. Lett., 2005, 7, 4787 CrossRef CAS PubMed; (b) D. Tsvelikhovsky and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 16917 CrossRef CAS PubMed; (c) X. Zhang, Y. Yang and Y. Liang, Tetrahedron Lett., 2012, 53, 6406 CrossRef CAS; (d) M. Ito, R. Kawasaki, K. S. Kanyiva and T. Shibata, Eur. J. Org. Chem., 2016, 5234 CrossRef CAS; (e) C.-W. Kuo, A. Konala, L. Lin, T.-T. Chiang, C.-Y. Huang, T.-H. Yang, V. Kavala and C.-F. Yao, Chem. Commun., 2016, 52, 7870 RSC; (f) H. Lam, J. Tsoung and M. Lautens, J. Org. Chem., 2017, 82, 6089 CrossRef CAS PubMed; (g) C.-Y. Huang, V. Kavala, C.-W. Kuo, A. Konala, T.-H. Yang and C.-F. Yao, J. Org. Chem., 2017, 82, 1961 CrossRef CAS PubMed; (h) T.-H. Yang, C.-W. Kuo, V. Kavala, A. Konala, C.-Y. Huang and C.-F. Yao, Chem. Commun., 2017, 53, 1676 RSC.
- For examples, see: (a) D. Tsvelikhovsky and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14048 CrossRef CAS PubMed; (b) N. Della Cá, G. Maestri, M. Malacria, E. Derat and M. Catellani, Angew. Chem., Int. Ed., 2011, 50, 12257 CrossRef PubMed; (c) E.-C. Elliott, E. R. Bowkett, J. L. Maggs, J. Bacsa, B. K. Park, S. L. Regan, P. M. O’Neil and A. V. Stachulski, Org. Lett., 2011, 13, 5592 CrossRef CAS PubMed; (d) E.-C. Elliott, J. L. Maggs, B. K. Park, P. M. O’Neil and A. V. Stachulski, Org. Biomol. Chem., 2013, 11, 8426 RSC; (e) T. Kotipalli, D. Janreddy, V. Kavala, C.-W. Kuo, T.-S. Kuo, M.-L. Chen, C.-H. He and C.-F. Yao, RSC Adv., 2014, 4, 47833 RSC; (f) A. Gini and O. G. Mancheño, Synlett, 2016, 2, 526 Search PubMed; (g) F. Lied, H. B. Žugelj, S. Kress, B. Štefane, F. Glorius and M. Lautens, ACS Catal., 2017, 7, 1378 CrossRef CAS; (h) S. S. lchake, A. Konala, V. Kavala, C.-W. Kuo and C.-F. Yao, Org. Lett., 2017, 19, 54 CrossRef PubMed.
- For examples, see: (a) D. Kaufmann, P. C. Fünfschilling, U. Beutler, P. Hoehn, O. Lohse and W. Zaugg, Tetrahedron Lett., 2004, 45, 5275 CrossRef CAS; (b) B. Eftekhari-Sis, M. Zirak and A. Akbari, Chem. Rev., 2013, 113, 2958 CrossRef CAS PubMed; (c) H. A. K. Abd El-Aal, ARKIVOC, 2015, 230 CAS; (d) L. M. Acosta-Quintero, J. Jurado, M. Nogueras, A. Palma and J. Cobo, Eur. J. Org. Chem., 2015, 5360 CrossRef CAS; (e) Z. Qureshi, J. Y. Kim, T. Bruum, H. Lam and M. Lautens, ACS Catal., 2016, 6, 4946 CrossRef CAS.
- For examples, see: (a) P. D. Thornton, N. Brown, D. Hill, B. Neuenswander, G. H. Lushington, C. Santini and K. R. Buszek, ACS Comb. Sci., 2011, 13, 443 CrossRef CAS PubMed; (b) K. H. Chung, C. M. So, S. M. Wong, C. H. Luk, Z. Zhou, C. P. Lau and F. Y. Kwong, Synlett, 2012, 1181 CAS.
- For examples, see: (a) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS PubMed; (b) N. C. Bruno, M. T. Tudge and S. L. Buchwald, Chem. Sci., 2013, 4, 916 RSC; (c) N. C. Bruno, N. Niljianskul and S. L. Buchwald, J. Org. Chem., 2014, 79, 4161 CrossRef CAS PubMed.
- For examples, see: (a) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC; (b) D. Maiti, B. P. Fors, J. L. Henderson, Y. Nakamura and S. L. Buchwald, Chem. Sci., 2011, 2, 57 RSC.
- For reviews, see: (a) P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564 CrossRef CAS PubMed; (b) N. Hazari, P. R. Melvin and M. M. Beromi, Nat. Rev. Chem., 2017, 1, 25 CrossRef PubMed.
- W. Huang and S. L. Buchwald, Chem.–Eur. J., 2016, 22, 14186 CrossRef CAS PubMed.
- For examples, see: (a) J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 6054 CrossRef CAS; (b) N. H. Park, G. Teverovskiy and S. L. Buchwald, Org. Lett., 2014, 16, 220 CrossRef CAS PubMed; (c) N. H. Park, E. V. Vinogradova, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2015, 54, 8259 CrossRef CAS PubMed.
- See the ESI‡ for details.
- A. N. Abeywickrema and A. L. J. Beckwith, J. Chem. Soc., Chem. Commun., 1986, 464 RSC.
- (a) D. R. Anton and R. H. Crabtree, Organometallics, 1983, 2, 855 CrossRef CAS; (b) M. Gupta, C. Hagen, R. J. Flesher, W. C. Kaska and C. M. Jensen, Chem. Commun., 1996, 2083 RSC; (c) M. Gupta, C. Hagen, W. C. Kaska, R. E. Cramer and C. M. Jensen, J. Am. Chem. Soc., 1997, 119, 840 CrossRef CAS; (d) S. Fu, Z. Shao, Y. Wang and Q. Liu, J. Am. Chem. Soc., 2017, 139, 11941 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Professor Qizhong Zhou on the occasion of his 45th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and NMR and HRMS (ESI) spectra. See DOI: 10.1039/c7ra07437a |
| This journal is © The Royal Society of Chemistry 2017 |