Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of Au@Ag–hemin decorated reduced graphene oxide sheets: a novel peroxidase mimetic for ultrasensitive colorimetric detection of hydrogen peroxide and glucose†
Sanjay Kumar a,
Pulak Bhushan
a,
Pulak Bhushan a and
Shantanu Bhattacharya
a and
Shantanu Bhattacharya *ab
*ab
aMicrosystems Fabrication Laboratory, Department of Mechanical Engineering, Indian Institute of Technology Kanpur, India. E-mail: bhattacs@iitk.ac.in
bDesign Programme, Indian Institute of Technology Kanpur, India
First published on 31st July 2017
Abstract
Herein we report the facile synthesis of a quaternary nanocomposite material (hemin–silver coated gold–graphene oxide) and evaluate its efficacy as a novel peroxidase mimetic. A strong synergistic coupling between the various components involved results in an excellent catalytic performance of this nanocomposite. A comparison of the different morphologies of the silver coated gold particles strongly indicates a greater sensitivity of the nanostar morphology over the nanoparticle morphology owing to its high surface-to-volume ratio. Furthermore, the immobilization of hemin and silver coated gold nanostars on a graphene oxide sheet framework imposes a nanoscale confinement, effectively augmenting the overall catalytic performance of the composite. The nanocomposite followed typical Michaelis–Menten theory and electrochemical analysis suggested facilitation of accelerated electron transfer between TMB and H2O2. A KM value of 2.75 mM−1 suggested a high affinity of the nanocomposite towards TMB. Furthermore, a 2.8 times increase in the maximum reaction rate compared to HRP established the high catalytic activity of the nanocomposite. The nanocomposite demonstrates a nanomolar range sensitivity towards hydrogen peroxide and glucose (limit of detection = 1.26 nM and 425 nM). The nanocomposites have also been employed to develop a paper-based point-of-care diagnostic device. The device has been utilized for detection of glucose in human blood serum samples with satisfactory results.
Introduction
Enzymes are biological catalysts serving a wide variety of disciplines in the paper, chemical and food processing industries, biochemical and medical fields, etc. Natural enzymes are extensively used as catalysts owing to their high catalytic efficiency and substrate specificity. However, their extreme susceptibility to denaturation in stringent conditions restricts their practical applications. Moreover, their complicated and expensive synthesis and storage has led to immense interest in discovering artificial enzyme mimetics.1 Hemin,2–4 cyclodextrin,5 porphyrin,6 graphene oxide,7,8 metal nanoparticles,9–11 etc., have attracted immense attention for the development of enzyme mimetics. Hemin, a natural metalloporphyrin and the active center of heme proteins, which include cytochromes, peroxidases, myoglobins and hemoglobins, exhibits a peroxidase-like activity.3,12 Nevertheless, it suffers from poor catalytic activity due to oxidative self-degradation, molecular aggregation to yield inactive dimers and low solubility in aqueous buffers.13 Immobilization of hemin on high surface area materials has led to improved efficiency by the virtue of effective site isolation.14Metal nanoparticles like gold, silver, etc. also possess unique catalytic activity accounted to their large surface area and other quantum effects. The intricate interplay between their surface structures, morphology and optical properties befit them for their deployment as chemical and biological sensors. These nanoparticles exhibit local surface plasmon resonance effect (LSPR), in which localized electromagnetic field peaks on a metallic surface on excitation by an incident light of specific wavelength. This effect is extremely sensitive to the morphology of the nanoparticles, their aggregation behavior and their engulfing surrounding media.15,16 Benefitting from these advantages, a variety of metal nanoparticles and their hybrids have been recently found to show excellent peroxidase-like activity, for example, AuNPs,17,18 AgNPs,19 Au@Ag core–shell NPs,20 chitosan-stabilized AgNPs,9 DNA–Ag/Pt nanoclusters,21 etc. Furthermore, an investigation into the nanoparticle morphology reported that nanoparticles with an overall lower symmetry possess a very high field enhancement factor.22 Recent technological advancements in the synthesis of nanoparticles have enabled the fabrication of anisotropic nanoparticles such as nanorods, nanotubes, nanoshells and nanostars. Among these, nanostars have received considerable attention owing to their distinguished characteristics such as wide range of tunable LSPR, excellent surface-enhanced Raman scattering (SERS) performance, superior optical detectability, multipeak scattering and high surface-to-volume ratio.23,24 Gold nanoparticles (AuNPs) have played a critical role in this domain owing to their excellent optical properties, biocompatibility and facile synthesis. In the past few decades, several efforts have been made to augment the catalytic activity and stability of AuNPs. An interesting approach has been coating of the gold nanoparticles with silver. The intrinsic properties of silver nanoparticles like higher extinction coefficient, higher scattering to extinction ratio and sharp extinction bands, result in the bimetallic nanoparticles having a broader plasmon resonance band than that of individual nanoparticles.25,26 Silver nanoparticles have been known to be effective scavengers of H2O2. However, their optical properties are rarely utilized albeit their high catalytic activity, owing to their self-coalescing property. Hence, a nanocomposite of Au@Ag can result in both superior catalytic activity and unique optical properties.
Recently, graphene oxide has also been reported to possess intrinsic peroxidase-like catalytic activity.7 On account of its high surface area and rapid electron transfer capability, it has been widely used as an anchoring substrate for various nanomaterials like gold/silver nanoparticles, hemin, iron oxide, etc. to enhance their catalytic efficiency.27,28 However, exploration of superior enzyme mimetics with higher stability and sensitivity is vital for the advancement of the biomimetic field. In addition, colorimetric detection techniques based on such artificial enzyme mimetics could facilitate a substantial improvement in the detection limits. Such techniques can allow for rapid, robust and inexpensive detection of various biomarkers obviating the need for laboratory sample evaluation.
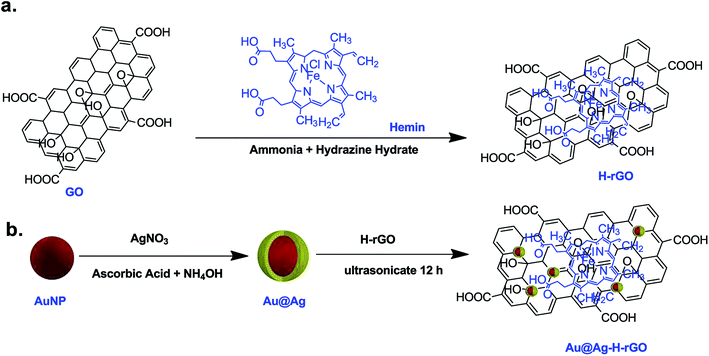
Herein, we have reported synthesis of a simple quaternary nanocomposite of gold, silver, hemin and graphene oxide (Au@Ag–H–rGO). The nanocomposite is an ensemble of graphene oxide serving as a matrix for anchoring hemin and silver coated gold nanoparticles/nanostars. A surface coating of silver helped enhance the stability of gold nanoparticles and may have changed its surface properties leading to a higher catalytic activity. Further, a synergistic interaction between the Au@Ag nanocomposites and hemin with graphene oxide led to a highly stable catalytic nanozyme. Interestingly, an investigation on the shape-dependent sensitivity of gold revealed that, the star-shaped morphology exhibit superior performance and stability over the spherical-shaped morphology. The synthesized nanocomposites are found to efficiently oxidize the peroxidase substrate TMB in the presence of H2O2, exhibiting an overall excellent catalytic activity. The exploitation of the nanostar morphology amalgamated with the embedment over GO sheet led to the development of a highly sensitive and selective colorimetric sensor for H2O2. It is known that H2O2 is produced by many oxidative enzymes (e.g. glucose, cholesterol, uric acid) when reacted with suitable substrates. The quantification of these enzymes depends on the amount of H2O2 produced in the reaction, necessitating its detection in trace amounts. Among these oxidative enzymes, glucose is a crucial biomarker for diagnosis of diseases like diabetes,29 cancer,30 etc. Hence, the H2O2 detection mechanism was further employed for the determination of glucose in human serum samples. The system showed high selectivity and sensitivity along with good reproducibility towards glucose, showing great potential for clinical diagnosis for future use. Exploiting this detection mechanism, a paper-based sensing platform was implemented for rapid and visual on-site monitoring of glucose in human serum samples.
Experimental section
Materials and reagents
Graphite flakes (acid treated, ≥99%), sodium chloride (NaCl), sulphuric acid (H2SO4, ≥98%), potassium permanganate (KMnO4, ≥99%), hydrogen peroxide (30 wt% in H2O), glucose oxidase (GOx), D-glucose, hydrochloric acid (35%), acetic acid, sodium acetate trihydrate (CH3COONa·3H2O, ≥99%), ammonia solution, tri-sodium citrate dihydrate (C6H5Na3O7·2H2O, ≥99%), sodium borohydride (NaBH4, ≥98%), silver nitrate (AgNO3, ≥99%), hemin, cetyltrimethyl ammonium bromide (CTAB, ≥98%) and Whatman qualitative filter paper grade 1 were purchased from Sigma Aldrich, India. Gold(III) chloride trihydrate (HAuCl4·3H2O, ≥99.9%), L-ascorbic acid (≥99%) and 3,5,3′,5′-tetramethylbenzidine (TMB) were obtained from Loba Chemie Pvt. Ltd., India. All chemicals and reagents used were of analytical grade. All aqueous solutions were prepared in high performance liquid chromatography (HPLC) water. Human blood serum samples were obtained from IIT Kanpur, India, and all analyses were performed at the Microsystems Fabrication Laboratory. All experiments were performed in compliance with the relevant laws and national guidelines (Ethical Guidelines for Biomedical Research on Human Participants, provided by Indian Council of Medical Research), and the ethical clearance for the same was provided by the GSVM Medical College, Kanpur. All the experiments with the samples were performed with informed consent obtained from the persons who provided the samples.Synthesis of AuNP@Ag–hemin–rGO and AuNS@Ag–hemin–rGO nanocomposites
Characterization
UV-visible spectra (UV-vis) were measured using an Evolution 300 spectrophotometer (Thermo Scientific). High resolution TEM (HR-TEM) and scanning-transmission-electron microscopy (STEM) images were recorded using a FEI Titan G2 60-300 TEM operated at 300 kV. The X-ray diffractometry (XRD) was performed on a Bruker D8 Advance X-ray diffractometer with Cu Kα (k = 0.154 nm) radiation (Bruker AXS, Germany). The Raman spectra were recorded on a confocal Raman microscope with 10× objective using an excitation laser light of wavelength 532 nm. Energy dispersive X-ray spectroscopy (EDS) (Oxford instruments) in conjunction with Sigma Field Emission Scanning Electron Microscope (CARL ZEISS) was used for chemical microanalysis of nanocomposites. Dynamic light scattering (DLS) and zeta potential measurements were carried out on a Beckman Coulter Delsa Nano C. Thermogravimetric analysis (TGA) profiles were obtained between 20 °C and 650 °C in O2 using a Simultaneous Thermal Analyzer (STA) 8000 (Perkin Elmer Ltd.) instrument. The cyclic voltammetry measurements were carried out by BASi Epsilon Eclipse™. The pH values of the buffer solutions were recorded using a Labman benchtop pH meter. Particle sizes were measured by image analysis of the TEM images using ImageJ software. Photographs of the samples in cuvettes and on paper were taken using a Nikon D3200 digital camera.Catalytic activity and kinetic state assays of AuNP/AuNS@Ag–H–rGO
In order to study the catalytic activity of the synthesized nanocomposites, 500 μL of 600 μM TMB and 500 μL of AuNP/AuNS@Ag–H–rGO (0.5 μg mL−1) solution was mixed in 0.02 M sodium acetate–acetic acid buffer (pH = 4.1) and vortexed for 10 min. Next, 1 mL of H2O2 solution (0.5 mM) was added to above solution. To analyze the steady state kinetics of the reaction, 500 μL of AuNP/AuNS@Ag–H–rGO (0.5 μg mL−1) mixed with 500 μL TMB or H2O2 (60 μM) as substrate was used. The solution was diluted in sodium acetate–acetic acid buffer (0.02 M). The spectral measurements were recorded at 652 nm using which the maximal reaction rate and the Michaelis–Menten constant were calculated.Colorimetric detection of H2O2 and glucose
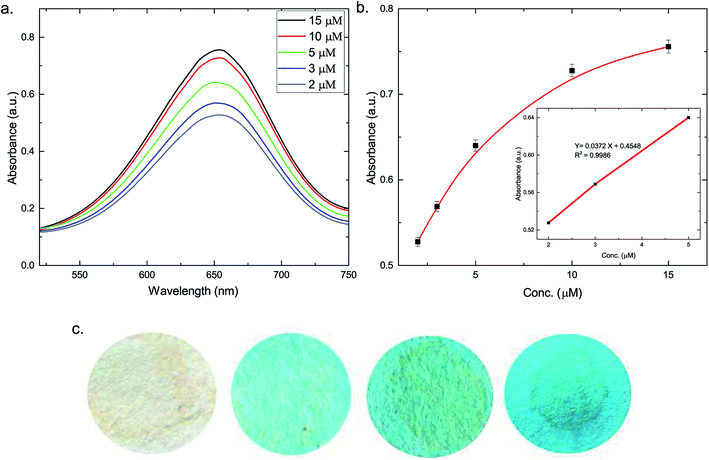
For H2O2 measurements, 500 μL TMB (60 μM) and 500 μL of AuNP/AuNS@Ag–H–rGO (0.5 μg mL−1) solution was mixed in 0.02 M sodium acetate–acetic acid buffer (pH = 4.1) and vortexed for 10 min. 1 mL of H2O2 of varying concentrations (10, 15, 20, 25, 35, 50, 75 and 100 nM) was added to the above solution, after which the spectral measurements were carried out. For glucose detection, the colorimetric detection mechanism of H2O2 was utilized based on the catalytic oxidization of glucose by GOx (glucose + O2 + GOx → gluconic acid + H2O2). In a typical experiment, a mixture of 50 μL of the as-synthesized AuNS@Ag–H–rGO nanocomposites (0.5 μg mL−1) and 20 μL of TMB (1 mM in methanol) was added to 3 mL of 0.02 M sodium acetate–acetic acid buffer (pH = 4.1) solution. Then, 15 μL of GOx aqueous solution (1 mg mL−1) and 150 μL of glucose with various concentrations (2, 3, 5, 10 and 15 μM) in sodium phosphate buffer (0.1 mM, pH = 7) were mixed to the above solution.Electrochemical study of AuNP/NS@Ag–H–rGO
For conducting the electrochemical measurements, the glassy carbon electrodes (GCEs) were polished subsequently with 0.3 and 0.05 μm alumina slurry, rinsed with HPLC water, washed successively with ethanol and HPLC water in an ultrasonic bath and dried in air. Then, the AuNP/AuNS@Ag–H–rGO modified GCEs were prepared by dropping 10 μL of AuNP/AuNS@Ag–H–rGO (3 μg mL−1) solution on the surface of the GCE, and air dried overnight.Preparation of human blood serum samples
To demonstrate the practical feasibility of the proposed detection assay, three human blood samples were collected and analyzed. All samples were centrifuged at 2000 rpm for 10 min to extract the serum. The supernatant was then diluted 20 times using sodium phosphate buffer (0.1 mM, pH = 7). Further, the samples were spiked with known concentrations of glucose to establish the selectivity of the assay.Results and discussion
Characterization of the AuNP/AuNS@Ag–hemin–rGO
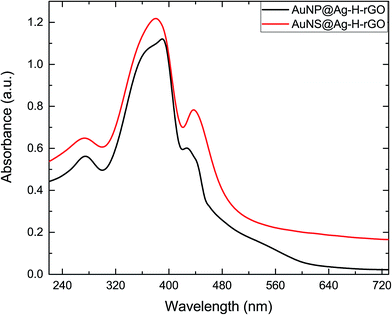
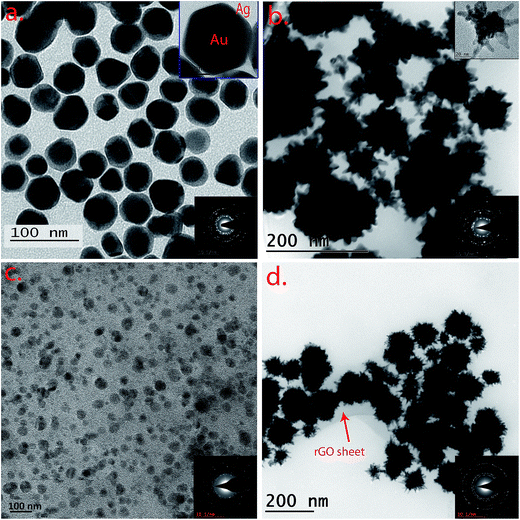
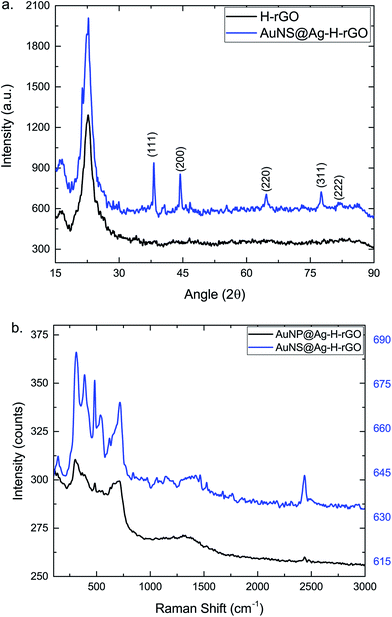
To monitor the successful synthesis of the nanocomposites, UV-visible spectroscopy (UV-vis) was utilized at every step. The spectrum of Au seeds showed an absorption maximum at 517 nm, confirming the synthesis of 12 nm sized particles (Fig. S1†). The absorption spectra of rGO, hemin and H–rGO are shown in Fig. S2a.† An aqueous dispersion of rGO exhibits a strong peak at 272 nm. In case of pure hemin the strong peak at 385 nm can be ascribed to the Soret band and a broad weak peak around 600 nm assigned to the Q-bands. Further, a red shift of the Soret band from 385 nm to 395 nm is observed in the H–rGO spectrum, which may be attributed to the ring π–π* transitions of the Soret band. This may be inferred as an interaction between the porphyrin moiety and the rGO sheet.34 Fig. S2b† illustrates the UV-vis spectra of AuNPs and AuNSs coated with varying amounts of silver, along with TEM images of a few representative samples. Initially, AuNPs and AuNSs showed respective strong peaks at 527 nm and 582 nm. But, with the increasing amount of coated silver, a progressive blue-shift in the surface plasmon wavelength has been observed. For example, the peak at 582 nm corresponding to AuNSs has been blue shifted to 437 nm on addition of 5 mL of silver nitrate. It has been observed from the TEM images that the silver first grows over the core region of the AuNS core and then over its branches. It has been seen that using a lower amount of Ag leads to the synthesis of AuNS structure with exposed branches. Hence, all the subsequent experiments and characterization were performed using AuNP@Ag-5–H–rGO and AuNS@Ag-5–H–rGO. The UV-vis spectra of AuNP@Ag–H–rGO and AuNS@Ag–H–rGO are presented in Fig. 1. The spectra showing prominent peaks of 270 and 385 nm confirms to the presence of rGO and hemin in the structures. In addition, the peaks at 428 and 437 nm correspond to AuNP@Ag and AuNS@Ag respectively. Fig. S3a and b† show the folded morphology of the single-layered rGO sheet and the successful adsorption of hemin over its surface respectively. The crystalline nature of rGO is represented by the SAED patterns shown as insets. Fig. S3c and d† illustrate the TEM images of as-synthesized AuNPs and AuNSs. The images revealed an average size of the AuNPs to be 30 nm and the AuNS core has been measured to be 20 nm with an average branch length of 15 nm. Fig. S4a and b† reveal the STEM images of AuNS@Ag and AuNS@Ag–H–rGO and the presence of the constituent elements have been further confirmed by elemental mapping using EDS technique. The TEM images of AuNP@Ag show an average size distribution of 50 nm of the synthesized gold nanoparticles. The silver coating over the gold nanoparticles can be clearly seen in the image inset (Fig. 2a). Fig. 2b shows the TEM image of silver coated gold nanostars. The crystallinity of the particles was established by the well-defined diffraction patterns (inset). Fig. 2c and d illustrate the morphology of the as-synthesized nanocomposites by TEM where, AuNP@Ag/AuNS@Ag and hemin can be seen localized and well dispersed over the rGO sheets. The acquired SAED patterns of the same confirm the crystalline structure of the nanocomposites (insets). H–rGO and AuNS@Ag–H–rGO nanocomposites have been further identified using X-ray diffraction (XRD) patterns as shown in Fig. 3a. The characteristic peaks corresponding to (002) at angle 22.82° confirms the existence of hexagonal structure of rGO. Further, five diffraction peaks (111), (200), (220), (311) and (222) confirms face centered cubic structure of Au and Ag (PDF 00-004-0783, PDF 00-004-0784). The diffraction peaks of gold and silver show high overlap with each other mostly owing to their similar lattice constants (Au = 0.4080 nm and Ag = 0.4086 nm).35 Shown in Fig. 3b is the Raman spectra of AuNP@Ag–H–rGO and AuNS@Ag–H–rGO nanocomposites. In its non-aggregated state, the AuNS@Ag–H–rGO shows approximately 2.5 ± 0.2 times higher signal intensity compared to AuNP@Ag–H–rGO. A major signal enhancement has been observed along with a blue-shift in the Raman band at five peaks corresponding to 299, 481, 712, 1309, 2436 cm−1 implying a synergistic interaction between H–rGO and AuNS@Ag. Energy dispersive X-ray spectroscopy (EDS) was utilized to analyze the elemental distribution of the as-synthesized nanocomposite (Fig. S5†). The peaks of Au, Ag, Cl and Fe suggested successful loading of hemin and AuNS@Ag on the rGO sheets. A strong negative charge of 24 mV and 57 mV revealed by zeta potential analysis indicated the high colloidal stability of AuNP@Ag–H–rGO and AuNS@Ag–H–rGO as a result of the acting intermolecular repulsion forces (Fig. S6†). The loading content of hemin, AuNP@Ag and AuNS@Ag calculated using thermal gravimetric analysis (TGA), was found to be about 10 wt%, 29 wt% and 30 wt% respectively (Fig. S7†).Catalytic activity of AuNP/AuNS@Ag–H–rGO
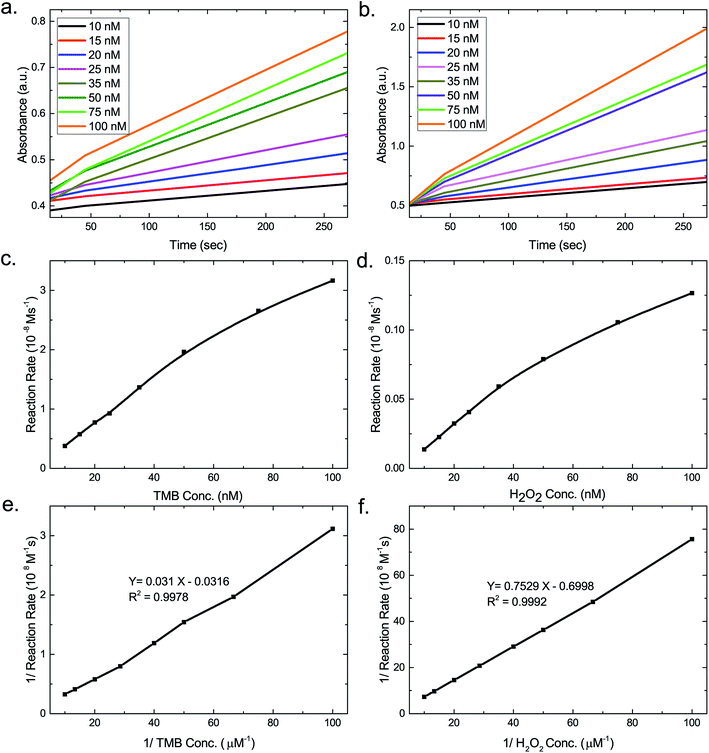
To evaluate the peroxidase-like activity of the as-prepared AuNP/AuNS@Ag–H–rGO nanocomposites, the catalysis of peroxidase substrate TMB in presence of H2O2 was tested and monitored by UV-vis spectroscopy (Fig. S8†). The catalytic properties of 10 wt% hemin in H–rGO, 29 wt% AuNP@Ag, 30 wt% AuNS@Ag and the final nanocomposites AuNP/AuNS@Ag–H–rGO were investigated. An addition of H2O2 to a solution mixture of TMB and AuNP/AuNS@Ag–H–rGO resulted in an overall blue coloration of the solution. In the absence of the synthesized nanocomposites, the absorbance was quite low and an SPR effect was observed at 652 nm, corresponding to oxidized TMB.36 On addition of H–rGO, an increase in the absorbance was observed, similar to when AuNP/AuNS@Ag was added. Since the loading of AuNP@Ag and AuNS@Ag was similar, it can be safely inferred from the graph that the nanostar morphology led to a higher catalytic activity than that of spherical morphology. Addition of AuNP@Ag–H–rGO showed a stronger catalytic activity, whilst, in the presence of the AuNS@Ag–H–rGO, the absorbance value was increased ten times. This confirmed a synergistic interaction between H–rGO and AuNS@Ag leading to a dramatic stimulation in the catalytic activity of the nanocomposites.The intrinsic peroxidase-like activity of AuNP/AuNS@Ag–H–rGO nanocomposites was further optimized by analyzing the effect of physical reaction conditions like pH, temperature and concentration of H2O2 on their catalytic efficiency (Fig. S9a–c†). The optimum values for pH, temperature and H2O2 concentration obtained were 4.1, 37 °C and 150 μM corresponding to which the maximum catalytic efficiency would be demonstrated. Thus, subsequent experiments were performed under these optimized set of conditions. Next, to investigate the catalytic activity of the as-synthesized nanocomposites, the steady state kinetics of the reaction was analyzed. Fig. 4a, b, S10a and b† show the time-dependent absorbance changes of the reaction solution at 652 nm with H2O2 and TMB as the substrates for AuNS@Ag–H–rGO and AuNP@Ag–H–rGO respectively. The typical Michaelis–Menten curves were obtained by evaluating the slopes (Fig. 4c, d, S10c and d†). Based on the calculated reaction rates, linear double reciprocal plots (Lineweaver–Burk plot) were acquired as shown in Fig. 4e, f, S10e and f.† These were used to find out the Michaelis–Menten constant (KM) and maximum reaction rate (Vmax). The slopes of all lines were parallel, indicating a characteristic ping-pong mechanism, as observed for native peroxidase (HRP).37 This indicates that the nanocomposites first bind and react with the first substrate, yielding the first product and then react with the second substrate.
As is evident from Table 1, the apparent KM values of 0.048 (TMB) and 2.75 (H2O2) for AuNS@Ag–H–rGO indicated its higher affinity to the substrates compared to AuNP@Ag–H–rGO which has KM values of 0.062 (TMB) and 2.82 (H2O2). This infers that both the nanocomposites exhibit higher affinity to TMB compared to H2O2. Also, the higher affinity of AuNS@Ag–H–rGO can be attributed to the surface morphology of the AuNS@Ag providing a higher surface-to-volume ratio as compared to AuNP@Ag. Further, the rGO sheet acts as a supporting matrix for hemin and AuNS@Ag inhibiting their self-aggregation. Moreover, it confines them in a region which is of nanometric dimensions allowing for the efficient adsorption of the substrates and also improving the catalytic efficiency. The Vmax of the synthesized nanocomposites showed a 2.8 and 2.1 times increase compared to HRP with TMB as the substrate. As a result of the superior performance of AuNS@Ag–H–rGO, the morphology of AuNS@Ag was further optimized and catalytic performance was evaluated for each of the morphologies. The amount of silver coating on the AuNSs was varied to achieve maximum sensitivity. Fig. S9d† corroborated that AuNS@Ag with an amount of 5 μL of Ag (i.e. AuNS@Ag-5), showed highest catalytic activity. This could be attributed to the fact that with Ag growing mainly on the core, the branches of the Au nanostar remain accessible, hence providing a higher surface-to-volume ratio for bioconjugation of TMB.
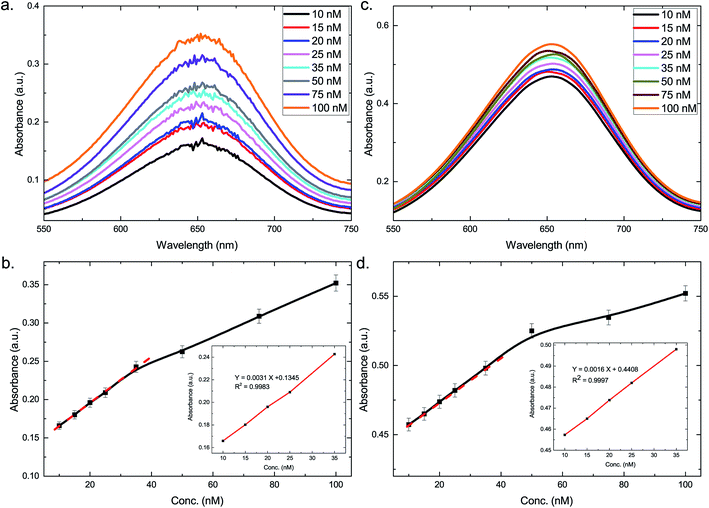
Henceforth, a colorimetric method was developed benefitting from the excellent catalytic activity of the synthesized nanocomposites. The response curves for a mixture of nanocomposites and TMB with varying concentrations of H2O2 were obtained (Fig. 5a and c). It was discerned that an increase in the H2O2 concentration led to an overall increase in the absorbance. Fig. 5b and d present the linear calibration plot for the varying concentrations of H2O2 at 652 nm in presence of AuNP@Ag–H–rGO and AuNS@Ag–H–rGO respectively. The correlation coefficients were calculated to be 0.9983 and 0.9997 representing a good linear behavior. The range of H2O2 varied linearly between 10 to 35 nM for both AuNP@Ag–H–rGO and AuNS@Ag–H–rGO, and the limit of detection were obtained as 3.096 nM and 1.26 nM, which are at least an order of magnitude lower than the detection limits reported hitherto (Table S1†).
Mechanism of AuNP/AuNS@Ag–H–rGO as peroxidase mimics
In order to explore a possible mechanism for the catalytic activity of AuNP/AuNS@Ag–H–rGO, an electrochemical study was carried out. Cyclic voltammetry plots were recorded to investigate the catalytic activity of the nanocomposites. Cyclic voltammograms of bare GCE electrode and AuNP/AuNS@Ag–H–rGO modified GCE electrodes in the presence of H2O2 are shown in Fig. S11.† The modified electrodes showed higher reduction current as compared to the bare GCE electrodes, suggesting promotion of electron transmission on their surface. An enhancement in the reduction current for the AuNS@Ag–H–rGO modified electrode is a characteristic of its higher electrocatalytic activity for H2O2 reduction. The high effective specific area of AuNS and the immobilization of hemin and AuNS@Ag on the rGO sheets, allows for an exposure of highly dense catalytically active centers. This may be conducive to an accelerated surface-confined electron transfer process occurring between the adsorbed TMB and H2O2 to efficiently carry out the oxidation of TMB.Practical implementation of the peroxidase mimetic for glucose detection
| Blood sample | OneTouch Verio Flex® glucose meter (mM) | Colorimetric method | Recovery (%) | ||
|---|---|---|---|---|---|
| Before spiking (mM) | Spiking concentration (μM) | After spiking (mM) | |||
| 1 | 3.81 | 3.64 | 20 | 3.59 | 98.08 |
| 2 | 5.47 | 5.78 | 40 | 5.90 | 101.3 |
| 3 | 6.95 | 7.01 | 80 | 7.02 | 99.01 |
Conclusions
In summary, we report a simple method for the synthesis of a synergistic AuNS@Ag–H–rGO nanocomposite that possesses excellent peroxidase-like activity. The novel nanomimetic exhibits several advantages over HRP such as, high stability, facile synthesis, higher catalytic efficiency, size/shape/composition dependent activity and lower cost. Furthermore, we demonstrate a simple and inexpensive colorimetric method for the detection of hydrogen peroxide and glucose with nanomolar sensitivity. The nanocomposite has also been used to develop a point-of-care diagnostic platform for detection of glucose in human blood serum. The synthesized nanocomposite has immense potential to be utilized extensively in the field of bionanotechnology and medical diagnostics.Conflict of interest
The authors declare no competing financial interest.Notes and references
- D. L. Nelson, A. L. Lehninger and M. M. Cox, Lehninger principles of biochemistry, Macmillan, 2008 Search PubMed.
- L. Fruk and C. M. Niemeyer, Angew. Chem., Int. Ed., 2005, 44, 2603–2606 CrossRef CAS PubMed.
- G. Zhang and P. K. Dasgupta, Anal. Chem., 1992, 64, 517–522 CrossRef CAS.
- T. Xue, S. Jiang, Y. Qu, Q. Su, R. Cheng, S. Dubin, C. Y. Chiu, R. Kaner, Y. Huang and X. Duan, Angew. Chem., 2012, 124, 3888–3891 CrossRef.
- Z. Liu, R. Cai, L. Mao, H. Huang and W. Ma, Analyst, 1999, 124, 173–176 RSC.
- R. P. Bonar-Law and J. K. Sanders, J. Am. Chem. Soc., 1995, 117, 259–271 CrossRef CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- F. Qu, T. Li and M. Yang, Biosens. Bioelectron., 2011, 26, 3927–3931 CrossRef CAS PubMed.
- H. Jiang, Z. Chen, H. Cao and Y. Huang, Analyst, 2012, 137, 5560–5564 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang and S. Perrett, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- Y. Jv, B. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- K. Yoshizumi, K. Aoki, I. Nouchi, T. Okita, T. Kobayashi, S. K. Amakura and M. Tajima, Atmos. Environ., 1984, 18, 395–401 CrossRef CAS.
- T. C. Bruice, Acc. Chem. Res., 1991, 24, 243–249 CrossRef CAS.
- F. Bedioui, Coord. Chem. Rev., 1995, 144, 39–68 CrossRef CAS.
- L. Shao, A. S. Susha, L. S. Cheung, T. K. Sau, A. L. Rogach and J. Wang, Langmuir, 2012, 28, 8979–8984 CrossRef CAS PubMed.
- H. Wei, S. M. H. Abtahi and P. J. Vikesland, Environ. Sci.: Nano, 2015, 2, 120–135 RSC.
- H.-H. Deng, G.-L. Hong, F.-L. Lin, A.-L. Liu, X.-H. Xia and W. Chen, Anal. Chim. Acta, 2016, 915, 74–80 CrossRef CAS PubMed.
- W. Luo, C. Zhu, S. Su, D. Li, Y. He, Q. Huang and C. Fan, ACS Nano, 2010, 4, 7451–7458 CrossRef CAS PubMed.
- L. Zhang and L. Li, Anal. Methods, 2016, 8, 6691–6695 RSC.
- X. Zhang, M. Wei, B. Lv, Y. Liu, X. Liu and W. Wei, RSC Adv., 2016, 6, 35001–35007 RSC.
- L.-L. Wu, L.-Y. Wang, Z.-J. Xie, F. Xue and C.-F. Peng, RSC Adv., 2016, 6, 75384–75389 RSC.
- T. Vo-Dinh, TrAC, Trends Anal. Chem., 1998, 17, 557–582 CrossRef CAS.
- L. Lu, K. Ai and Y. Ozaki, Langmuir, 2008, 24, 1058–1063 CrossRef CAS PubMed.
- A. M. Fales, H. Yuan and T. Vo-Dinh, J. Phys. Chem. C, 2014, 118, 3708–3715 CAS.
- J.-S. Lee, A. K. Lytton-Jean, S. J. Hurst and C. A. Mirkin, Nano Lett., 2007, 7, 2112 CrossRef CAS PubMed.
- X. Wang, Z. Zhang and G. V. Hartland, J. Phys. Chem. B, 2005, 109, 20324–20330 CrossRef CAS PubMed.
- L. Song, C. Huang, W. Zhang, M. Ma, Z. Chen, N. Gu and Y. Zhang, Colloids Surf., A, 2016, 506, 747–755 CrossRef CAS.
- B. Garg, T. Bisht and Y.-C. Ling, Molecules, 2015, 20, 14155–14190 CrossRef CAS PubMed.
- A. D. Association, Diabetes Care, 2014, 37, S81–S90 CrossRef PubMed.
- T. N. Seyfried, R. E. Flores, A. M. Poff and D. P. D'Agostino, Carcinogenesis, 2014, 35, 515–527 CrossRef CAS PubMed.
- K. Yang, L. Feng, H. Hong, W. Cai and Z. Liu, Nat. Protoc., 2013, 8, 2392–2403 CrossRef CAS PubMed.
- X. Jiang, Y. Chai, H. Wang and R. Yuan, Biosens. Bioelectron., 2014, 54, 20–26 CrossRef CAS PubMed.
- N. G. Bastús, J. Comenge and V. Puntes, Langmuir, 2011, 27, 11098–11105 CrossRef PubMed.
- X. Lv and J. Weng, Sci. Rep., 2013, 3, 3285 CrossRef PubMed.
- M. Tsuji, M. Matsunaga, H. Kumagai, M. Ogino, S. Hikino, Y. Yoshida and T. Ishizaki, CrystEngComm, 2013, 15, 1345–1351 RSC.
- L. A. Marquez and H. B. Dunford, Biochemistry, 1997, 36, 9349–9355 CrossRef CAS PubMed.
- D. Porter and H. Bright, J. Biol. Chem., 1983, 258, 9913–9924 CAS.
- N. Lu, M. Zhang, L. Ding, J. Zheng, C. Zeng, Y. Wen, G. Liu, A. Aldalbahi, J. Shi and S. Song, Nanoscale, 2017, 9, 4508–4515 RSC.
- C. Lu, X. Liu, Y. Li, F. Yu, L. Tang, Y. Hu and Y. Ying, ACS Appl. Mater. Interfaces, 2015, 7, 15395–15402 CAS.
- A. Dalui, B. Pradhan, U. Thupakula, A. H. Khan, G. S. Kumar, T. Ghosh, B. Satpati and S. Acharya, Nanoscale, 2015, 7, 9062–9074 RSC.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2009, 82, 3–10 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06973a |
| This journal is © The Royal Society of Chemistry 2017 |