Open Access Article
Open Access ArticleFabrication of mechanically robust superhydrophobic steel surface with corrosion resistance property†
Peng
Wang
 *,
Tao
Yao
,
Bo
Sun
,
Tiejun
Ci
,
Xiaoliang
Fan
and
Huilong
Han
*,
Tao
Yao
,
Bo
Sun
,
Tiejun
Ci
,
Xiaoliang
Fan
and
Huilong
Han
School of Energy, Power and Mechanical Engineering, North China Electric Power University, Baoding, 071000, China. E-mail: wang.peng.ncepu@foxmail.com
First published on 14th August 2017
Abstract
In this research, a simple and cheap method was presented to make steel superhydrophobic. A mixture of antiformin solution and hydrogen peroxide was utilized to grow a porous structure on steel foil; the antiformin solution is much cheaper and more environmentally friendly than the use of strong acids. More interestingly, lotus-leaf-like hierarchical micro-nanostructures were formed after ultrasonic treatment. The superhydrophobic surface can be further prepared by surface modification, which could withstand sandpaper abrasion for 2.24 m under a pressure of 24.50 kPa without losing superhydrophobicity. Moreover, the as-prepared superhydrophobic surface exhibits excellent self-cleaning and anti-corrosion properties.
Introduction
As is well known, steel has achieved extensive industrial applications due to its easy accessibility, cost efficiency, high fatigue strength and excellent machinability. Nevertheless, steel is easily oxidized and highly susceptible to corrosion in damp environments. It has been founded that the corrosion of steel is partly due to the contact with air and moisture.1,2 One possible solution is to make the steel surface superhydrophobic, which not only provides corrosion resistance property but also adds self-cleaning function to the surface.In nature, water droplets exhibit a nearly spherical shape on the surface of lotus leaves and immediately roll off because of low adhesion. The rolling motion carries away dust and contaminants, which results in a self-cleaning property. Inspired by this phenomenon, superhydrophobic surfaces with large contact angles (>150°) and small sliding angles (<10°) have attracted a large amount of attention because of their potential applications in self-cleaning,3,4 oil/water separation,5–8 anti-icing,9,10 anti-corrosion,11 and so forth. Among these applications, to improve the anti-corrosion performance on metal surface is an important research direction. Many methods have been introduced to prepare superhydrophobic metal surfaces, such as electrodepositing film,12,13 nanocasting technique,14 coating SiO2 or TiO2 nanoparticle,15,16 and chemical etching.17,18 Because of the simplicity, cost efficiency, and suitable for industrial applications, chemical etching is thought to be an effective method for preparing large-area superhydrophobic steel surfaces.19–21 Wang et al. prepared a superhydrophobic surface on steel through the combined etching of H2O2 and HCl/HNO3.22 Li et al. fabricated a superhydrophobic steel surface by HF/H2O2 etching.23 Nevertheless, preparing a superhydrophobic surface on steel using antiformin solution is still scarcely reported.
A crucial factor hindering the large scale application of superhydrophobic steel surfaces is their weak mechanical abrasion resistance. Although a large amount of superhydrophobic surfaces have been introduced, most of them are prone to be destroyed after a slight scratch, rubbing, and even finger contact. The reason may be that mechanical damage on the superhydrophobic surfaces tends to destroy the fragile micro-nano hierarchical structures. Recently, some researchers began to evaluate the mechanical durability.22–26 She et al. presented a robust superhydrophobic surface on magnesium alloy substrate, and the prepared surface showed a maximum abrasion distance of 0.70 m under a 1.2 kPa pressure.25 Wang et al. prepared a robust superhydrophobic steel surface by H2O2 and HCl/HNO3 etching, which could not endure the abrasion distance over 1.10 m under a 16 kPa pressure.22 To the best of our knowledge, few studies about robust superhydrophobic steel surfaces under large pressure (24.5 kPa) have been reported in the literature.
In this research, antiformin solution is introduced to prepare robust superhydrophobic steel surfaces. The fabrication process is composed from three steps: chemical immersion through the combination of antiformin and H2O2, ultrasonic treatment and surface modification. The present approach could be easily applied for large scale production, as the procedure is performed in an economical aqueous solution. In addition, the as-prepared surface exhibited superior anti-corrosion, anti-abrasion and self-cleaning properties, which get rid of the major neckbottle for its practical application.
Experimental
Materials
1045 steel (composition: C, 0.42–0.50; Si, 0.17–0.37; Mn, 0.50–0.80) with the size of 20 mm × 20 mm × 1 mm was used as the substrates. Ethanol (AR), acetone (AR), hexane (AR), H2O2 (30 wt% in water), antiformin (CP, free alkali 7.0–8.0%, active chlorine ≥ 5.2%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. 1H,1H,2H,2H-perfluorooctyltriethoxysilane (C8F13H4Si(OCH2CH3)3, FAS) were purchased from Aladdin Reagent Co., Ltd, Shanghai, China. Polydimethylsiloxane (PDMS) is a two part crosslinkable resin (Sylgard®184, Dow Corning Co., Michigan, USA).The preparation of micro nano roughness
Before using, steel substrates were ultrasonically cleaned in acetone, ethanol and deionized water, respectively, then dried in air for 30 min. The etching solution was formed by adding 12.50 ml antiformin solution into 10.00 ml H2O2 solution. Then, the cleaned steel substrate was immersed gently into the etching solution for 4 h. After reaction, the substrate was rinsed with deionized water, and then dried in air.Ultrasonic treatment
The steel substrate was immersed in deionized water, and then ultrasonically cleaned by a 100 W ultrasonic cleaner for 3 min. The ultrasonic cleaned steel was further dried in air.Surface modification
First, the aforementioned sample was immersed in an ethanol solution which contains 2.0 wt% FAS for 4 h. Next, the sample was immersed in PDMS solution including 0.5 g PDMS, 0.1 g curing agent and 9.5 g hexane. After 3 min, the sample was taken out, and cured at 80 °C for 2 h.Characterization
The surface morphologies of as-prepared samples were observed with a scanning electron microscope (SEM, Hitachi S3400N). Prior to SEM measurements, a thin Au layer (ca. 5 nm) was deposited on the specimens by sputtering. The water contact angles (CAs) and sliding angles (SAs) were measured with a 5 µl droplet of deionized water at ambient temperature on a contact angle measurement instrument (JC2000D, China). The average water CA and SA values were obtained by measuring the same sample at five different positions. The polarization curves were measured in 3.5 wt% NaCl aqueous solution through a CHI600D electrochemical workstation (Shanghai CH instruments). A three-electrode system was adopted. The sample and a platinum electrode were employed as the working and counter electrodes, respectively. A saturated calomel electrode (SCE) was used as reference electrode. The scanning rate of polarization curves was 0.5 mV s−1.Results and discussion
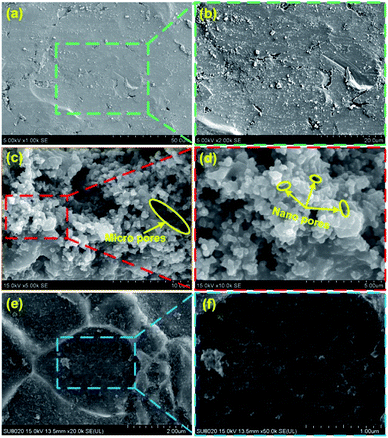
The surface morphology plays a key role in the preparation of superhydrophobic surfaces. Fig. 1a and b show the SEM images of surface morphologies of untreated steel at low and high magnifications, respectively. Most areas of the surface were smooth, and a small amount of defects exist. Fig. S1† shows the typical surface morphology of the steel substrates etched for different time intervals. After 4 h of etching in the mixture of NaClO and H2O2 solution, the steel surface showed a porous structure. As shown in Fig. 1c and d, the pore size ranged from nanoscale to microscale. We attributed this to the existence of H2O2, which generated O2 in the etching process. More interesting, ultrasonic treatment was adopted as a complementary texturing mechanism which can improve the surface's mechanical stability rather than degrade it. After ultrasonic treatment, micro sized cavities were presented on the surface as shown in Fig. 1e. Furthermore, nano sized features were created on the micro sized cavities as shown in Fig. 1f. This hierarchical morphology extensively mimicks the lotus leaf surface, where the rough microscale structure is covered by a nanoscale structure.Chemical composition is another key factor to determine the superhydrophobicity of the surface. After ultrasonic treatment, the steel surface was further chemical modified to create superhydrophobicity. The EDS spectrum of modified steel surface is displayed in Fig. 2. From Fig. 2b, it is found that the Fe element exhibited a uniform distribution. The presence of fluorine element (Fig. 2c) confirmed the successful grafting of the FAS molecules. Moreover, the F element showed a heterogeneous distribution, which is basically in coordination with the bright area shown in the SEM image. The O element also exhibited a heterogeneous distribution. Through the comparison of Fig. 2c and d, it is found that the distribution of O and F showed a consistency. Then it is hard to detect F element in the regions without O element. We attribute this to the –OH groups which existed on the oxidized surface, which is found to be the prerequisite for surface grafting of silane.22 Therefore, it is reasonable to deduce that the surface modification of FAS could only be performed at oxidized region, which is similar with other researches.27–30
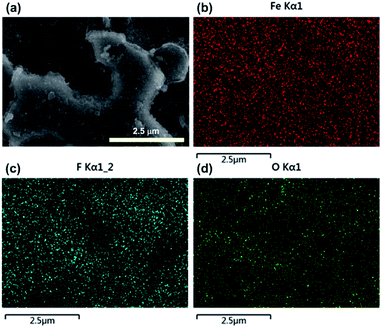 | ||
| Fig. 2 EDS mapping images of Fe (b), F (c), and O (d) elements from the superhydrophobic surface (a). | ||
The formation mechanism of the as-prepared superhydrophobic surface can be further elaborated in Fig. 3. As known, both NaClO and H2O2 show strong oxidizing behaviour in the reacting solution, which means that Fe could be oxidized into Fe2O3. Due to the existence of defect and crystallinity in the steel surface, the oxidizing process is not uniform and micro-cavity structure tended to be formed. Moreover, a large amount of bubbles would generate in the etching process. We attribute this phenomenon to the existence of H2O2, which could generate O2 gas in the oxidizing process. The generation of bubbles further led to the formation of porous structure in the steel surface (Fig. 3b). After ultrasonic treatment, the fragile structure was removed. Then the steel surface was changed to lotus-leaf-like hierarchical micro-nanostructures (Fig. 3c). After the surface treatment of FAS and PDMS, a layer with low surface energy covered the hierarchical micro-nanostructures (Fig. 3d). In this research, the superhydrophobicity of the as-prepared surface derives from its rough surface with lotus-leaf-like hierarchical micro-nanostructures and the presence of low-surface-energy layer on it. Fig. 4 shows the wetting state for water droplets with different diameters on the as-prepared superhydrophobic surface, while all droplets keep nearly spherical shapes. According to the contact angle measurement, this kind of superhydrophobic coating has a high water contact angle of 163 ± 2° (Fig. 4). In addition, the water droplets can easily roll down the sample surface when the sliding angle is 6°, indicating a low contact angle hysteresis.31,32
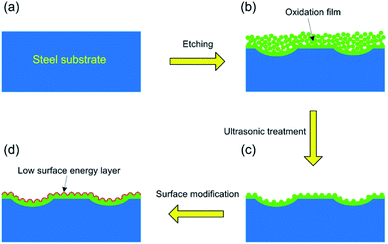 | ||
| Fig. 3 The formation mechanism of the as-prepared superhydrophobic surface with hierarchical structure. | ||
 | ||
| Fig. 4 The formation mechanism of the as-prepared superhydrophobic surface with hierarchical structure. | ||
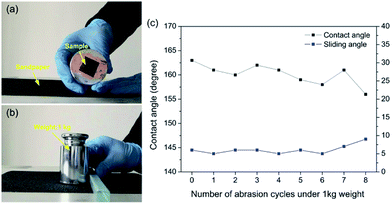
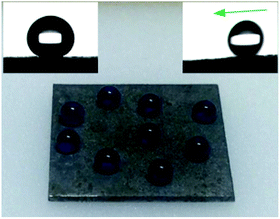
The mechanical durability of superhydrophobic surfaces is a crucial factor which limits the widespread applications. Nevertheless, superhydrophobic surfaces with special micro/nano structures are usually mechanically weak and easily destroyed.32,33 To solve this problem, lotus-leaf-like hierarchical micro-nanostructures were prepared on the steel surface. Using this structure, robust microscale bumps can provide protection to a more fragile nanoscale roughness.34 In this research, the sandpaper abrasion test was performed to systematically study the mechanically stability of the as-prepared superhydrophobic steel surface. As shown in Fig. 5a, the superhydrophobic steel (2 cm × 2 cm) was attached to a 1 kg weight (a pressure of 24.5 kPa) with the help of commercial adhesive. Then, the sample facing down sandpaper (grit no. 150) was moved for 28 cm along the ruler by an external drawing force (Fig. 5b and Movie S1†). The water contact angles and sliding angles were measured after each abrasion test cycle. Fig. 5c exhibits the change in contact angles and sliding angles as a function of the number of abrasion cycles. It can be found that the water contact angles were between 150° and 165°, and sliding angles varied between 5° and 9° through the abrasion cycles. When the abrasion cycle was further increased to 9, some bad points could be found in Movie S1† where water droplets could not slip away. Therefore, the as-prepared steel surface retained superhydrophobicity after 9 sandpaper abrasion cycles (2.24 m) under a pressure of 24.5 kPa. It should be noted that the results displayed in this research showed better mechanical durability than other reported superhydrophobic metal surfaces,22–26 as shown in Table 1.
| Substrate | Method | Anti-abrasion test | Reference | ||
|---|---|---|---|---|---|
| Roughness | Surface modification | Load | Distance | ||
| Mg | Electrodopositing Ni | Stearic acid | 1.2 kPa | 0.70 m | 25 |
| Cu | Electrodepositing Ni | FAS | 4.8 kPa | 1.00 m | 26 |
| Si | Two step etching | FAS | 3.45 kPa | 2.00 m | 24 |
| Steel | HF and H2O2 etching | Stearic acid | 500 g | 1.00 m | 23 |
| Steel | HCl/HNO3 and H2O2 etching | FAS | 500 g (16 kPa) | 1.10 m | 22 |
| Steel | NaClO and H2O2 etching | FAS | 1 kg (24.5 kPa) | 2.24 m | This work |
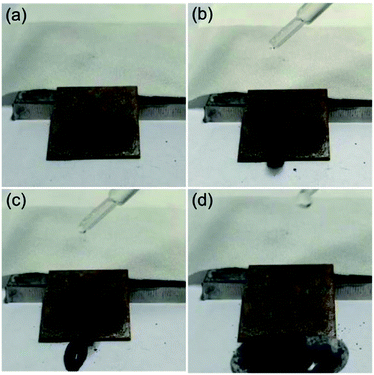
The self-cleaning ability is a crucial character of superhydrophobic surfaces for practical application. A self-cleaning test for the as-prepared superhydrophobic surface was carried out (Fig. 6 and Movie S2, ESI†). Here, the carbon black powder was utilized as characteristic dust particles.35 As shown in Fig. 6a, the dust particles were spreaded onto the superhydrophobic steel surface (Fig. 6b). When a water droplet was dripped on the sample, they can smoothly roll down the surface and take away the dust at the same time (Fig. 6b and c). After water pouring processes, water droplets carried away the dust particles completely and left a clean surface (Fig. 6d). Therefore, the as-prepared steel superhydrophobic surface exhibited excellent self-cleaning property.
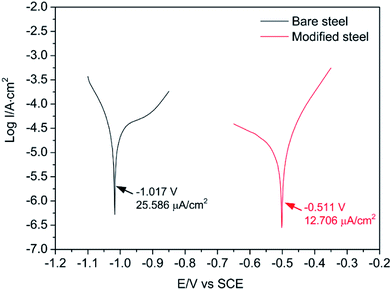
The polarization curves are useful methods to explore the impact of the superhydrophobic surfaces on the corrosion resistance of the steel substrate. Fig. 7 shows the polarization curves of bare steel substrate and modified superhydrophobic steel surface. Parameters such as corrosion potential (Ecorr) and corrosion current (Icorr) can be obtained using the Tafel extrapolation. As shown in Fig. 7, the anti-corrosion ability was found to be improved on the superhydrophobic surface due to the lower Icorr (12.706 µm cm−2) and higher Ecorr (−0.511 V) as compared to those of bare substrate (Icorr = 25.586 µm cm−2, Ecorr = −1.017 V), suggesting a good corrosion protection for the steel substrate. This result is consistent with previously published reports that superhydrophobic surfaces exhibit excellent anti-corrosion ability.36–38 When the superhydrophobic surfaces are immersed in a corrosive solution, air tends to be trapped in the lotus-leaf-like hierarchical micro-nanostructure of the as-prepared superhydrophobic surface, and the trapped air behaves as a dielectric for a pure parallel plate capacitor and prevents the electron transfer between the electrolyte and the steel substrate.38
Conclusions
In summary, mechanically robust superhydrophobic surface was fabricated on steel by a simple and low cost three-step method. Immersing steel into the mixture of antiformin and hydrogen peroxide was used to create porous structure. More interesting, lotus-leaf-like hierarchical micro-nanostructures were obtained after subsequent ultrasonic treatment. The superhydrophobic surface was further prepared by surface modification. Particularly, this superhydrophobic surface could withstand the sandpaper abrasion for 2.24 m under a pressure of 24.50 kPa. Moreover, this superhydrophobic surface shows distinguishing self-cleaning and anti-corrosion properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Beijing Natural Science Foundation (3174058), National Nature Science Foundation of China (51607067), the Natural Science Foundation of Hebei Province (No. E2015502023), and the Fundamental Research Funds for the Central Universities (2017MS149).Notes and references
- Y. Lu, S. Sathasivam, J. Song, F. Chen, W. Xu, C. J. Carmalt and I. P. Parkin, J. Mater. Chem. A, 2014, 2, 11628 CAS.
- K. Liu and L. Jiang, Annu. Rev. Mater. Res., 2012, 42, 231 CrossRef CAS.
- S. Nishimoto and B. Bhushan, RSC Adv., 2013, 3, 671 RSC.
- D. Li and Z. Guo, RSC Adv., 2017, 7, 9169 RSC.
- S. Taleb, T. Darmanin and F. Guittard, RSC Adv., 2014, 4, 3550 RSC.
- C. R. Reshmi, S. P. Sundaran, A. Juraij and S. Athiyanathil, RSC Adv., 2017, 7, 2092 RSC.
- J. Li, R. Kang, X. Tang, H. She, Y. Yang and F. Zha, Nanoscale, 2016, 8, 7638 RSC.
- J. Li, L. Yan, X. Tang, H. Feng, D. Hu and F. Zha, Adv. Mater. Interfaces, 2016, 3, 1500770 CrossRef.
- Y. Shen, J. Tao, H. Tao, S. Chen, L. Pan and T. Wang, RSC Adv., 2015, 5, 32813 RSC.
- T. Moriya, K. Manabe, M. Tenjimbayashi, K. Suwabe, H. Tsuchiya, T. Matsubayashi, W. Navarrini and S. Shiratori, RSC Adv., 2016, 6, 92197 RSC.
- J. Li, R. Wu, Z. Jing, L. Yan, F. Zha and Z. Lei, Langmuir, 2015, 31, 10702 CrossRef CAS PubMed.
- S. Khorsanda, K. Raeissi, F. Ashrafizadeh, M. A. Arenas and A. Conde, Appl. Surf. Sci., 2016, 364, 349 CrossRef.
- A. V. Rao, S. S. Latthe, S. A. Mahadik and C. Kappenstein, Appl. Surf. Sci., 2011, 257, 5772 CrossRef CAS.
- C. Peng, K. Chang, C. Weng, M. Lai, C. Hsu, S. Hsu, Y. Hsu, W. Hung, Y. Wei and J. Yeh, Electrochim. Acta, 2013, 95, 192 CrossRef CAS.
- S. A. Mahadik, F. Pedraza and R. S. Vhatkar, J. Alloys Compd., 2016, 663, 487 CrossRef CAS.
- T. Isimjan, T. T. Wang and S. Rohani, Chem. Eng. J., 2012, 210, 182 CrossRef CAS.
- C. Chen, S. Yang, L. Liu, H. Xie, H. Liu, L. Zhu and X. Xu, J. Alloys Compd., 2017, 711, 506 CrossRef CAS.
- S. R. Yu, J. A. Liu, W. Diao and W. Li, J. Alloys Compd., 2014, 585, 689 CrossRef CAS.
- L. Liu, W. Liu, R. Chen, X. Li and X. Xie, Chem. Eng. J., 2015, 281, 804 CrossRef CAS.
- L. Liu, X. Feng and M. Guo, J. Phys. Chem. C, 2013, 117, 25519 CAS.
- L. Liu, F. Xu and L. Ma, J. Phys. Chem. C, 2012, 116, 18722 CAS.
- N. Wang, D. Xiong, Y. Deng, Y. Shi and K. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6260 CAS.
- H. Zhang, J. Yang, B. Chen, C. Liu, M. Zhang and C. Li, Appl. Surf. Sci., 2015, 359, 905 CrossRef CAS.
- Y. Xiu, Y. Liu, D. W. Hess and C. Wong, Nanotechnology, 2010, 21, 155705 CrossRef PubMed.
- Z. She, Q. Li, Z. Wang, L. Li, F. Chen and J. Zhou, Chem. Eng. J., 2013, 228, 415 CrossRef CAS.
- F. Su and K. Yao, ACS Appl. Mater. Interfaces, 2014, 6, 8762 CAS.
- Z. Guo, F. Zhou, J. Hao and W. Liu, J. Am. Chem. Soc., 2005, 127, 15670 CrossRef CAS PubMed.
- N. Saleema, S. Sarkar, R. Paynter and X. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 2500 CAS.
- A. Hozumi, B. Kim and T. J. McCarthy, Langmuir, 2009, 25, 6834 CrossRef CAS PubMed.
- J. Li, Z. Jing, Y. Yang, F. Zha, L. Yan and Z. Lei, Appl. Surf. Sci., 2014, 289, 1 CrossRef CAS.
- P. Wang, M. Chen, H. Han, X. Fan, Q. Liu and J. Wang, J. Mater. Chem. A, 2016, 4, 7869 CAS.
- X. Tian, T. Verho and R. H. A. Ras, Science, 2016, 352, 142 CrossRef CAS PubMed.
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132 CrossRef CAS PubMed.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2011, 23, 673 CrossRef CAS PubMed.
- P. Wang, T. Yao, B. Sun, X. Fan, S. Dong, Y. Bai and Y. Shi, Colloids Surf., A, 2017, 513, 396 CrossRef CAS.
- P. Wang, H. Han, J. Li, X. Fan, H. Ding and J. Wang, Appl. Phys. A, 2016, 122, 53 CrossRef.
- M. Liang, Y. Wei, L. Hou, H. Wang, Y. Lia and C. Guo, J. Alloys Compd., 2016, 656, 311 CrossRef CAS.
- Y. Qing, C. Yang, C. Hu, Y. Zheng and C. Liu, Appl. Surf. Sci., 2015, 326, 48 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06836k |
| This journal is © The Royal Society of Chemistry 2017 |