Open Access Article
Open Access ArticleImpeding phonon transport through superlattices of organic–inorganic halide perovskites
Rahul Singh and
Ganesh Balasubramanian *
*
Department of Mechanical Engineering, Iowa State University, 2092 Black Engineering, Ames, IA 50011, USA. E-mail: bganesh@iastate.edu
First published on 25th July 2017
Abstract
Superlattice structures present a strategy to impede lattice thermal transport through organic–inorganic halide perovskites and improve their potential for thermoelectric applications. We investigate the phonon characteristics of such novel configurations and compare against predictions of the simple perovskite lattices using first principle calculations. Our results show the existence of structural instabilities due to distortions in the octahedral cage surrounding the methylammonium ion. In the superlattices, a strong phonon incoherence reduces the group velocities while interfacial resistance enhances scattering and limits the phonons impeding heat conduction. Although heat transfer is anisotropic in these perovskites, the interfaces in the superlattice obstruct phonon transport along all directions.
Introduction
Organic–inorganic halide perovskites have received significant attention over the past half a decade because of their excellent photovoltaic conversion efficiencies.1–7 One of the building blocks of these hybrid chemistries is the central cation. Amongst the perovskites, methylammonium lead iodide (MAPbI3), where MA representing the CH3NH3+ cation is a molecular ion, has demonstrated a very high potential for solar cells as well thermoelectric applications.8–14 Reducing the thermal conductivity (κ), especially that due to lattice vibrations (phonons), with minimal or no deterioration to electrical properties is considered the key to improving the thermoelectric figure of merit. Introducing interfaces through superlattice structural forms for increased interfacial thermal resistance is a strategy to decrease κ.15–18 The decrease in κ is achieved with the destruction of the lattice periodicity, as described previously in numerous studies on different types of superlattices such as those of Si/Ge17,19–21 and Bi2Te3/Sb2Te3.22–24 These efforts have shown considerable decrease in κ relative to the parent materials with simple lattices. In superlattices, thermal transport is predominantly governed by the scattering of long and short wavelength phonons, and hence the thermal conductivity can be controlled by both the period and the total thickness of the structures.25,26 Therefore, thermal transport can be potentially modified by manipulating these two characteristic geometric properties of superlattice structures.Here, we employ the same concept of introducing interfaces by creating superlattice structures of organic–inorganic halide perovskites. Materials with perovskite type lattices assume complex structural landscapes because the atoms arrange accordingly for the ABX3 representation, where the cation A is at the center of BX6 octahedra located at the corners of the cubic lattice. Hybrid organic–inorganic perovskites have a very high Seebeck coefficient and low thermal conductivity,27–29 the latter being attributed to a number of factors. Some studies have suggested the cause to be the rotational motion of the organic cation,29 due to the inorganic atoms30 as well as by the coupling between the organic and inorganic parts of the molecule.29 Here, we show that the thermal transport is further reduced in heterogeneous structures (superlattices) of hybrid organic–inorganic halide perovskites. We have already proved that electrical conductivity and Seebeck coefficient persists with values in superlattice structures similar to those determined for simple lattice configurations.31 Hence, the objective of this paper is to understand the phonon transport mechanisms in the superlattices and suggest opportunities to engineer these materials for energy applications. The limited literature only provides κ predictions of MAPbI3 and suggests of the transitional states due to instabilities arising from certain soft modes.27–30,32–38 We investigate the phonon dynamics through dispersion curves, density of states (DOS) and group velocities, and predict the specific heats for a set of superlattice structures of MAPbI3 and methylammonium tin iodide (MASnI3) relative to the simple perovskite forms. The presence of interfaces decreases the phonon group velocities, creating localized regions of phonons that contribute to increased scattering and reduced κ.
Computational methods
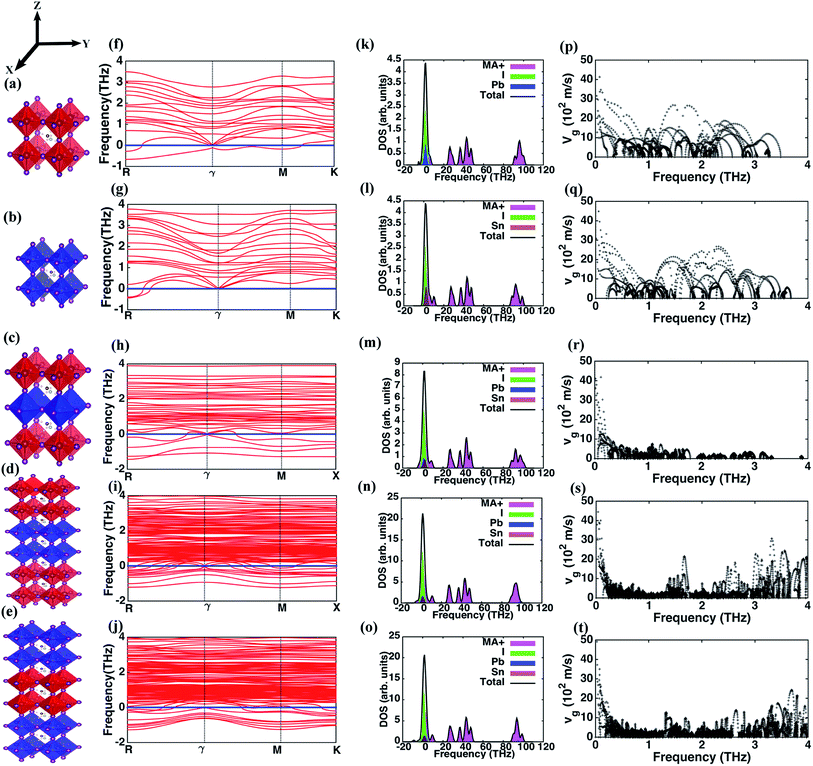
We employ density functional theory (DFT) calculations for optimizing the geometry of the perovskite and superlattice structures followed by computations of phonon properties. Self-consistent pseudo-potential plane wave approximation, as implemented in the Vienna Ab initio Simulation Package (VASP),39,40 is used. The exchange correlation functional is approximated using Perdew–Burke–Ernzerhof (PBE) Generalized Gradient Approximation (GGA).41 The five structures (I through V) investigated here are illustrated in Fig. 1(a–e). We choose pseudo-cubic phases for these structures while the MA molecules are arranged in 〈1 1 1〉 direction. We choose an 800 eV cut-off for the energy minimization with a plane wave basis set and consider a 4 × 4 × 4, 4 × 4 × 4, 5 × 5 × 2, 5 × 5 × 3 and 5 × 5 × 3 Monkhorst–Pack k-point mesh for the different structures, respectively. An optimization criterion for the computed force on each atom of less than 0.01 eV Å−1 is used in all the calculations. The details about the structure and the k mesh convergence tests are provided in Table 1. The experimental demonstration of the superlattices has been provided in our earlier work where scanning electron microscopy images and characterization of the energy dispersion validate the successful creation of these superlattice structures.31| Structure | Lattice constants | No. of atoms/unit cell | ||
|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||
| I | 6.432 | 6.515 | 6.445 | 12 |
| II | 6.302 | 6.428 | 6.303 | 12 |
| III | 6.401 | 6.514 | 12.809 | 24 |
| IV | 6.372 | 6.492 | 31.811 | 60 |
| V | 6.378 | 6.507 | 31.780 | 60 |
| Convergence tests for k-mesh | |||||
|---|---|---|---|---|---|
| Structure | k-Point mesh (reported) | k-Point mesh (denser) | Energy (eV) (reported k) | Energy (eV) (denser k) | % difference |
| I | 4 × 4 × 4 | 6 × 6 × 6 | −50.85 | −50.96 | 0.20 |
| II | 4 × 4 × 4 | 6 × 6 × 6 | −50.90 | −50.93 | 0.05 |
| III | 5 × 5 × 2 | 10 × 10 × 2 | −101.42 | −101.85 | 0.40 |
| IV | 5 × 5 × 3 | 10 × 10 × 4 | −254.40 | −254.60 | 0.07 |
| V | 5 × 5 × 3 | 10 × 10 × 4 | −254.40 | −254.60 | 0.07 |
Phonon dispersion is obtained from Phonopy42 within the harmonic approximation and with the force constants derived from DFT. The calculations are performed by the supercell approach within the framework of density functional perturbation theory43 and frozen cell method.44 The supercell chosen for our study is 2 × 2 × 2 for structures I, II, III and 1 × 1 × 1 for structures IV and V. In both cases, the second order derivative of total energy required to calculate the force constants is computed relative to atomic displacements. Using these force constants, phonon frequencies are obtained that subsequently provide us with the phonon dispersion and the DOS. Specific heat42 (C) is evaluated from 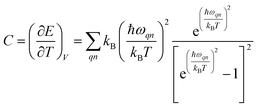 , where kB is the Boltzmann constant, n the band index, q the reciprocal lattice vector, ω the phonon frequency, T the temperature and E the total energy. Likewise, group velocity42
, where kB is the Boltzmann constant, n the band index, q the reciprocal lattice vector, ω the phonon frequency, T the temperature and E the total energy. Likewise, group velocity42 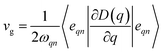 , where D is the dynamical matrix, and e the eigenvector at the band index n and reciprocal point q.
, where D is the dynamical matrix, and e the eigenvector at the band index n and reciprocal point q.
Results and discussion
The phonon dispersion curves for the different perovskites, presented in Fig. 1(f–j), show that all the structures have negative frequencies (soft modes) indicative of their respective transitional states. Comparable results for MAPbI3 have been reported elsewhere that show negative frequencies at the boundary k points and similar phonon dispersion curves.33,35 Perovskite structures assume different crystalline phases at different temperatures and minimal distortion in the structure induces a transitional state causing instability. MAPbI3 and MASnI3 exist in three structural phases at different temperatures – below 162 K MAPbI3 assumes the orthorhombic phase, transforms to the tetragonal phase at higher temperatures and transitions from tetragonal to cubic at 327 K.45 Similarly, MASnI3 undergoes three temperature dependent phase transitions – it is in a monoclinic form at low temperatures, changes to rhombohedral at 331 K and subsequently to cubic phase beyond 463 K.46,47 These instabilities are more pronounced in superlattice structures with greater number of negative frequencies noted in structures IV and V. The per-atom negative frequencies for structures I, II, III, IV and V at k-point R are 1/6, 1/6, 1/6, 1/5, and 1/4 respectively. Likewise, at ϒ point the per-atom negative frequencies observed are 0, 0, 1/12, 1/6 and 1/5. As different perovskite structures are introduced in the primitive cell, these instabilities increase because the various layers independently show dissimilar lattice dynamics behavior under the same thermodynamic conditions. The relative stability of these structures is analyzed based on the binding energies Eb, derived using the free energies of structures I and II, as| Eb = Estr − (nEI + mEII) |
Next, we investigate the phonon DOS for the different structures and present in Fig. 1(k–o) the contributions of different atoms in the lattice. The DOS for all the structures reveal the presence of three distinct sub-domains of high, intermediate and low frequencies. The high and intermediate frequencies are due to the phonons arising from the MA+ ion with a minor contribution of the Pb atom in structures IV and V, while the predominant low frequency contributions are from Pb–I and Sn–I pairs. As shown in Fig. 1(m–o), for structures III, IV and V the effect of Sn and Pb atoms are identical. We predict that characteristic vibrational frequencies due to the metal atoms are similar and thus might permit energy transfer across the interfaces of dissimilar perovskites in the superlattice using two-phonon scattering processes. Hence, interfacial thermal resistance within the superlattice structure is weakly influenced by the characteristic phonon modes of the metal atoms in these hybrid perovskites. Fig. 1(k–o) also reveal the underlying causes driving the structural instability in these structures. Since the negative frequency phonons are predominantly due to MA+ ion and I, structural disorder arises in the MA+ ion and Pb/Sn–I sub-lattices. Atoms in these sub-lattices occupy more than one non-equivalent lattice site.48 While MAPbI3 and MASnI3 exhibit disorder in their structures with MA+ ion occupying non-equivalent positions in adjacent cages,48 the octahedral cage (here Pb/Sn–I sub-lattices) are known to show disorder even in other perovskites such as CaTiO3, SrTiO3 and BaTiO3.49 Additionally, the extra degree of freedom in the angular orientation of MA+ ion that occurs in organic–inorganic halide perovskites creates further hindrance to an optimal configuration for an overall stable structure with suitable positioning of the MA+ ion and Pb/Sn–I sub-lattices.
The lattice contribution to κ is directly proportional to the average group velocity of phonons. Fig. 1(p–t) present the variation of the group velocity with frequency for the five structures. The group velocities in this figure are reported for every q and n, where n is the band index, q is the reciprocal lattice vector. The phonons in the low frequency range of 0–4 THz that have non-zero group velocities predominantly contribute to heat conduction. The number density (ratio of the number of phonons at each frequency level to the number of atoms in the primitive cell of the structure) of such phonons with finite group velocities is highest in structures I and II relative to the superlattices. The phonon number density in the frequency range from 0–1 THz for group velocities greater than 500 m s−1 is 23, 27, 8, 5 and 7 phonons per unit cell for I, II, III, IV and V respectively. These phonons with low scattering rates and relatively longer mean free paths conduct thermal energy across extended distances in the perovskite lattice. There is a strong decrease in the number of these phonons in the superlattice structures. The presence of different layers in the superlattice imposes dissimilar behavior of the phonons across the adjacent layers, effectively reducing the number density. Thus, the total energy transport by phonons is reduced including the fraction of lattice vibrations with long mean free path, and this impedes heat conduction. So, the reduction in phonon number density is still observed even after considering that different metal toms (Pb and Sn) show similar frequencies as per the density of states (Fig. 1(k–o)). Therefore, we corroborate that phonon contribution to κ in superlattices is significantly diminished relative to that in the simple perovskite lattices represented by structures I and II. Similar predictions derived in the frequencies between 1 and 2 THz are 26, 24, 8, 3 and 4 phonons per unit cell and between 2 and 3 THz are 24, 25, 0, 2 and 3 phonons per unit cell for the five structures, I through V, respectively.
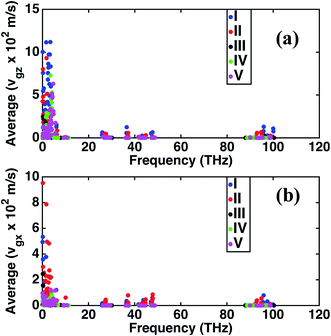
The frequency dependence of the mean in-plane (vgx) and cross-plane (vgz) group velocities are presented in Fig. 2. The average group velocities represent the mean over all the band indices calculated by dividing the sum of group velocities at each frequency with the number of bands. The superlattice structures have low group velocities over the entire frequency domain. Lattice κ is related to the group velocity as (κ = Cvg2τ), where τ is the average relaxation time and vg is the phonon group velocity averaged over all polarizations and directions. κ being directly proportional to the square of the group velocity, superlattice structures have poor heat conduction through them. Together with the interfacial thermal resistance, superlattices break the phonon coherence and reduce κ in a direction normal to the interacting surfaces between dissimilar perovskites. Fig. 2 shows that structure I has the highest vgz, while vgx is maximum for structure II. Structure III has the minimum vgz suggesting a strong phonon incoherence relative to the other structures. Previous experiments50 and theoretical modeling51 show that in superlattices heat conduction is diminished in both the in-plane and cross-plane directions. Our calculations reveal that group velocities along the in-plane direction are significantly lower than that perpendicular to the interfaces, especially in the low frequency range for the superlattice structures IV and V. The reduction in in-plane group velocities as observed in superlattices is due to the change in phonon spectrum with the introduction of interfaces. Fig. 1 shows the spectrum of structures 1 to 5 and the value of frequency at various k points. Especially the spectrum between ϒ → M → X is responsible for in-plane properties. The spectrum becomes flat in the superlattice structures at boundary k-points and results in the low group velocities. Among the structures considered in this study, structure IV is predicted to have the lowest group velocity and is hence predicted to have the lowest thermal conductance. On one hand, we predict that superlattices have reduced κ relative to structures I and II, on the other hand the distribution of the group velocities reveal a strong anisotropic feature of thermal transport for all the perovskites considered. Although interfaces are present normal to the longitudinal z-direction, group velocities and hence heat conduction along the transverse directions are also reduced in the superlattices relative to the simple perovskite structures.
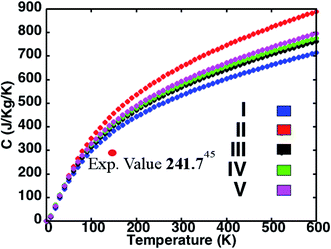
The variation of specific heat C with temperature for the five structures in presented in Fig. 3. While C of structure II is highest and that of structure I is lowest among the perovskites investigated, the predictions for the superlattices lie in between. In accord with the simple Debye model, we observe that at low temperatures C is proportional to T3. Also, as the density of a material is inversely proportional to the C and the density order of the investigated perovskite structures being I > IV > V > II, we find the order for the specific heat to be I < IV < V < II. Structures with an average lighter mass require relatively lesser amount of energy than those with heavier atoms for excitation at the lattice sites and facilitate heat conduction by phonons across the material. This amount of energy reflects in the C predictions for the different perovskites investigated here. While our predictions differ from some corresponding measurements in the literature,52 the results do compare well with recent experimental reports.53 For instance, the computed C of 301.62 J kg−1 K−1 compares well with the measured result of 241.94 J kg−1 K−1 at 150 K.
Conclusions
In summary, we investigate the lattice dynamics of superlattice structures of organic–inorganic halide perovskites and compare their phonon dispersion, density of states, group velocities and specific heat against the corresponding predictions for the simple lattice forms using first principle calculations. The phonon vibrational spectra of the superlattices reveals the presence of negative frequencies or soft modes indicating transitional states in the structural configurations. Through the partial density of states, we find that the I and MA+ ion predominantly contribute to these soft modes. Our results suggest that these perovskite lattices are sensitive to the distortions in the octahedral cage surrounding the MA+ ion and rotations of the MA+ ion, and the future need for methods beyond the harmonic approximation for an accurate treatment of phonons. We predict that a strong phonon incoherence causes significant reductions in the group velocities of the superlattice configurations, both parallel to and perpendicular to the interfaces, relative to the simple forms. Similar results noted for the number density of phonons implies that an enhanced scattering and interfacial resistance to energy transport by lattice vibrations impedes heat conduction and diminishes thermal conductivity. While the frequency dependence of mean group velocities ascertains the anisotropic heat transfer characteristics of these structures, the interfaces between dissimilar perovskites obstruct phonon transport in both the in-plane and cross-plane directions. The specific heat of the structures agrees with the Debye model at low temperatures as well as inverse dependence on the material density. Overall, our investigation highlights that superlattice perovskite structures reduce lattice thermal transport that could contribute towards employing organic–inorganic halide perovskites as potential thermoelectrics.Acknowledgements
We thank Prof. Vikram Dalal of the Microelectronics Research Center for his encouragement and the Catron Center for Solar Energy Research for the graduate fellowship. The research was partly supported by the National Science Foundation (NSF) grant no. CMMI-1404938 and the High Performance Computing HPC@ISU equipment at Iowa State University, some of which has been purchased through NSF funding under MRI grant no. CNS 1229081 and CRI grant no. 1205413.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 Search PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- H. A. Abbas, R. Kottokkaran, B. Ganapathy, M. Samiee, L. Zhang, A. Kitahara, M. Noack and V. L. Dalal, APL Mater., 2015, 3, 016105 CrossRef.
- L. Etgar, P. Gao, Z. Xue, Q. Peng, A. K. Chandiran, B. Liu, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2012, 134, 17396–17399 CrossRef CAS PubMed.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H. Kim, A. Sarkar, K. NazeeruddinMd, M. Gratzel and S. Il Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–320 CrossRef CAS PubMed.
- Q. Chen, N. De Marco, Y. Yang, T. Bin Song, C. C. Chen, H. Zhao, Z. Hong, H. Zhou and Y. Yang, Nano Today, 2015, 10, 355–396 CrossRef CAS.
- J. Bisquert, J. Phys. Chem. Lett., 2013, 4, 2597–2598 CrossRef CAS.
- N. G. Park, J. Phys. Chem. Lett., 2013, 4, 2423–2429 CrossRef CAS.
- NREL, Best Research-Cell Efficiencies, Retrieved from, https://www.nrel.gov/pv/assets/images/efficiency-chart.png.
- Y. He and G. Galli, Chem. Mater., 2014, 26, 5394–5400 CrossRef CAS.
- C. Lee, J. Hong, A. Stroppa, M.-H. Whangbo and J. H. Shim, RSC Adv., 2015, 5, 78701–78707 RSC.
- X. Mettan, R. Pisoni, P. Matus, A. Pisoni, J. Jacimovic, B. Náfrádi, M. Spina, D. Pavuna, L. Forró and E. Horváth, J. Phys. Chem. C, 2015, 3, 2488–2492 Search PubMed.
- J. Garg and G. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 1–5 CrossRef.
- L. Hu, G. Shao, T. Jiang, D. Li, X. Lv, H. Wang, X. Liu, H. Song, J. Tang and H. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 25113–25120 CAS.
- S.-M. Lee, D. G. Cahill and R. Venkatasubramanian, Appl. Phys. Lett., 1997, 70, 2957 CrossRef CAS.
- G. Balasubramanian and I. K. Puri, Appl. Phys. Lett., 2011, 99, 013116 CrossRef.
- I. Savic, D. Donadio, F. Gygi and G. Galli, Appl. Phys. Lett., 2013, 102, 073113 CrossRef.
- T. A. R. Adetic and R. O. G. Ronsky, Superlattices Microstruct., 2000, 28, 199–206 CrossRef.
- P. Ferrando-villalba, A. F. Lopeandía, F. X. Alvarez, B. Paul, C. De and J. Rodriguez-viejo, Nano Res., 2015, 8, 2833–2841 CrossRef CAS.
- M. Winkler, X. Liu, U. Schürmann, J. D. König, L. Kienle, W. Bensch and H. Böttner, Mater. Res. Soc. Symp. Proc., 2013, 1490, 205–222 CrossRef.
- F. He, W. Wu and Y. Wang, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 1–5 CrossRef CAS.
- M. N. Touzelbaev and P. Zhou, J. Appl. Phys., 2001, 90, 763 CrossRef CAS.
- Y. Chen, D. Li, J. R. Lukes, Z. Ni and M. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 174302, 1–6 Search PubMed.
- J. Jiang, J. Wang and B. Wang, Appl. Phys. Lett., 2011, 43109, 97–100 Search PubMed.
- S. Y. Yue, X. Zhang, G. Qin, J. Yang and M. Hu, Phys. Rev. B: Condens. Matter Mater. Phys., 2016, 94, 1–10 Search PubMed.
- X. Qian, X. Gu and R. Yang, Appl. Phys. Lett., 2016, 108, 063902 CrossRef.
- T. Hata, G. Giorgi and K. Yamashita, Nano Lett., 2016, 16, 2749–2753 CrossRef CAS PubMed.
- C. Caddeo, C. Melis, M. I. Saba, A. Filippetti, L. Colombo and A. Mattoni, Phys. Chem. Chem. Phys., 2016, 18, 24318–24324 RSC.
- R. Singh, R. Kottokkaran, V. L. Dalal and G. Balasubramanian, Nanoscale, 2017, 9, 8600–8607 RSC.
- A. Pisoni, J. Jaćimović, O. S. Barišić, M. Spina, R. Gaál, L. Forró and E. Horváth, J. Phys. Chem. Lett., 2014, 5, 2488–2492 CrossRef CAS PubMed.
- F. Brivio, J. M. Frost, J. M. Skelton, A. J. Jackson, O. J. Weber, M. T. Weller, A. R. Goni, A. M. A. Leguy, P. R. F. Barnes and A. Walsh, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 1–8 CrossRef.
- Y.-Y. Zhang, S. Chen, P. Xu, H. Xiang, X.-G. Gong, A. Walsh and S. Wei, Nat. Commun., 2015, 11 Search PubMed.
- M. Wang and S. Lin, Adv. Funct. Mater., 2016, 26, 5297–5306 CrossRef CAS.
- A. Mattoni, A. Filippetti, M. I. Saba, C. Caddeo and P. Delugas, J. Phys. Chem. Lett., 2016, 7, 529–535 CrossRef CAS PubMed.
- D. Vanderbilt and W. Zhong, Ferroelectrics, 1998, 206, 181–204 CrossRef.
- E. Cockayne and B. P. Burton, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 3743, 3735–3743 CrossRef.
- G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
- S. Baroni, S. De Gironcoli, A. Dal Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515–562 CrossRef CAS.
- R. P. Stoffel, C. Wessel, M. W. Lumey and R. Dronskowski, Angew. Chem., Int. Ed., 2010, 49, 5242–5266 CrossRef CAS PubMed.
- M. T. Weller, O. J. Weber, P. F. Henry, M. Di Pumpo and T. C. Hansen, Chem. Commun., 2015, 51, 4180–4183 RSC.
- I. Borriello, G. Cantele and D. Ninno, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 1–9 CrossRef.
- A. Poglitsch and D. Weber, J. Chem. Phys., 1987, 87, 6373 CrossRef CAS.
- T. Baikie, Y. Fang, J. M. Kadro, M. Schreyer, F. Wei, S. G. Mhaisalkar, M. Gratzel and T. J. White, J. Mater. Chem. A, 2013, 1, 5628 CAS.
- M. Johnsson and P. Lemmens, Crystallography and Chemistry of Perovskites, John Wiley & Sons, Ltd., New York, 2007, p. 11 Search PubMed.
- T. Yao, Appl. Phys. Lett., 1987, 51, 1798–1800 CrossRef CAS.
- G. Chen, J. Heat Transfer, 1997, 119, 220 CrossRef CAS.
- N. Onoda-Yamamuro, T. Matsuo and H. Suga, J. Phys. Chem. Solids, 1990, 51, 1383–1395 CrossRef CAS.
- D. H. Fabini, T. Hogan, H. A. Evans, C. C. Stoumpos, M. G. Kanatzidis and R. Seshadri, J. Phys. Chem. Lett., 2016, 7, 376–381 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |