Open Access Article
Open Access ArticleBimetallic copper and zinc-catalyzed oxidative cycloaddition of 3-aminopyridazines and nitriles: a direct synthesis of 1,2,4-triazolo[1,5-b]pyridazines via C–N and N–N bond-forming process†
Qiu-Chao Muab,
Ji-Yuan Lvb,
Mu-Yi Chenb,
Xing-Feng Baiab,
Jing Chen*a,
Chun-Gu Xiaa and
Li-Wen Xu *ab
*ab
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, University of the Chinese Academy of Sciences, P. R. China. E-mail: liwenxu@hznu.edu.cn
bKey Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou 311121, P. R. China. Fax: +86 2886 5135; Tel: +86 2886 5135
First published on 27th July 2017
Abstract
One-pot formation of the 1,2,4-triazolo[1,5-b]pyridazine nucleus and its derivatives is presented in this manuscript, in which the desired targets are offered easily via cooperative Cu(I) and Zn(II)-catalyzed tandem C–N addition and subsequent I2/KI-mediated intramolecular oxidative N–N bond formation.
The 1,2,4-triazolo[1,5-b]pyridazine scaffolds have been recognized an important structural motif in medicinal chemistry, especially showing excellent anti-asthmatic activity.1 In recent decades, a series of synthetic methods for the synthesis of 1,2,4-triazolo[1,5-b]pyridazine derivatives have been elaborated. To date, the preparation of 1,2,4-triazolo[1,5-b]pyridazines is typically involved multistep reaction sequences. For example, as shown in Scheme 1, the 3-aminopyridazine derivatives could be converted into N,N-dimethylaminomethylene derivatives by treatment with N,N-dimethylaminoformamide dimethyl acetal (DMFDMA), and then reacted with hydroxylamine hydrochloride to afford formamide oxime, which treated with polyphosphoric acid to give the fused 1,2,4-triazolo derivatives.1,2 In addition, the treatment of 3-aminopyridazine or 3-amino-6-chloropyridazine with O-mesitylenesulfonylhydroxylamine (MSH), and the subsequent cyclization with acylating agents, such as formic acid, acetic anhydride and benzoyl chloride, was also an effective procedure to the preparation of 1,2,4-triazolo[1,5-b]pyridazine derivatives.3 Although the 1,2,4-triazolo[1,5-b]pyridazine derivatives and its analogues could be obtained successfully with multistep reaction sequences in the past decades,4 some disadvantages existed in these classic methods, including high temperature, low total yields and highly toxic. Thus the development of one-pot novel methods to access the 1,2,4-triazole nucleus is highly desired.
Recently, one-pot formation of 1,2,4-triazoles nucleus and its analogues using copper-catalyzed tandem addition have also been presented.5 And notably, metal-free oxidative N–N bond formation to synthesize 1,2,4-triazoles skeleton was proved to an interesting method for the oxidative construction of heterocycles in the past years.6 In comparison to the previously reported multistep methods, these catalytic one-pot transformations provided a simple process for the synthesis of 1,2,4-triazoles. However, to the best of our knowledge, there is no reports on the copper-catalyzed oxidative cycloaddition of 3-aminopyridazines and nitrile for the preparation of 1,2,4-triazolo[1,5-b]pyridazine scaffolds. In addition, the development of synthetic methods for the construction of substituted of 1,2,4-triazoles and its derivatives has been of longstanding importance in medicinal chemistry.
Inspired by previous works on the triazoles,5–7 we envisioned a straightforward strategy for the synthesis of the 1,2,4-triazolo[1,5-b]pyridazine nucleus by one-pot oxidative cycloaddition reaction of 3-aminopyridazine derivatives and nitriles involving copper-catalyzed C–N bond formation and I2/KI-mediated oxidative N–N coupling. Herein we disclose a new and efficient methodology for the preparation of chlorine-containing 1,2,4-triazolo[1,5-b]pyridazine scaffolds that difficultly achieved by previous reported methods.
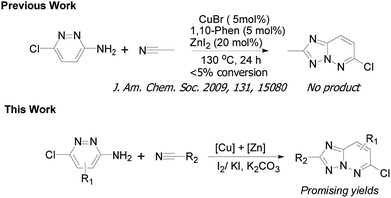
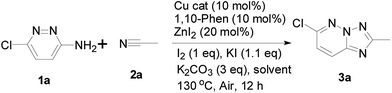
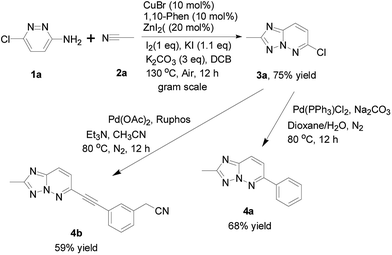
Initially, to explore the feasibility of the copper-based catalytic approach that reported by Ueda and Nagasawa in 2009,8 we selected 3-amino-6-chloropyridazine and acetonitrile as a model substrate for reaction evaluation. According to the classic reaction conditions, when 1a and 2a were reacted in the presence of 5 mol% of CuBr and 5 mol% of 1,10-phenanthroline as well as 10 mol% ZnI2 in dichlorobenzene (DCB), at 130 °C for 24 h under air (Scheme 1). Unfortunately, almost no desired product was obtained in this case (Table 1, entry 1). Therefore, the copper-catalyzed oxidative cycloaddition of 3-amino-6-chloropyridazine is different from the copper-catalyzed tandem addition-oxidative cyclization of 2-aminopyridines and nitriles because of its low reactivity under the reported reaction conditions. To explore this approach with copper catalysis, we continued to optimized the cycloaddition reaction of 3-amino-6-chloropyridazine. On the basis of the optimization of reaction conditions, we were pleased to find that the addition of a stoichiometric amount of I2 or KI to the reaction system is crucial to the formation of desired product 3a in the presence of K2CO3. We hypothesized that the cooperative activation by both CuBr and ZnI2 was the key step in the addition of 3-amino-6-chloropyridazine to nitriles. To improve the oxidative cycloaddition of 3-amino-6-chloropyridazine to nitrile, we further added I2 and KI to the reaction system. To our delight, the reaction gave the desired product in promising yield. Similarly to previous report,9 I2/KI-mediated oxidative cyclization is generally worked via the KI3-promoted N–N bond formation. Notably, no predicted product was observed in the absence of I2 or K2CO3. In addition, the reaction also proceeded in toluene and DMSO, albeit in lower yields. Interestingly, the other copper sources, such as CuI and Cu(OAc)2, proved to be a negative impact (Table 1, entries 11 and 12).
| Entry | Catalyst | Solvent | Additive | Base | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1 mmol, 1 eq.), 2a (10 mL), CuBr (10 mol%), 1,10-phenanthroline (10 mol%), ZnI2 (20 mol%), I2 (1 eq.), KI (1.1 eq.), K2CO3 (3 eq.), DCB (2 mL).b Determined by LC-MS yield.c CuBr (5 mol%), 1,10-phenanthroline (5 mol%), ZnI2 (10 mol%). DCB is dichlorobenzene. | |||||
| 1c | CuBr | DCB | ZnI2/—/— | — | 0 |
| 2 | CuBr | DCB | ZnI2/—/KI | K2CO3 | 0 |
| 3 | CuBr | DCB | —/I2/KI | K2CO3 | 6 |
| 4 | CuBr | DCB | ZnI2/I2/— | K2CO3 | 14 |
| 5 | CuBr | DCB | ZnI2/I2/KI | — | 0 |
| 6 | CuBr | Toluene | ZnI2/I2/KI | K2CO3 | 29 |
| 7 | CuBr | DMSO | ZnI2/I2/KI | K2CO3 | 24 |
| 8 | CuBr | DCB | ZnBr2/I2/KI | K2CO3 | 5 |
| 9 | CuBr | DCB | ZnI2/I2/KI | K2CO3 | 40 |
| 10 | CuBr | DCB | —/I2/— | K2CO3 | Trace |
| 11 | CuI | DCB | ZnI2/I2/KI | K2CO3 | 8 |
| 12 | Cu(OAc)2 | DCB | ZnI2/I2/KI | K2CO3 | Trace |
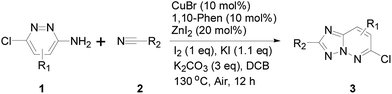
With the optimized cyclization conditions established above, we further investigated the scope and generality of the synthetic methodology. As shown in Table 2, benzonitriles bearing halogen or trifluoromethyl groups on aromatic rings afforded the corresponding product in moderate yields, while electron-rich benzonitriles reacted with 3-amino-6-chloropyridazine to offer relatively low yields. Delightfully, the heterocyclic nitriles, such as cyanopyridines and cyanothiophene, could also be employed to give moderate yields of products. The reactions performed smoothly when acetonitrile and benzonitriles were employed as substrates to offer desired products in moderate yields. 3-Amino-6-chloropyridazine with electron-donating substituent, such as methoxy group, provided yield slightly higher than that of halogen-containing substrates. In fact, the conversion of 3-aminopyridazines was almost completed, and the starting material was not observed after the reaction. The major reasons included: (1) the desired products were not good in solubility in general organic solvents, thus the corresponding yield was sacrificed during purification by silica gel column chromatography; (2) the side product might be insoluble carbon-based material because this reaction was carried out at high temperature.
| Entry | R1 | R2 | Product | Yieldb (%) | Conversionc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.77 mmol), 2 (1.93 mmol), CuBr (10 mol%), 1,10-phenanthroline (10 mol%), ZnI2 (20 mol%), I2 (0.77 mmol), KI (0.85 mmol), K2CO3 (2.31 mmol), DCB (5 mL), 130 °C, 12 h, ambient air.b Isolated yields.c Determined by GC-MS and HPLC. | |||||
| 1 | H | CH3 | 3a | 63 | >99 |
| 2 | H | Ph | 3b | 63 | >99 |
| 3 | H | 4-FPh | 3c | 52 | >99 |
| 4 | H | 4-MePh | 3d | 36 | >99 |
| 5 | H | 4-OCF3Ph | 3e | 18 | >99 |
| 6 | H | 2-FPh | 3f | 34 | >99 |
| 7 | H | 2-ClPh | 3g | 22 | >99 |
| 8 | H | 2-OMePh | 3h | 22 | >99 |
| 9 | H | 2-CF3Ph | 3i | 39 | >99 |
| 10 | H | 3-OMePh | 3j | 27 | >99 |
| 11 | H | 2-Thiophen | 3k | 30 | >99 |
| 12 | H | 3-Pyridin | 3l | 39 | >99 |
| 13 | H | 2,4-Cl2Ph | 3m | 22 | >99 |
| 14 | 4-OMe | CH3 | 3n | 33 | >99 |
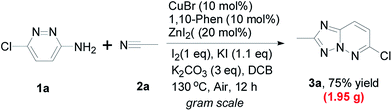
Notably, the gram-scale synthesis was also carried out. Gratifyingly, the corresponding target 3a could be obtained in the better yield (75% isolated yield, it is better than that with above small-scale experiment, versus 63% yield) under the standard conditions (Scheme 2), which revealed the practical usefulness of present method in the preparation of large amount of target product. In addition, to demonstrate the synthetic utility of this method, 3a was treated with phenylboronic acid and arylethynylene to evaluate its reactivity toward the known Suzuki reaction and Sonogashira reaction. To our delight, the corresponding products were obtained in moderate yields, which may enhance the value of this newly developed method (Scheme 3).
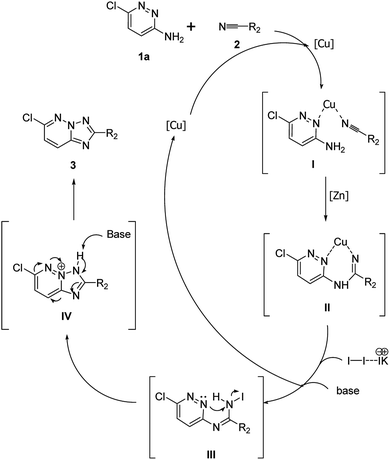
On the basis of the observed results and the LC-MS analysis (see ESI†), we found the side products of this reaction is quite complicated because of the formation of amidine intermediate. Therefore, we suggested a plausible mechanism for the cyclization process of 1,2,4-triazolo[1,5-b]pyridazine framework (Scheme 4). The initial amidine intermediate II may be formed firstly via copper-catalyzed intramolecular nucleophilic attack of 1a on the nitrile 2.10 Next, amidine intermediate II might provide an iodide intermediate III, promoted by base (K2CO3) via the subsequent nucleophilic attack of N atom of the pyridazine ring to afford ammonium ion 5, with cleavage of N–I bond.6c,9 At the lasts step, the desired 1,2,4-triazolo[1,5-b]pyridazine framework 3 was obtained after the deprotonation and aromatization (Scheme 4).
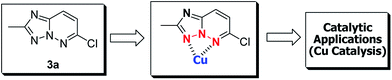
As described above, there is no reports on the copper-catalyzed oxidative cycloaddition of 3-aminopyridazines and nitrile for the preparation of 1,2,4-triazolo[1,5-b]pyridazine scaffolds, which promoted us to consider it as a nitrogen-based ligand for copper catalysis because of three nitrogen atoms on this scaffold (Fig. 1). Accordingly, with such a series of 1,2,4-triazolo[1,5-b]pyridazine derivatives in hand, we hypothesized that whether the products 3 bearing four nitrogen centers to be worked as ligands in transition metal catalysis. Although no works about 1,2,4-triazolo[1,5-b]pyridazine skeleton as ligands was reported in the past, we continued to evaluate its possibility in the copper-catalyzed cycloaddition of carbon dioxide (CO2) with epoxide. It is well-known that conversion or fixation of CO2 to synthetically useful compounds has been an important topic for synthetic chemists.11 Among numerous transformations of CO2, one of the most attractive and direct synthetic goals starting from carbon dioxide is the construction of five-membered cyclic carbonates because it has widely synthetic uses. And in generally, five-membered cyclic carbonates were achieved from the corresponding diols and phosgene or related compounds.11,12 In the past years, we have also demonstrated several catalytic systems for the cycloaddition of epoxides with CO2 with high efficiency.13 However, for most examples in the previous studies, the cycloaddition of CO2 to epoxides for the preparation of corresponding cyclic carbonates was generally conducted at relatively high reaction temperatures and high pressures (CO2) in the presence of ionic liquids, metal halides, or metal complexes as catalysts.14 Therefore, the development of a simple and efficient methodology, especially at atmosphere pressure, for the activation or fixation of CO2 is a demanding challenge and useful process for organic chemists.
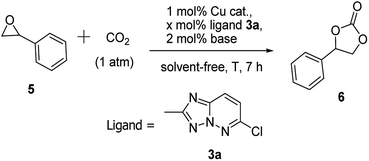
In this part, we would like to applied the cycloaddition of epoxide with carbon dioxide as a model reaction to evaluate the performance of 1,2,4-triazolo[1,5-b]pyridazine derivative 3a as a ligand in the copper catalysis. Initially, many attempts was carried out using 3a as a ligand, to our delight, the cycloaddition of carbon dioxide with styrene oxide was smoothly took place, and numerous screening experiments about the effect of temperature, pressure of CO2, as well as the ratio of copper salts with ligand were investigated (Table 3). Under the optimized reaction conditions, the styrene carbonate was obtained in an excellent yield (82%), as shown in Table 3. Therefore present finding provided an alternative method for the copper-catalyzed synthesis of cyclic carbonates from CO2 and epoxides,15 which would be an environmentally benign catalyst system in this reaction.
| Entry | Cu catalyst | Base | Temp (°C) | Pressure (atm) | Yieldd (%) |
|---|---|---|---|---|---|
a Note: the ratio of Cu salt with ligand is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, except note.b With 1 mol% of ligand (3a).c With 3 mol% of ligand (3a).d Determined by GC-MS and it is the GC yield.e With the ligand 7. 2, except note.b With 1 mol% of ligand (3a).c With 3 mol% of ligand (3a).d Determined by GC-MS and it is the GC yield.e With the ligand 7. |
|||||
| 1 | CuCl2 | DMF | 100 | 1 | 30 |
| 2 | CuCl2 | DMAP | 100 | 1 | 82 |
| 3 | CuCl2 | TBAB | 100 | 1 | 61 |
| 4 | CuCl2 | TBAI | 100 | 1 | 35 |
| 5 | CuCl2 | DMAP | 70 | 1 | 25 |
| 6 | CuCl2 | DMAP | 130 | 1 | 78 |
| 7 | CuCl2 | DMAP | 160 | 1 | 56 |
| 8b | CuCl2 | DMAP | 100 | 1 | 21 |
| 9c | CuCl2 | DMAP | 100 | 1 | 52 |
| 10 | CuBr2 | DMAP | 100 | 1 | 78 |
| 11 | Cu(OAc)2 | DMAP | 100 | 1 | 34 |
| 12 | CuCl2 | DMAP | 100 | 2 | 80 |
| 13e | CuCl2 | DMAP | 100 | 1 | 20 |
| 14e | CuCl2 | — | 100 | 1 | NR |
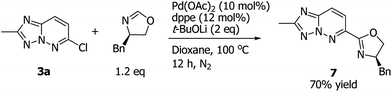
To demonstrate the potential application of 1,2,4-triazolo[1,5-b]pyridazine derivative 3a to the synthesis of other new ligand, the palladium-catalyzed C–H heteroarylation of oxazoline16 was also utilized as a strategy to give a new 1,2,4-triazolo[1,5-b]pyridazine-derived oxazoline ligand 7 (Scheme 5, 70% yield). Catalytic alkynylation of trifluoromethyl ketones is an important reaction in organic synthesis and organofluorine chemistry, which provided a facile process to the preparation of fluorinated propargylic alcohols.17 And in the past years, many methods have been reported for the catalytic synthesis of such fluorinated alcohols by catalytic alkynylation of trifluoromethyl ketones.18 However, development of highly efficient benign methodologies for alkynylation of trifluoromethyl ketones is still very important to the advancement of synthetic organic chemistry. In this work, under the Shibasaki's reported reaction conditions,18b we found the new 1,2,4-triazolo[1,5-b]pyridazine-derived oxazoline 7 was a highly efficient ligand in this reaction (>99% yield, see Scheme 6), albeit without enantioselectivity in this case. We believed that the described reactions in this work could be extended to the synthesis of structurally diverse propargyl alcohols.
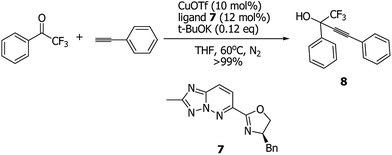 | ||
| Scheme 6 Copper-catalyzed alkynylation of 2,2,2-trifluoro-1-phenylethanone with 1-ethynylbenzene in the presence of new 1,2,4-triazolo[1,5-b]pyridazine-derived oxazoline ligand 7. | ||
In summary, we have presented a novel and simple metal-catalyzed tandem oxidative cycloaddition reaction, in which the desired targets are offered easily via cooperative Cu(I) and Zn(II)-catalyzed tandem C–N addition and subsequent I2/KI-mediated intramolecular oxidative N–N bond formation. Although the yields of the desired products were not perfect at present, the method will be an attractive alternative for the preparation of potentially biological active 1,2,4-triazolo[1,5-b]pyridazine derivatives, with features such as the broad substrate scope within short reaction time. The future applications of this compound class and its derivatives in organic synthesis, medicinal chemistry, and pesticide chemistry are still ongoing in our laboratory.
Acknowledgements
This project was supported by the National Natural Science Founder of China (No. 21472031 and 21503060), and Zhejiang Provincial Natural Science Foundation of China (LY17E030003 and LY17B030005). This work is also supported partially by Science and Technology Department of Zhejiang Province (2015C31138 and 2014C31131), and Hangzhou Science and Technology Bureau of China (20170533B08 and 20160432B08).Notes and references
- (a) M. Kuwahara, Y. Kawano, M. Kajino, Y. Ashida and A. Miyake, Chem. Pharm. Bull., 1997, 45, 1447 CrossRef CAS PubMed; (b) M. Gyoten, H. Nagaya, S. Fukuda, Y. Ashida and Y. Kawano, Chem. Pharm. Bull., 2003, 51, 122 CrossRef CAS PubMed; (c) S. D. Edmondson, A. Mastracchio, R. J. Mathvink, J. He, B. Harper, Y. J. Park, M. Beconi, J. D. Salvo, G. J. Eiermann, H. He, B. Leitting, J. F. Leone, D. A. Levorse, K. Lyons, R. A. Patel, S. B. Patel, A. Petrov, G. Scapin, J. Shang, R. S. Roy, A. Smith, J. K. Wu, S. Xu, B. Zhu, N. A. Thornberry and A. E. Weber, J. Med. Chem., 2006, 49, 3614 CrossRef CAS PubMed; (d) C. J. Menet, S. R. Fletcher, G. V. Lommen, R. Geney, J. Blanc, K. Smits, N. Jouannigot, P. Deprez, E. M. van der Aar, P. Clement-Lacroix, L. Lepescheux, R. Galien, B. Vayssiere, L. Nelles, T. Christophe, R. Brys, M. Uhring, F. Ciesielski and L. V. Rompaey, J. Med. Chem., 2014, 57, 9323 CrossRef CAS PubMed.
- (a) S. Polanc, B. Vercek, B. Stanovnik and M. Tisler, Tetrahedron Lett., 1972, 1677 Search PubMed; (b) S. Polanc, B. Vercek, B. Stanovnik and M. Tisler, J. Org. Chem., 1974, 39, 2143 CrossRef CAS; (c) B. Stanovnik, A. Stimac, M. Tisler and B. Vercek, J. Heterocycl. Chem., 1982, 19, 577 CrossRef CAS; (d) B. Stanovnik, V. Stibilj and M. Tisler, Synthesis, 1986, 807 CrossRef CAS.
- Y. Tamura, J. H. Kim and M. Ikeda, J. Heterocycl. Chem., 1975, 12, 107 CrossRef CAS.
- (a) M. Zupan, B. Stanovnik and M. Tisler, Tetrahedron Lett., 1972, 4179 CrossRef CAS; (b) G. Schneider and M. Nettekoven, J. Comb. Chem., 2003, 5, 233 CrossRef CAS PubMed; (c) D. G. Yu, M. Suri and F. Glorius, J. Am. Chem. Soc., 2013, 135, 8802 CrossRef CAS PubMed; (d) K. L. Stevens, M. J. Reno, J. B. Alberti, D. J. Price, L. S. Kane-Carson, V. B. Knick, L. M. A. M. Hassell, J. M. Veal, S. T. Davis, R. J. Griffin and M. R. Peel, Bioorg. Med. Chem. Lett., 2008, 18, 5758 CrossRef CAS PubMed; (e) W. M. Abdou, N. A. Ganoub and E. Sabry, Beilstein J. Org. Chem., 2013, 9, 1730 CrossRef PubMed; (f) T. Irrgan and R. Kempe, Eur. J. Org. Chem., 2005, 4382 CrossRef.
- (a) J.-P. Zhang, Y. Y. Lin, X. C. Huang and X. M. Chen, J. Am. Chem. Soc., 2005, 127, 5495 CrossRef CAS PubMed; (b) X. Meng, C. Yu and P. Zhao, RSC Adv., 2014, 4, 8612 RSC; (c) B. Bartels, C. G. Bolas, P. Cueni, S. Fantasia, N. Gaeng and A. S. Trita, J. Org. Chem., 2015, 80, 1249 CrossRef CAS PubMed; For recent reviews on copper-catalyzed organic reactions, see: (d) A. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef PubMed.
- (a) O. Prakash, H. K. Gujral, N. Rani and S. P. Sing, Synth. Commun., 2000, 30, 417 CrossRef CAS; (b) Z. Zheng, S. Ma, L. Tang, D. Z. Negrerie, Y. Du and K. Zhao, J. Org. Chem., 2014, 79, 4686 Search PubMed; (c) L. Song, X. Tian, Z. Lv, E. Li, J. Wu, Y. Liu, W. Yu and J. Chang, J. Org. Chem., 2015, 80, 7219 CrossRef CAS PubMed.
- (a) Z. J. Zheng, F. Ye, L. S. Zheng, K. F. Yang, G. Q. Lai and L. W. Xu, Chem.–Eur. J., 2012, 18, 14094 CrossRef CAS PubMed; (b) C. Y. Wang, J. F. Zou, Z. J. Zheng, W. S. Huang, L. Li and L. W. Xu, RSC Adv., 2014, 4, 54256 RSC; (c) T. Song, L. Li, W. Zhou, Z. J. Zheng, Y. Deng, Z. Xu and L. W. Xu, Chem.–Eur. J., 2015, 21, 554 CrossRef CAS PubMed; (d) M. Y. Chen, T. Song, Z. J. Zheng, Z. Xu, Y. M. Cui and L. W. Xu, RSC Adv., 2016, 6, 58698 RSC.
- S. Ueda and H. Nagasawa, J. Am. Chem. Soc., 2009, 131, 15080 CrossRef CAS PubMed.
- (a) P. H. Svensson and L. Kloo, Chem. Rev., 2003, 103, 1650 CrossRef PubMed; (b) P. Gogoi and D. Konwar, Org. Biomol. Chem., 2005, 3, 3473 RSC; (c) X. Tian, L. Song, M. Wang, Z. Lv, J. Wu, W. Yu and J. Chang, Chem.–Eur. J., 2016, 22, 7617 CrossRef CAS PubMed.
- (a) G. Rousselet, P. Capdevielle and M. Maumy, Tetrahedron Lett., 1993, 34, 6395 CrossRef CAS; (b) W. Yin, C. Wang and Y. Huang, Org. Lett., 2013, 15, 1850 CrossRef CAS PubMed; (c) Z. Zheng, S. Ma, L. Tang, D. Zhang-Negrerie, Y. Du and K. Zhao, J. Org. Chem., 2014, 79, 4687 CrossRef CAS PubMed; (d) H. Xu, S. Ma, Y. Xu, L. Bian, T. Ding, X. Fang, W. Zhang and Y. Ren, J. Org. Chem., 2015, 80, 1789 CrossRef CAS PubMed; (e) C.-y. Chen, G. Tang, F. He, Z. Wang, H. Jing and R. Faessler, Org. Lett., 2016, 18, 1690 CrossRef CAS PubMed.
- For representative reviews, see: (a) D. H. Dibson, Chem. Rev., 1996, 96, 2063 CrossRef; (b) X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462 RSC; (c) A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. Dubois, M. DuPuis, J. G. Ferry, E. Fujita, R. Hille and P. J. A. Kenis, Chem. Rev., 2013, 113, 6621 CrossRef CAS PubMed; (d) J. F. Shi, Y. J. Jiang, Z. Y. Jiang, X. Y. Wang, X. L. Wang, S. H. Zhang, P. P. Han and C. Yang, Chem. Soc. Rev., 2015, 44, 5981 RSC; (e) H. Wang, J. J. Peng and J. Li, Chem. Rec., 2016, 16, 1298 CrossRef CAS PubMed; (f) L. J. Guo, Y. J. Wang and T. He, Chem. Rec., 2016, 16, 1918 CrossRef CAS PubMed; (g) J. Hasegawa, R. Miyazaki, C. Maeda and T. Ema, Chem. Rec., 2016, 16, 2260 CrossRef CAS PubMed; For recent examples, see: (h) G. P. Ji, Z. Z. Yang, H. Y. Zhang, Y. F. Zhao, B. Yu, Z. S. Ma and Z. M. Liu, Angew. Chem., Int. Ed., 2016, 55, 9684 Search PubMed; (i) W. Y. Gao, H. F. Wu, K. Y. Leng, Y. Y. Sun and S. Q. Ma, Angew. Chem., Int. Ed., 2016, 55, 5472 CrossRef CAS PubMed; (j) J. Rintjema, R. Epping, G. Fiorani, E. Martin, E. C. Escudero-Adan and A. W. Kleiji, Angew. Chem., Int. Ed., 2016, 55, 3972 CrossRef CAS PubMed; (k) D. Y. Zhang, S. K. Boopathi, N. Hadjichristidia, Y. Gnanou and X. S. Feng, J. Am. Chem. Soc., 2016, 138, 11117 CrossRef CAS PubMed; (l) C. Romain, Y. Q. Zhu, P. Dingwall, S. Paul, H. S. Rzepa, A. Buchard and C. K. Williams, J. Am. Chem. Soc., 2016, 138, 4120 CrossRef CAS PubMed.
- (a) V. Amarnath and A. D. Broom, Chem. Rev., 1977, 77, 183 CrossRef CAS; (b) M. Pena-Lopez, H. Neumann and M. Beller, Eur. J. Org. Chem., 2016, 3721 CrossRef CAS; (c) G. L. Gregory, M. Ulmann and A. Buchard, RSC Adv., 2015, 5, 39404 RSC; (d) F. D. Bobbink, W. Gruszka, M. Hulla, S. Das and P. J. Dyson, Chem. Commun., 2016, 52, 10787 RSC; (e) S. B. Wang and X. C. Wang, Angew. Chem., Int. Ed., 2016, 55, 2308 CrossRef CAS PubMed.
- (a) F. W. Li, C. G. Xia, L. W. Xu, C. G. Xia, W. Sun and G. X. Chen, Chem. Commun., 2003, 2042 RSC; (b) F. W. Li, L. F. Xiao, C. G. Xia and B. Hu, Tetrahedron Lett., 2004, 45, 8307 CrossRef CAS; (c) L. W. Xu, M. S. Yang, J. X. Jiang, H. Y. Qiu and G. Q. Lai, Cent. Eur. J. Chem., 2007, 5, 1073 CAS; (d) F. Wang, C. Z. Xu, Z. Li, C. G. Xia and J. Chen, J. Mol. Catal. A: Chem., 2014, 385, 133 CrossRef CAS; (e) H. L. Liu, Z. Huang, Z. Han, K. L. Ding, H. C. Liu, C. G. Xia and J. Chen, Green Chem., 2015, 17, 4281 RSC.
- (a) M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514 RSC; (b) T. Sakakura and K. Kohno, Chem. Commun., 2009, 1312 RSC; (c) A. Decortes, A. M. Castilla and A. W. Kleij, Angew. Chem., Int. Ed., 2010, 49, 9822 CrossRef CAS PubMed; (d) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed.
- (a) L. Wu, H. Yang, H. Wang and J. Lu, RSC Adv., 2015, 5, 23189 RSC; (b) M. Y. Wang, Q. W. Song, R. Ma, J. N. Xie and L. N. He, Green Chem., 2016, 18, 282 RSC.
- T. Xi, Y. Mei and Z. Lu, Org. Lett., 2015, 17, 5939 CrossRef CAS PubMed.
- (a) C. J. Li, Acc. Chem. Res., 2010, 43, 581 CrossRef CAS PubMed; (b) J. Nie, H. C. Guo, D. Cahard and J. A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed.
- (a) R. Motoki, D. Tomita, M. Kanai and M. Shibasaki, Tetrahedron Lett., 2006, 47, 8083 CrossRef CAS; (b) R. Motoki, M. Kanai and M. Shibasaki, Org. Lett., 2007, 9, 2997 CrossRef CAS PubMed; (c) G. J. Deng and C. J. Li, Synlett, 2008, 1571 CAS; (d) G. W. Zhang, W. Meng, H. Ma, J. Nie, W. Q. Zhang and J. A. Ma, Angew. Chem., Int. Ed., 2011, 50, 3538–3542 CrossRef CAS PubMed; (e) V. R. Chintareddy, K. Wadhwa and J. G. Verkade, J. Org. Chem., 2011, 76, 4482 CrossRef CAS PubMed; (f) C. A. Correia, D. T. McQuade and P. H. Seeberger, Adv. Synth. Catal., 2011, 355, 3517 CrossRef; (g) H. Wang, K. F. Yang, L. Li, Y. Bai, Z. J. Zheng, W. Q. Zhang, Z. W. Gao and L. W. Xu, ChemCatChem, 2014, 6, 580 CrossRef CAS; (h) L. Wang, N. Liu, B. Dai, X. Ma and L. Shi, RSC Adv., 2015, 5, 10089 RSC; (i) F. Lazreg, M. Lesieur, A. J. Samson and C. S. J. Cazin, ChemCatChem, 2016, 8, 209 CrossRef CAS; (j) P. Czerwinski, E. Molga, L. Cavallo, A. Poater and M. Michalak, Chem.–Eur. J., 2016, 22, 8089 CrossRef CAS PubMed; (k) J.-i. Ito, S. Ubukata, S. Muraoka and H. Nishiyama, Chem.–Eur. J., 2016, 22, 16801 CrossRef CAS PubMed; (l) Y. Zheng, Y. Tan, K. Harms, M. Marsch, R. Riedel, L. Zhang and E. Meggers, J. Am. Chem. Soc., 2017, 139, 4322 CrossRef CAS PubMed; (m) F. L. Li, L. Wang, C. H. Li, N. Liu and B. Dai, ACS Omega, 2017, 2, 1104 CrossRef CAS; (n) X. D. Li, H. Y. Ma, C. H. Xing and L. Lu, Tetrahedron Lett., 2017, 58, 1564 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06727e |
| This journal is © The Royal Society of Chemistry 2017 |