Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
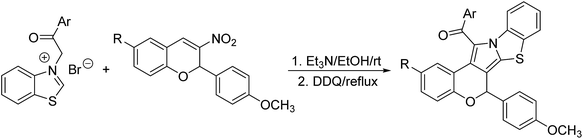
Diastereoselective synthesis of benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles via cycloaddition reaction of benzothiazolium salts with 3-nitrochromenes†
Wang Jiang,
Jing Sun and
Chao-Guo Yan *
*
College of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225002, P. R. China. E-mail: cgyan@yzu.edu.cn; Fax: +86-514-87975244; Tel: +86-514-87975531
First published on 1st September 2017
Abstract
The triethylamine mediated 1,3-dipolar cycloaddition reaction of 2-phenacyl- or 2-alkoxycarbonylmethylbenzothiazolium bromides with 3-nitrochromenes in ethanol at room temperature afforded functionalized tetrahydrobenzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles in 83–95% yields and with high diastereoselectivity. The corresponding dehydrogenated benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles were also easily obtained by sequential oxidation with DDQ. The stereochemistry of the polycyclic compounds was clearly elucidated by analysis of NMR spectroscopy results and determination of single crystal X-ray structures. The reaction is believed to proceed via endo-[3 + 2] cycloaddition of the in situ generated anti-form ylides to the cyclic dipolarophiles.
Introduction
Chromane (benzopyran) is one of the most important oxygen-containing heterocycles. Its derivatives are not only widespread in many natural products, but also are known as the biological compounds which exhibit a wide range of biological properties such as anticancer, diuretic, anticoagulant, and anti-anaphylactic activity.1,2 For examples, benzopyranopyrrolidine derivatives are antagonistic towards 5-HT2C receptors and adrenoreceptor antagonists.3 Many chromane derivatives are also natural lipophilic antioxidants and radical scavengers in the vitamin E family.4 Therefore, continuous efforts have been devoted to the development of more efficient synthetic methodologies for this class of compound.5–8 Over the last few decades, heteroaromatic N-ylides have been drawing the attention of chemists due to their wide spectrum of utilities in numerous chemical transformations.9 The cycloaddition of the heteroaromatic N-ylides to various dipolarophiles showed very interesting chemical, regio- and stereo-selectivity, from which indolizine, cyclopropane, dihydrofuran and zwitterionic derivatives as well as other polycyclic systems can be selectively obtained by adjusting molecular structures of the substrates and the reaction conditions.10 Particularly, the 1,3-dipolar cycloaddition reaction of the heteroaromatic N-ylides with nitroolefins have been widely investigated in the literature.11 On this respect, the well-known conjugated 3-nitrochromenes, which could be derived from the condensation reaction of substituted salicylaldehyde with nitroolefins, were also widely employed as Michael acceptors and dipolarophiles in various reactions.12,13 Recently, we have successfully found that N-phenacylisoquinolinium salts reacted with 3-nitrochromenes in basic system to give complex tetrahydro-6H-5-oxa-12a-azadibenzo[a,g]fluorene derivatives.14 Against this background and in continuation of our aim to exploit the versatile synthetic applications of the heteroaromatic N-ylides for diverse heterocyclic compounds,15,16 herein we wish to report the base promoted 1,3-dipolar cycloaddition reaction of various benzothiazolium salts with 3-nitrochromenes for diastereoselective synthesis of tetrahydrobenzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazole and their aromatic derivatives.Results and discussions
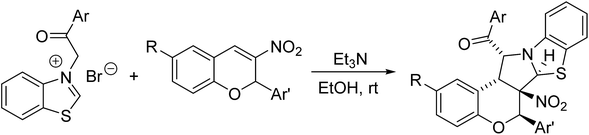
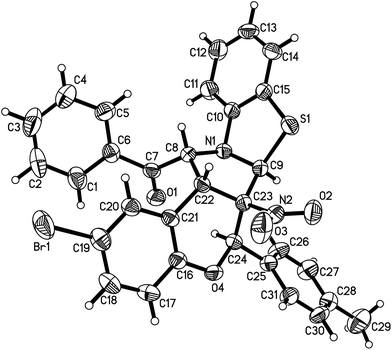
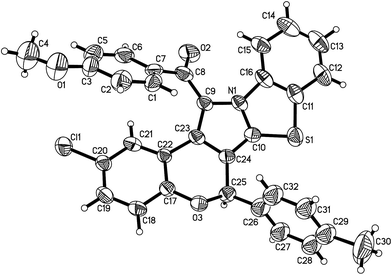
According to the previously reported reaction conditions of the various heteroaromatic N-ylides with nitroalkenes,11 a mixture of 3-phenacylbenzothiazolium bromide (1) with 3-nitrochromene (2) in ethanol in the presence of triethylamine was stirred at room temperature. TLC monitor indicated that the reaction proceeded smoothly in three hours. The resulting products were readily precipitated from solution. After filtration and washing with cold alcohol, the pure products were easily obtained from this very simple procedure. The other organic bases such as piperidine and DABCO were also effective for the reaction. Because triethylamine is the most cheap and common organic base, thus, it is employed as the base for this reaction. The results are summarized in Table 1. It can be seen that all reactions afforded the polycyclic products in satisfactory yields. The substituent on the aromatic ring showed very little effect on the yields. The structures of the products 3a–3t were fully characterized by IR, HRMS, 1H NMR and 13C NMR spectra. There are five chiral carbon atoms in the polycyclic compounds, therefore, several diastereoisomers might be formed in the reaction. We were pleased to find that the 1H NMR and 13C NMR spectra of 3a–3t clearly reveals one set of characterized absorptions for the groups and scaffolds in the molecules, which strongly indicated that only one diastereoisomer was predominately formed in this 1,3-dipolar cycloaddition reaction. In order to determine the relative configuration of the obtained products, four single crystal structures of the compounds 3a (Fig. 1), 3e, 3n and 3r (Fig. s1–s3†) were successfully determined by X-ray crystallographic method. The single crystals suitable for X-ray crystallographic analysis were usually obtained by slower evaporation the solution of the compounds in a mixed chloroform and ethanol. The four molecules actually have same configuration. Thus, it can be certainly conclude that all compounds 3a–3t have this kind of configuration and this [3 + 2] cycloaddition reaction has very high diastereoselectivity. From the Fig. 1, it can be seen that the benzoyl group and the benzo-moiety of chromene exist in trans-position in the newly formed pyrrolidine ring. The nitro group and phenylsulfanyl group stand on the trans-position in the newly formed pyrrolidine ring. Additionally, the aryl group at C1-position of the moiety of chromene also exists in a cis relationship with the NO2 group. On the other hand, the benzopyran and benzothiazole rings are found on the opposite faces of the central pyrrolidine ring. Thus, the 1,3-dipolar cycloaddition reaction proceeded in a highly diastereoselective fashion. According to the stereochemical precedent established by Tsuge and co-workers,11 the heteroaromatic ylide usually adopted the anti-form configuration to proceed with the endo-addition type in [3 + 2] cycloaddition reaction. In accord with the stereochemical precedent established by Tsuge and co-workers,11 the products are formed via a single regioisomeric endo transitions state involving the anti-form of the ylide.| Entry | Compd | Ar | R | Ar′ | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: benzothiazolium bromide (1.0 mmol), 3-nitrochromene (1.0 mmol), Et3N (1.2 mmol), EtOH (10.0 mL), rt, 3 h.b Isolated yield. | |||||
| 1 | 3a | C6H5 | Br | p-CH3C6H4 | 87 |
| 2 | 3b | p-CH3C6H4 | H | p-CH3C6H4 | 89 |
| 3 | 3c | p-CH3C6H4 | H | p-CH3OC6H4 | 91 |
| 4 | 3d | p-CH3C6H4 | Cl | p-CH3C6H4 | 91 |
| 5 | 3e | p-CH3C6H4 | Br | p-CH3C6H4 | 89 |
| 6 | 3f | p-CH3OC6H4 | H | C6H5 | 91 |
| 7 | 3g | p-CH3OC6H4 | H | p-CH3C6H4 | 87 |
| 8 | 3h | p-CH3OC6H4 | H | p-CH3OC6H4 | 88 |
| 9 | 3i | p-CH3OC6H4 | H | p-ClC6H4 | 93 |
| 10 | 3j | p-CH3OC6H4 | Cl | C6H5 | 86 |
| 11 | 3k | p-CH3OC6H4 | Cl | p-CH3C6H4 | 89 |
| 12 | 3l | p-CH3OC6H4 | Cl | p-CH3OC6H4 | 86 |
| 13 | 3m | p-CH3OC6H4 | Cl | p-ClC6H4 | 89 |
| 14 | 3n | p-CH3OC6H4 | Br | C6H5 | 83 |
| 15 | 3o | p-CH3OC6H4 | Br | p-CH3C6H4 | 88 |
| 16 | 3p | p-CH3OC6H4 | Br | p-CH3OC6H4 | 92 |
| 17 | 3q | p-CH3OC6H4 | Br | p-ClC6H4 | 86 |
| 18 | 3r | p-ClC6H4 | Br | p-CH3C6H4 | 95 |
| 19 | 3s | p-NO2C6H4 | H | p-ClC6H4 | 87 |
| 20 | 3t | p-NO2C6H4 | Br | p-CH3C6H4 | 83 |
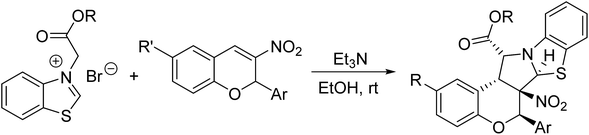
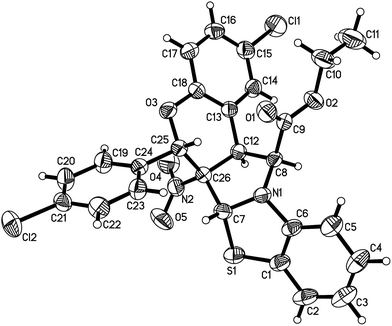
For developing the scope of this reaction, 2-alkoxycarbonylmethylbenzothiazolium bromides were also employed in the reaction. However, the reactivity of the corresponding 2-alkoxycarbonylmethylbenzothiazolium bromides is obviously lower than the above mentioned 2-phenacylbenzothiazolium bromides. The reaction of the benzothiazolium bromides bearing methoxy and ethoxy groups with 3-nitrochromenes could be finished overnight at room temperature to give the expected benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles 4a–4d in moderated yields (Table 2). The reaction of 2-(tert-butoxycarbonylmethyl)benzothiazolium bromides with 3-nitrochromenes cannot afforded the desired products both at room temperature and in refluxing ethanol, which might be the steric effect of the t-butyl ester group on the energy of the cycloaddition transition state. The structures of the polycyclic products 4a–4d were established by the spectroscopic analyses. The crystal structure of the compound 4d was successfully determined by X-ray diffraction (Fig. 2). We were pleased to find that the compound 4d has same configuration to that of the above mentioned compounds 3a–3t, in which the benzoyl group is just replaced by the ethoxycarbonyl group. This result also revealed that the 1,3-dipolar cycloaddition of the base promoted benzothiazolium ylides with 3-nitrochromenes has same controlling effect on the stereochemistry.
For developing the molecular diversity of the reaction, a dehydrogenation process was employed. After finishing the base promoted 1,3-dipolar cycloaddition reaction, an excess of DDQ was added and the reaction mixture was refluxed in ethanol for four hours. After workup, the aromatized benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles 5a–5i were obtained in satisfactory yields by dehydrogenation and elimination of nitro group process (Table 3). Because the aromatic pyrrole ring was formed, there is only one stereogenic C2 carbon atom in the molecules 5a–5i. The molecular structures of the compounds 5a–5i were easily established by the spectroscopy. Additionally, four single crystal structures 5c (Fig. 3), 5e, 5g and 5h (Fig. s4–s6†) were successfully determined by X-ray crystallographic method. Therefore, the functionalized benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles were efficiently prepared by one-pot two-step 1,3-dipolar cycloaddition and oxidation procedure.
| Entry | Compd | R | Ar′ | Yieldb (%) |
|---|---|---|---|---|
| a Reaction condition: benzothiazolium bromide (1.0 mmol), 3-nitrochromene (1.0 mmol), Et3N (1.0 mmol), EtOH (10.0 mL), rt, 3 h; DDQ (2.2 mmol), reflux, 4 h.b Isolated yield. | ||||
| 1 | 5a | H | C6H5 | 67 |
| 2 | 5b | Cl | C6H5 | 77 |
| 3 | 5c | Cl | p-CH3C6H4 | 79 |
| 4 | 5d | Cl | p-CH3OC6H4 | 83 |
| 5 | 5e | Cl | p-ClC6H4 | 81 |
| 6 | 5f | Br | C6H5 | 74 |
| 7 | 5g | Br | p-CH3C6H4 | 75 |
| 8 | 5h | Br | p-CH3OC6H4 | 81 |
| 9 | 5i | Br | p-ClC6H4 | 79 |
Conclusion
In summary, we have developed a base promoted 1,3-dipolar cycloaddition reaction of benzothiazolium bromides with 3-nitrochromenes for the diastereoselective synthesis of tetrahydrobenzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles in high yields. The corresponding benzo[d]chromeno[3′,4′:3,4]pyrrolo[2,1-b]thiazoles were also efficiently prepared by the one-pot sequential 1,3-dipolar cycloaddition and oxidation procedure. The reaction has the advantages of readily available starting material, mild reaction conditions, operational simplicity, diastereoselectivity and molecular diversity. This reaction not only provided efficient synthetic method for complex polycyclic compounds containing N, O, S heteroatoms, but also developed potential applications of the heteroaromatic N-ylides in synthetic and medicinal chemistry. Further expansions of this methodology for the preparation of complex nitrogen-containing heterocycles are in progress in our laboratory.Experimental section
General procedure for the preparing the compounds 3a–3t and 4a–4d
A mixture of 2-phenacylbenzothiazolium bromide (1.0 mmol) and 3-nitrochromene (1.0 mmol) and triethylamine (1.2 mmol) in ethanol (10.0 mL) was stirred at room temperature for three hours. The resulting precipitates were collected by filtration and washed with cold alcohol to give the pure product for analysis. The same procedure was used for preparation of compounds 4a–4d excepting prolonging the reaction time to twelve hours.General procedure for the preparing the compounds 5a–5i
A mixture of 2-phenacylbenzothiazolium bromide (1.0 mmol) and 3-nitrochromene (1.0 mmol) and triethylamine (2.0 mmol) in ethanol (10.0 mL) was stirred at room temperature for three hours. Then, DDQ (2.2 mmol) was added. The mixture was heated to refluxing in ethanol for four hours. After cooling, the resulting precipitates were collected by filtration and washed with cold alcohol to give the pure product for analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21372192, 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank Analysis and Test Center of Yangzhou University providing all analytical instruments.References
-
(a) A. Goel, A. Kumar and A. Raghuvanshi, Chem. Rev., 2013, 113, 1614–1640 CrossRef CAS PubMed
; (b) R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476–10526 CrossRef CAS PubMed
.
-
(a) J. P. Cueva, G. Giorgioni, R. A. Grubbs, B. R. Chemel, V. J. Watts and D. E. Nichols, J. Med. Chem., 2006, 49, 6848–6857 CrossRef CAS PubMed
; (b) D. T. Zhang, Y. T. Ma, Y. Liu and Z. P. Liu, Arch. Pharm., 2014, 347, 576–588 CrossRef CAS PubMed
; (c) G. Q. Xiao, B. X. Liang, S. H. Chen, T. M. Ou, X. Z. Bu and M. Yan, Arch. Pharm., 2012, 345, 767–770 CrossRef CAS PubMed
.
-
(a) T. Dubuffet, O. Muller, S. S. Simonef, J.-J. Descomhes, M. Laubie, T. J. Verheuren and G. Lavidle, Bioorg. Med. Chem. Lett., 1996, 6, 349–352 CrossRef CAS
; (b) T. Dubuffet, A. Newman-Tancerdi, D. Cussac, V. Audinot, A. Loutz, M. J. Millan and G. Lavielle, Bioorg. Med. Chem. Lett., 1999, 9, 2059–2064 CrossRef CAS PubMed
.
-
(a) H. G. Pars, F. E. Granchelli, R. K. Razdan, J. K. Keller, D. G. Teiger, F. J. Rosenberg and L. S. Harris, J. Med. Chem., 1976, 19, 445 CrossRef CAS PubMed
; (b) A. R. Haight, A. E. Bailey, W. S. Baker, M. H. Cain, R. R. Copp, J. A. DeMattei, K. L. Ford, R. F. Henry, M. C. Hsu, R. F. Keyes, S. A. King, M. A. McLaughlin, L. M. Melcher, W. R. Nadler, P. A. Oliver, S. I. Parekh, H. H. Patel, L. S. Seif, M. A. Staeger, G. S. Wayne, S. J. Wittenberger and W. Zhang, Org. Process Res. Dev., 2004, 8, 897 CrossRef CAS
; (c) K. Ishikawa, Y. Tamura and S. Maruta, J. Biochem., 2014, 155, 195–206 CrossRef CAS PubMed
.
-
(a) A. Shaabani, R. Ghadari, A. Sarvary and A. H. Rezayan, J. Org. Chem., 2009, 74, 4372–4374 CrossRef CAS PubMed
; (b) D. Amantini, F. Fringuelli and F. Pizzo, J. Org. Chem., 2002, 67, 7238–7243 CrossRef CAS PubMed
; (c) M. A. Terzidis, T. Zarganes-Tzitzikas, C. Tsimenidis, J. Stephanidou-Stephanatou, C. A. Tsoleridis and G. E. Kostakis, J. Org. Chem., 2012, 77, 9018–9028 CrossRef CAS PubMed
; (d) R. G. Soengas, H. Rodríguez-Solla, A. M. S. Silva, R. Llavona and F. A. A. Paz, J. Org. Chem., 2013, 78, 12831–12836 CrossRef CAS PubMed
; (e) X. S. Shang, N. T. Li, H. X. Siyang and P. N. Liu, J. Org. Chem., 2015, 80, 4808–4815 CrossRef CAS PubMed
; (f) K. Tangdenpaisal, K. Chuayboonsong, S. Ruchirawat and P. Ploypradith, J. Org. Chem., 2017, 82, 2672–2688 CrossRef CAS PubMed
.
-
(a) M. R. P. Queiroz, A. S. Abreu, P. M. T. Ferreira, M. M. Oliveira, R. Dubest, J. Aubard and A. Samat, Org. Lett., 2005, 7, 4811–4814 CrossRef CAS PubMed
; (b) J. Peng, X. Huang, P. F. Zheng and Y. C. Chen, Org. Lett., 2013, 15, 5534–5537 CrossRef CAS PubMed
; (c) J. Fei, Q. Q. Qian, X. H. Sun, X. D. Gu, C. C. Zou and J. X. Ye, Org. Lett., 2015, 17, 5296–5299 CrossRef CAS PubMed
; (d) Y. T. Gu, F. L. Li, P. F. Hu, D. H. Liao and X. F. Tong, Org. Lett., 2015, 17, 1106–1109 CrossRef CAS PubMed
; (e) J. M. Gil-Negrete, J. P. Sestelo and L. A. Sarandeses, Org. Lett., 2016, 18, 4316–4319 CrossRef CAS PubMed
.
-
(a) R. P. K. Kodukulla, S. Hariharan and G. K. Trivedi, Tetrahedron, 1994, 50, 4623–4634 CrossRef CAS
; (b) A. Virányi, G. Marth, A. Dancsó, G. Blaskó, L. Tőke and M. Nyerges, Tetrahedron, 2006, 62, 8720–8730 CrossRef
; (c) T. K. Chaitanya and R. Nagarajan, Tetrahedron Lett., 2007, 48, 2489–2492 CrossRef CAS
; (d) J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2015, 56, 2276–2279 CrossRef
.
-
(a) D. Q. Xu, Y. F. Wang, S. P. Luo, S. Zhang, A. G. Zhong, H. Chen and Z. Y. Xu, Adv. Synth. Catal., 2008, 350, 2610–2616 CrossRef CAS
; (b) E. H. Granados-Covarrubias and L. A. Maldonado, J. Org. Chem., 2009, 74, 5097–5099 CrossRef CAS PubMed
; (c) S. Z. Nie, Z. P. Hu, Y. N. Xuan, J. J. Wang, X. M. Li and M. Yan, Tetrahedron: Asymmetry, 2010, 21, 2055–2059 CrossRef CAS
; (d) J. W. Xie, L. P. Fan, H. Su, X. S. Li and D. C. Xu, Org. Biomol. Chem., 2010, 8, 2117–2122 RSC
; (e) L. Zhang, Y. M. Wang, H. B. Song, Z. H. Zhou and C. C. Tang, Eur. J. Org. Chem., 2014, 3163–3169 CrossRef CAS
; (f) L. L. Zhang, J. Sun and C. G. Yan, Chin. J. Chem., 2013, 31, 1546–1550 CrossRef CAS
; (g) J. Fang and C. G. Yan, J. Heterocycl. Chem., 2016, 53, 800–804 CrossRef CAS
.
-
(a) F. Kröhnke and W. Zecher, Angew. Chem., Int. Ed., 1962, 1, 626–632 CrossRef
; (b) F. Kröhnke, Angew. Chem., Int. Ed., 1963, 2, 225–238 CrossRef
; (c) O. Tsuge, S. Kanemasa and S. Takenaka, Bull. Chem. Soc. Jpn., 1987, 60, 1489–1495 CrossRef CAS
; (d) O. Tsuge, S. Kanemasa, K. Sakamoto and S. Takenaka, Bull. Chem. Soc. Jpn., 1988, 61, 2513–2544 CrossRef CAS
.
-
(a) D. S. Allgäuer, P. Mayer and H. Mayr, J. Am. Chem. Soc., 2013, 135, 15216–15224 CrossRef PubMed
; (b) D. S. Allgäuer and H. Mayr, Eur. J. Org. Chem., 2014, 2956–2963 CrossRef
; (c) Y. Liu and J. W. Sun, J. Org. Chem., 2012, 77, 1191–1197 CrossRef CAS PubMed
; (d) V. A. Osyanin, D. V. Osipov and Y. N. Klimochkin, J. Org. Chem., 2013, 78, 5505–5520 CrossRef CAS PubMed
; (e) F. Y. Wang, Y. M. Shen, H. Y. Hu, X. S. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 9556–9566 CrossRef CAS PubMed
.
-
(a) O. Tsuge, S. Kanemasa and S. Takenaka, Bull. Chem. Soc. Jpn., 1985, 58, 3137–3157 CrossRef CAS
; (b) Y. Tominaga, Y. Ichihara and A. Hosomi, Heterocycles, 1988, 27, 2345–2348 CrossRef CAS
; (c) A. M. Shestopalov, K. S. Chunikhin and L. A. Rodinovskaya, Chem. Heterocycl. Compd., 2002, 38, 310–313 CrossRef CAS
; (d) B. K. Ni, Q. Y. Zhang and A. D. Headley, Tetrahedron Lett., 2008, 49, 1249–1252 CrossRef CAS
; (e) S. B. Nosachev, K. A. Del'netskaya, N. A. Solov'ev and A. G. Tyrkov, Russ. J. Org. Chem., 2010, 46, 1427–1429 CrossRef CAS
; (f) K. P. Chen, Y. J. Chen and C. P. Chuang, Eur. J. Org. Chem., 2010, 5292–5300 CrossRef CAS
; (g) M. Kucukdisli and T. Opatz, Eur. J. Org. Chem., 2012, 4555–4564 CrossRef CAS
; (h) Z. M. Liu, J. Fang and C. G. Yan, Chem. Res. Chin. Univ., 2013, 29, 1089–1093 CrossRef CAS
; (i) F. Shi, Y. Zhang, Z. L. Lu, X. L. Zhu, W. Q. Kan, X. Wang and H. Y. Hu, Synthesis, 2016, 48, 413–420 CAS
; (j) G. Jin, J. Sun, R. Y. Yang and C. G. Yan, Sci. Rep., 2017, 7, 46470 CrossRef PubMed
.
-
(a) M. C. Yan, Y. J. Jang and C. F. Yao, Tetrahedron Lett., 2001, 42, 2717–2721 CrossRef CAS
; (b) C. F. Yao, Y. J. Jang and M. C. Yan, Tetrahedron Lett., 2003, 44, 3813–3816 CrossRef CAS
; (c) A. Viranyi, G. Marth, A. D. Blaskò, L. Tòke and M. Nyerges, Tetrahedron, 2006, 26, 8720–8730 CrossRef
; (d) K. Thandavamurthy and S. Sethuraman, Tetrahedron: Asymmetry, 2008, 19, 2741–2745 Search PubMed; (e) P. Li, L. L. Luo, X. S. Li and J. W. Xie, Tetrahedron, 2010, 66, 7590–7594 CrossRef CAS
; (f) J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2013, 54, 6568–6573 CrossRef
; (g) V. Y. Korotaev, A. Y. Barkov, V. S. Moshkin, E. G. Matochkina, M. I. Kodess and V. Y. Sosnovskikh, Tetrahedron, 2013, 69, 8602–8608 CrossRef CAS
.
-
(a) R. Muruganantham, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2007, 9, 125–1128 CrossRef PubMed
; (b) R. Ballini, G. Bosica, D. Fiorini and A. Palmieri, Green Chem., 2005, 7, 825–827 RSC
; (c) J. W. Xie, L. P. Fan, H. Su, X. S. Li and D. C. Xu, Org. Biomol. Chem., 2010, 8, 2117–2122 RSC
; (d) Z. W. Guo, X. S. Li, W. D. Zhu and J. W. Xie, Eur. J. Org. Chem., 2012, 6924–6932 CrossRef CAS
; (e) L. L. Zhang, J. Sun and C. G. Yan, Chin. J. Chem., 2013, 31, 1546–1550 CrossRef CAS
; (f) Y. Jia and D. M. Du, RSC Adv., 2013, 3, 1970–1975 RSC
; (g) Z. K. Fu, J. Y. Pan, D. C. Xu and J. W. Xie, RSC Adv., 2014, 4, 51548–51557 RSC
; (h) J. Fang and C. G. Yan, J. Heterocycl. Chem., 2016, 53, 800–805 CrossRef CAS
.
- J. Fang and C. G. Yan, Mol. Diversity, 2014, 18, 91–99 CrossRef CAS PubMed
.
-
(a) C. G. Yan, X. K. Song, Q. F. Wang, J. Sun, U. Siemeling and C. Bruhn, Chem. Commun., 2008, 1440–1442 RSC
; (b) Q. F. Wang, X. K. Song, J. Chen and C. G. Yan, J. Comb. Chem., 2009, 11, 1007–1010 CrossRef CAS PubMed
; (c) Q. F. Wang, H. Hou, L. Hui and C. G. Yan, J. Org. Chem., 2009, 74, 7403–7406 CrossRef CAS PubMed
; (d) C. G. Yan, Q. F. Wang, X. K. Song and J. Sun, J. Org. Chem., 2009, 74, 710–718 CrossRef CAS PubMed
; (e) Q. F. Wang, L. Hui, H. Hou and C. G. Yan, J. Comb. Chem., 2010, 12, 260–265 CrossRef CAS PubMed
; (f) H. Hou, Y. Zhang and C. G. Yan, Chem. Commun., 2012, 48, 4492–4494 RSC
; (g) L. Z. Liu, X. Y. Wang, J. Sun and C. G. Yan, Tetrahedron Lett., 2015, 56, 6711–6714 CrossRef
.
-
(a) L. Wu, J. Sun and C. G. Yan, Org. Biomol. Chem., 2012, 10, 9452–9463 RSC
; (b) Q. Fu and C. G. Yan, Tetrahedron, 2013, 69, 5841–5849 CrossRef CAS
; (c) L. J. Lu, Q. Fu, J. Sun and C. G. Yan, Tetrahedron, 2014, 70, 2537–2545 CrossRef CAS
; (d) G. L. Shen, J. Sun and C. G. Yan, Org. Biomol. Chem., 2015, 13, 10929–10938 RSC
; (e) L. J. Lu and C. G. Yan, Chin. J. Chem., 2015, 33, 1178–1188 CrossRef CAS
; (f) L. J. Lu and C. G. Yan, J. Heterocycl. Chem., 2015, 52, 1513–1517 CrossRef
; (g) G. L. Shen, J. Sun and C. G. Yan, RSC Adv., 2015, 5, 4475–4483 RSC
; (h) J. Sun, G. L. Shen, Y. Huang and C. G. Yan, Sci. Rep., 2017, 7, 41024 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data 3a (CCDC 1546830), 3e (CCDC 1546831), 3n (CCDC1546832), 3r (CCDC 1546833), 4d (CCDC 1546834), 5c (CCDC 1546835), 5e (CCDC 1546836), 5g (CCDC 1546837), and 5h (CCDC 1546838). CCDC 1546830–1546838. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra06548e |
| This journal is © The Royal Society of Chemistry 2017 |