Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
K3LaTe2O9:Er: a novel green up-conversion luminescence material†
Hong Wang ,
Xiumei Yin
,
Xiumei Yin ,
Mingming Xing
,
Mingming Xing ,
Yao Fu
,
Yao Fu ,
Ying Tian
,
Ying Tian *,
Xin Feng
*,
Xin Feng ,
Tao Jiang
,
Tao Jiang and
Xixian Luo
and
Xixian Luo *
*
Physics Department, Dalian Maritime University, Dalian, Liaoning 116026, PR China. E-mail: tianying15@hotmail.com; luoxixiandl@126.com; Fax: +86 411 84723626; Tel: +86 411 84723626
First published on 21st July 2017
Abstract
A novel green up-conversion luminescence material, K3LaTe2O9:Er, was synthesised via a solid-state reaction method. K3LaTe2O9:Er phosphors were characterised by X-ray diffraction, reflectance spectroscopy, Raman spectroscopy, photoluminescence spectroscopy, up-conversion spectroscopy and temperature sensing performance analysis. The diffraction pattern of the hexagonal K3LaTe2O9:0.02Er microcrystals was indexed with Miller indices and the lattice constants were a = b = 0.60636 ± 0.00018 nm, and c = 1.49543 ± 0.00037 nm. The photoluminescence under 380 nm excitation and the up-conversion luminescence under 980 and 1550 nm pumping were investigated. The influence of Er3+ ion concentration and excitation power on the luminescence properties of K3LaTe2O9:Er was also discussed. K3LaTe2O9:Er phosphor presented green down-shifting emission and up-conversion luminescence under 380, and 980 nm excitation and yellow–green up-conversion luminescence under 1550 nm pumping, respectively, and the red emission component was enhanced with the increment in excitation wavelength. The quenching concentration of Er3+ ions in K3LaTe2O9:Er was much higher than that in normal phosphors. This result can be attributed to the suppression of energy migration because the shortest (0.606 nm) and average distance (0.9720 nm) between Er3+ ions were significantly large in K3LaTe2O9. Therefore, the electric quadrupole–quadrupole interactions between Er3+ ions are the dominant energy transfer process in down-shift emission, and the UCL mechanism can be regarded as the excited state absorption in K3LaTe2O9:Er. Furthermore, the doping concentration of Er3+ ions influenced the temperature sensitivity of K3LaTe2O9:Er.
1. Introduction
Rare-earth (RE)-doped luminescence materials play an important role in illumination, display, temperature sensor, security and biomedicine.1–3 Selecting appropriate materials with excellent luminescence performance based on practical applications is important. In general, the luminescence properties of phosphors are regulated through the selection of matrix materials. Matrix materials can bond doped RE ions, and the symmetry and strength of the crystal field exert significant effects on the optical properties of RE ions. Moreover, the practical applications of matrix materials are largely dependent on their physicochemical properties.Tellurates with rich chemical structures and unique physical properties have attracted considerable attention. RE-doped tellurite glass with relatively low phonon energy, high reflection index, and excellent thermal stability has become a popular research topic.4–7 However, tellurate phosphors have seldom been reported. For instance, Sobczyk et al.8 studied the optical properties of Y2Te4O11:Sm3+. Zhang et al.9 investigated the structure and luminescence properties of Li3Y3Te2O12:Eu3+.
K3LaTe2O9 is a new quaternary tellurite material with medium phonon energy.10 In the present work, the diffraction pattern of K3LaTe2O9 microcrystals was indexed with Miller indices. The down-shifting luminescence (DSL, λem = 380 nm) and up-conversion luminescence (UCL, λem = 980 and 1550 nm) of K3LaTe2O9:Er were reported for the first time. In addition, the thermal quenching and temperature sensor of K3LaTe2O9:Er were investigated.
2. Experimental
2.1 Sample preparation
K3LaTe2O9:Er phosphors were synthesized via the solid-state reaction method. The starting raw materials La2O3 (99.99%), Er2O3 (99.99%), K2CO3 (A. R.), and TeO2 (A. R.) were weighed according to stoichiometric ratio. Secondly, the above-mentioned materials were thoroughly mixed and ground using an agate ball mill for 20 min. Finally, all samples were sintered at 650 °C for 5 h. K3LaTe2O9:xEr samples with different Er3+ concentrations were then obtained (x = 0, 2, 8, 14, 20, 26, 32, 38, 44 mol%).2.2 Characterisation technique
X-ray powder diffraction was performed at 40 kV and 40 mA from 10° to 70° using a SHIMADZU-6000 X-ray generator with Cu Kα (λ = 0.154184 nm) radiation. The DSL and UCL spectra (400 nm to 900 nm) were recorded on a Hitachi F-4600 fluorescence spectrophotometer equipped with a power-tunable 980 and 1550 nm fibre laser diode (LD). The highest available power for the LD was approximately 800 and 700 mW, respectively. The beam of the LD was focused by the convex lens before measurement. The temperature dependence of the DSL spectra was tested by a self-assembly temperature control system with a XMT-4000 programmable temperature controller. The reflection spectra of the samples were obtained with a UV-3600 SHIMADZU UV-Vis-NIR spectrophotometer. The maximum phonon energy of the K3LaTe2O9 host lattice was obtained by a micro-Raman spectroscope (Jobin Yvon HR800, excited by 633 nm He–Ne laser with a laser spot size of 1 μm2, in line mapping mode). The scanning electron microscope (SEM) and Energy Dispersive Spectrometer (EDS) of the sample were obtained by JEOL-6360LV field emission gun scanning electron microscopy. The X-ray photoelectron spectroscopy (XPS) spectrum of the sample was obtained by ESCALAB250 surface analysis system.3. Results and discussion
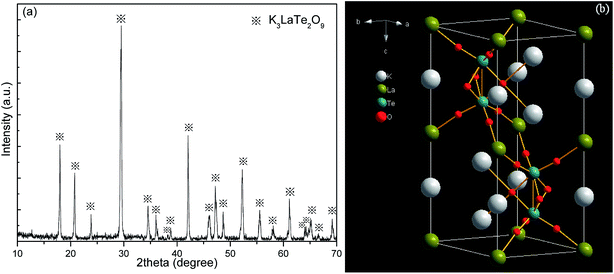
Fig. 1a shows the XRD pattern of the K3LaTe2O9:0.02Er sample. The diffraction peaks of the present sample are similar to those in ref. 10, indicating that the hexagonal K3LaTe2O9:0.02Er was synthesised. The micrograph and EDS of the K3LaTe2O9 crystal shown in Fig. S1.† The concentration of element in the K3LaTe2O crystal shown in Table S1.† The molar ratio of K, La, Te, and O element is 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.7 (Table S1†). The diffraction peaks in the XPS pattern are assigned to K+, O2−, Te6+, and La3+, respectively (Fig. S2†). The relative concentration values calculated by elemental sensitivity factor method of K, O, Te, and La atoms are 61.88, 18.47, 12.31, and 7.34%, respectively, which are close to the theoretical calculated values in the K3LaTe2O9. However, the standard PDF data of K3LaTe2O9:Er are not available, and ref. 10 did not provide the index data of K3LaTe2O9. The lattice parameters of K3LaTe2O9:0.02Er were calculated by the least square method in accordance with the data in Table S2,† and the crystal lattice parameters of the K3LaTe2O9:0.02Er sample were obtained: a = b = 0.60636 ± 0.00018 nm, c = 1.49543 ± 0.00037 nm, V = 0.47617 nm3. K3LaTe2O9 is a 3D framework structure (Fig. 1b). K atoms are two sites that coordinate with nine and twelve O atoms forming distorted cuboctahedra, respectively. Te atoms are linked to six O atoms to form [TeO6] octahedral, and two [TeO6] octahedral are connected to form a face-sharing [Te2O9]6− anion group. La atoms are surrounded by six O atoms in regular octahedra, and La3+ ions have a high-ordered state in the crystal lattice.
7.7 (Table S1†). The diffraction peaks in the XPS pattern are assigned to K+, O2−, Te6+, and La3+, respectively (Fig. S2†). The relative concentration values calculated by elemental sensitivity factor method of K, O, Te, and La atoms are 61.88, 18.47, 12.31, and 7.34%, respectively, which are close to the theoretical calculated values in the K3LaTe2O9. However, the standard PDF data of K3LaTe2O9:Er are not available, and ref. 10 did not provide the index data of K3LaTe2O9. The lattice parameters of K3LaTe2O9:0.02Er were calculated by the least square method in accordance with the data in Table S2,† and the crystal lattice parameters of the K3LaTe2O9:0.02Er sample were obtained: a = b = 0.60636 ± 0.00018 nm, c = 1.49543 ± 0.00037 nm, V = 0.47617 nm3. K3LaTe2O9 is a 3D framework structure (Fig. 1b). K atoms are two sites that coordinate with nine and twelve O atoms forming distorted cuboctahedra, respectively. Te atoms are linked to six O atoms to form [TeO6] octahedral, and two [TeO6] octahedral are connected to form a face-sharing [Te2O9]6− anion group. La atoms are surrounded by six O atoms in regular octahedra, and La3+ ions have a high-ordered state in the crystal lattice.
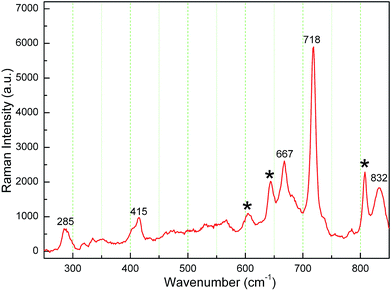
Fig. 2 shows the Raman spectrum of the K3LaTe2O9:0.02Er sample. The peaks situated at 285, 415, 667, 718, and 832 cm−1 corresponding to the K3LaTe2O9 host shown in ref. 10. Singh11 reported that the range of 500–580 cm−1, 620–680 cm−1, and 780–900 cm−1 are the characteristic peaks of Er3+ ion. Therefore, several peaks (remarked as *) are related to Er3+ ions. Some weak peaks in Raman spectrum are also observed due to defects in the lattice of K3LaTe2O9:Er.12 Raman peaks shift to the right side, which results from Er3+ doping. The maximum phonon energy of the K3LaTe2O9 host lattice is ℏω = 832 cm−1. Thus, K3LaTe2O9 with an appropriate phonon energy can be considered as an alternative for the luminescence host material.
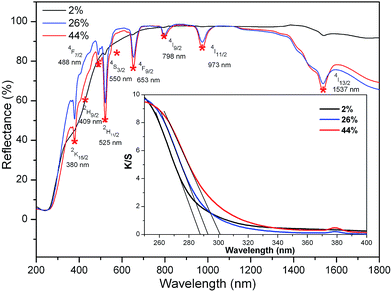
Fig. 3 shows the diffuse reflectance spectra of K3LaTe2O9:xEr (x = 2, 26, 44 mol%). As shown in the figure, the absorption peaks become stronger with increasing Er3+ ion concentration. The absorption peaks at 380, 409, 486, 525, 550, 653, 798, 973, and 1537 nm can be assigned to transition from an Er3+ ground state 5I15/2 to excited states 2K15/2, 2H9/2, 4F7/2, 2H11/2, 4S3/2, 4F9/2, 4I9/2, 4I11/2, and 4I13/2 levels. The 4I13/2 level of Er3+ ions has a strong absorption to 980 nm photons. The absorption efficiency of the 4I13/2 level of Er3+ ions to 1550 nm photons reached 89% of the 1537 nm. Therefore, Er3+ ions can be effectively sensitised by 1550 nm exciting light. The absorption edge of K3LaTe2O9:Er can be obtained in absorption spectra. The reflectance spectra of K3LaTe2O9:Er was converted into the absorption spectra based on the Kubelka–Munk formula13 (inset in Fig. 3):
| F(R) = (1 − R)2/2R = K/S, | (1) |
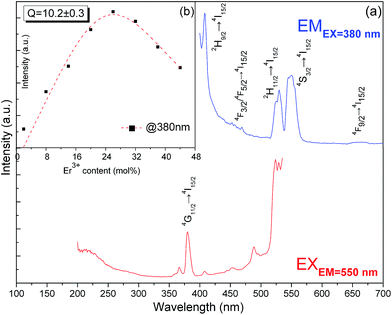
Fig. 4a shows the excitation and DSL spectra of K3LaTe2O9:Er. The excitation spectrum of K3LaTe2O9:Er presents a strong peak at ∼380 nm, which corresponds to the 4G11/2 → 4I15/2 transition by monitoring the 550 nm emission of Er3+ ions. K3LaTe2O9:Er under 380 nm excitation (K3LaTe2O9:Er at 380 nm) exhibits pure green emission (IG, 2H11/2/4S3/2 → 4I15/2), and the red irradiation (IR, 4F9/2 → 4I15/2) is very weak with the green and red fluorescence intensity ratio (IG/IR) IG/IR = 21.3. K3LaTe2O9:Er at 380 nm has a relatively strong peak at ∼410 nm, which is assigned to the 4H9/2 → 4I15/2 transition of Er3+ ions. In addition, the emission spectra present a weak band located at 450–470 nm, which corresponds to the 4F3/2/4F5/2 → 4I15/2 transition. The doping concentration of the optimum Er3+ ions is 26 mol% (Fig. 4b). The integrated intensity initially increases with increasing doping concentration, reaches its maximum at around 26 mol%, and then decreases. The quenching concentration is much higher than that of conventional materials.14 The relationship between the DSL intensity and doping concentration of the luminescence centre can be described by the Van Uitert model.15
| I(C) = C/κ(1 + βCQ/3), | (2) |
 | ||
| Fig. 4 Excitation and DSL spectra of K3LaTe2O9:Er (a) and dependence of 2H11/2/4S3/2 → 4I15/2 green DSL integrated intensity on Er3+ ion concentration (b). | ||
The shortest distance between La3+–La3+ ions is 0.606 nm in the K3LaTe2O9 matrix, which is significantly larger than that of Y3+–Y3+ in Y2O3 (0.351 nm). The average distance ![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) between doping Er3+ ions can be expressed as17
between doping Er3+ ions can be expressed as17
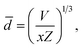 | (3) |
![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) = 0.9711 nm, which is also significantly larger than that in Gd2O2S:10% Er3+ (0.753 nm) and β-NaYF4:25% Er3+ (0.663 nm).17 Therefore, the shortest distance and average distance between Er3+ ions are large in the K3LaTe2O9 matrix. The energy migration is affected by matrix.18 The K3LaTe2O9 host can provide long lattice sites for Er3+ ions, which is helpful in obtaining heavy dopes and effectively reduces the harmful ET process. Moreover, it is beneficial in improving the absorption of incident light.
= 0.9711 nm, which is also significantly larger than that in Gd2O2S:10% Er3+ (0.753 nm) and β-NaYF4:25% Er3+ (0.663 nm).17 Therefore, the shortest distance and average distance between Er3+ ions are large in the K3LaTe2O9 matrix. The energy migration is affected by matrix.18 The K3LaTe2O9 host can provide long lattice sites for Er3+ ions, which is helpful in obtaining heavy dopes and effectively reduces the harmful ET process. Moreover, it is beneficial in improving the absorption of incident light.
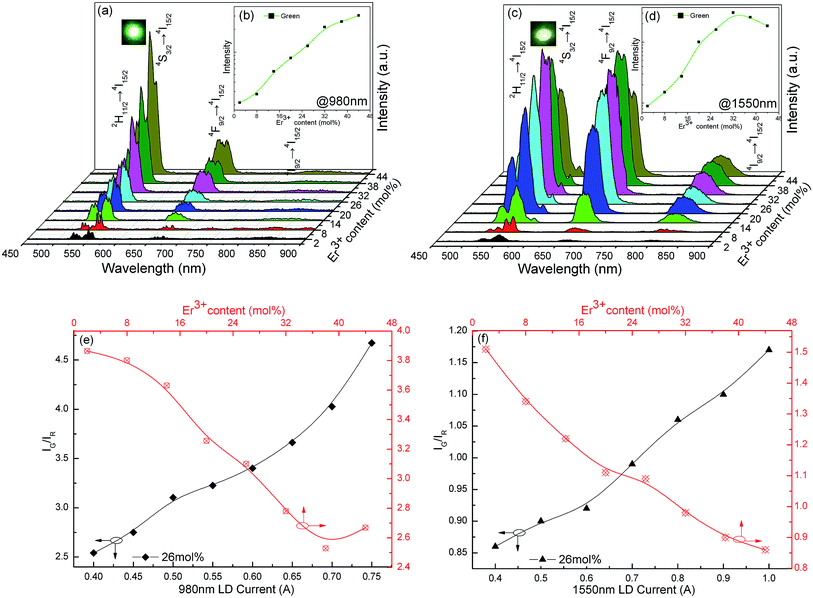
Under 980 nm pumping, K3LaTe2O9:Er presents the green UCL (K3LaTe2O9:Er at 980 nm) (inset of Fig. 5a). The UCL spectra for K3LaTe2O9:xEr at 980 nm are shown in Fig. 5a (x = 2, 8, 14, 20, 26, 32, 44 mol%). The spectra consist of basically two groups in the visual regions at an interval of 500–800 nm: (1) the strongest green emission at ∼525 nm and ∼550 nm can be attributed to the joint contributions of the Er3+ ion 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transition, and (2) the second strongest red emission at ∼665 nm can be attributed to the Er3+ ion 4F9/2 → 4I15/2 transition. The emission peaks intensity of K3LaTe2O9:Er at 980 nm are successively enhanced with increasing concentration of Er3+ ions. The concentration quenching phenomenon is not observed when the concentration of Er3+ ion reaches 44 mol%. Compared with the DSL spectra in Fig. 4a, the red emission is improved significantly, and its IG/IR ratio is reduced from 21.3 (DSL) to 2.5–4.7 (UCL, 980 nm). The luminescence colour can be tuned by the doping concentration of Er3+ ions and the excitation power under 980 nm excitation. As the Er3+ ion concentration continues to increase from 2 mol% to 38 mol%, the red light component is enhanced, and its IG/IR ratio is reduced from 3.9 to 2.7. By contrast, the green component can be effectively enhanced and the IG/IR ratio is increased from 2.5 to 4.7 with increasing excitation power (Fig. 5e). Under high excitation power pumping, more photons can be absorbed by Er3+ ions, which results in Er3+ ions populating high levels easily.
In addition, the dependence of luminescence-integrated intensity on the Er3+ ion concentration of K3LaTe2O9:Er at 980 nm is nearly linear when the concentration is low (2–26 mol%), which indicates that the UCL mechanism can be regarded as excited state absorption (ESA). This phenomenon is owed to the long distance between the RE ions in the K3LaTe2O9 structure resulting in the suppression of the ET.19 K3LaTe2O9:Er at 980 nm presents an approximate nonlinear relation when Er3+ > 26 mol%, indicating that the ET between Er3+ ion pairs also plays an important role in the UC process (Fig. 5b).
K3LaTe2O9:Er exhibits yellow–green emission under 1550 excitation (K3LaTe2O9:Er at 1550 nm) (inset of Fig. 5b). The UCL spectra in the visible region of K3LaTe2O9:Er at 1550 nm have the same peak positions and shape with different relative intensities compared with those at 980 nm excitation. However, the red emission intensity is significantly improved excited by 1550 nm, and its IG/IR ratio is reduced to 0.86–1.17. Whilst a relatively strong NIR emission peak at ∼800 nm is observed, the optimum doping concentration of Er3+ ions is 32 mol% at 1550 nm pumping (Fig. 5c). When the Er3+ ion concentration is increased from 2 mol% to 44 mol%, the IG/IR ratio is reduced from 1.5 to 0.86. On the contrary, the green emission can be significantly enhanced, and the IG/IR ratio is increased from 0.86 to 1.17 with increasing excitation power (Fig. 5f). The UCL mechanism of K3LaTe2O9:Er at 1550 nm is mainly based on the ESA process when the concentration of Er3+ ions is less than 16 mol%. Subsequently, the ET process begins to dominate (Er3+ > 16 mol%, Fig. 5d). Compared with DSL, the red component of K3LaTe2O9:Er is increased, particularly under 1550 nm pumping. However, the red UCL mechanism is still controversial.20,21
The non-radiation multiphonon relaxation rate (ωP) between the energy levels of RE ions can be expressed using the Miyakawa–Dexter theory:22,23
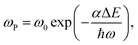 | (4) |
The energy gaps of 4S3/2–4F9/2 and 4I11/2–4I13/2 are ΔE ≈ 2980 and 3400 cm−1, corresponding to ΔE/ℏω = 3.6 and 4.1. The strong green emission (2H11/2/4S3/2 → 4I15/2) and almost no red emission (4F9/2 → 4I15/2) of K3LaTe2O9:Er at 380 nm are observed directly excited at 380 nm, indicating that the non-radiative relaxation 4S3/2 → 4F9/2 is weak (Fig. 4a). However, the non-radiative relaxation probability of 4I11/2 → 4I13/2 is smaller than that of the 4S3/2 → 4F9/2, that is, the 4S3/2 → 4F9/2 non-radiative relaxation process is not the main reason for the red UCL in K3LaTe2O9:Er at 980 nm. Therefore, the mechanism of red intensity enhancement can only be the cross relaxation (CR) between Er3+ ions. The red emission intensity is very weak even at high Er3+ concentrations for DSL because the CR between Er3+ ions is restrained in K3LaTe2O9 owing to the joint contributions of the long distance between the RE ions and the lack of intermediate metastable energy level below the 4S3/2 level. However, the long-level lifetime of the intermediate levels is inspired, and the CR processes significantly enhance the red emission in the UCL compared with DSL. The short-level lifetime of the 4I9/2 level and the small energy gap of 4I9/2–4I11/2 (ΔE ≈ 1950 cm−1, ΔE/ℏω = 2.3) are the major factors influencing the red radiation enhancement of K3LaTe2O9:Er at 1550 nm.24,25 Therefore, the difference in UCL spectra and quenching concentration for DSL and UCL results mainly from the different energy level population of the Er3+ ions caused by the different excitation routes.
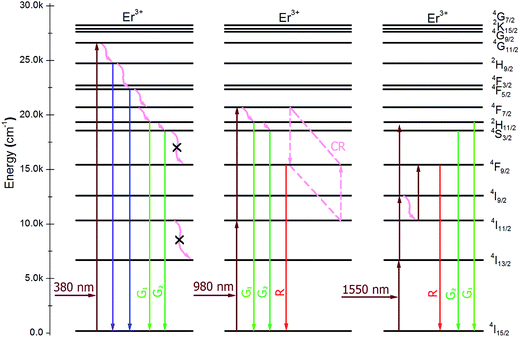
The different optimum Er3+ concentrations of K3LaTe2O9 under various wavelengths pumping is caused by the different excitation paths, level lifetime, and absorption cross section of levels. The transition model of the Er3+ ions in the K3LaTe2O9 phosphor excited at 380, 980, and 1550 nm is established to describe the luminescence process in Fig. 6. Red emission (4F9/2 → 4I15/2) of Er3+ ions was very weak in the DSL spectrum under 380 nm pumping (Fig. 4a). The green UCL process for K3LaTe2O9:Er at 380 nm is as follows: 4I15/2 + hν980 nm → 4G11/2, 4G11/2 → 4H9/2 + multiphonon relaxation + hν410 nm, 4H9/2 → 4F3/2/4F5/2 + multiphonon relaxation + hν450–470 nm, 4F3/2/4F5/2 → 2H11/2/4S3/2 + multiphonon relaxation, 2H11/2/4S3/2 → 4I15/2 + hν525 nm/hν550 nm. The green UCL process for K3LaTe2O9:Er at 980 nm is as follows: 4I15/2 + hν980 nm → 2I11/2, 4I11/2 + hν980 nm → 4F7/2, 4F7/2 → 2H11/2/4S3/2 + multiphonon relaxation, and 2H11/2/4S3/2 → 4I15/2 + hν525 nm/hν550 nm. Its red UCL mechanism is the 4F7/2 + 4I11/2 → 4F9/2 + 4F9/2 CR process. The red and NIR intensity of K3LaTe2O9:Er at 1550 nm is significantly enhanced. The UCL mechanism is as follows: 4I15/2 + hν1550 nm → 2I13/2, 4I13/2 + hν1550 nm → 4I9/2, 4I9/2 + hν1550 nm → 2H11/2, 4I9/2 + multiphonon relaxation → 4I11/2, 4I11/2 + hν1550 nm → 4F9/2, and 4F9/2 → 4I15/2 + hν660 nm, 4I9/2 → 4I15/2 + hν800 nm.
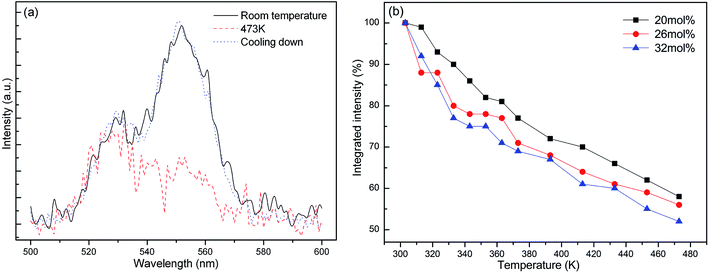
As shown in Fig. 7a, the K3LaTe2O9:0.26Er sample can nearly recover to its original intensity after cooling down to room temperature from 473 K, indicating that K3LaTe2O9:Er phosphors have a high resistance to heating damage by using a 380 nm xenon lamp as the excitation source. Fig. 7b shows the integrated intensities of K3LaTe2O9:xEr phosphors as a function of temperature. The integrated emission intensities are normalized at room temperature for K3LaTe2O9:xEr (x = 20, 26, 32 mol%). With the successive increase in the heating temperature, the green UCL intensity of different concentration samples gradually decreases. At 473 K, the integrated intensity of the K3LaTe2O9:xEr (x = 20, 26, 32 mol%) samples can retain 52%, 56%, and 58% at room temperature, respectively. Therefore, K3LaTe2O9:Er is a good matrix material to achieve a stable DSL. The higher Er3+ ion concentration, the more obvious is the thermal quenching phenomenon of the sample. The thermal quenching mechanism of the luminescent material is usually different, containing ET, CR, and non-radiative transition.26 The integrated intensity curves for various content samples have a similar slope. Thus, the ET rates between Er3+ ions causing heating temperature are similar. In addition, the probability of 4S3/2 non-radiative relaxation to near-low energy level is small. Therefore, CR is mainly responsible for the luminescent thermal quenching.
According to Boltzmann's distribution, the intensity ratio (RHS) between the thermal equilibrium levels of 2H11/2 and 4S3/2, (IH/IS), can be expressed as
RHS = IH/IS = C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/κT), exp(−ΔE/κT),
| (5) |
| S = dR/dT = R(−ΔE/κT2), | (6) |
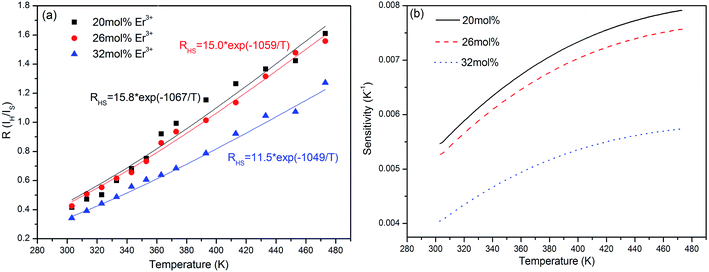 | ||
| Fig. 8 Relationships between 2H11/2/4S3/2 level green emission intensity radio and the heating temperature (a), and sensitivity curves of the K3LaTe2O9:xEr samples (b) (x = 20, 26, 32 mol%). | ||
In general, the luminescence intensity caused by doping concentration exerts a slight effect on sensitivity. However, in the present study, the temperature sensitivity of the K3LaTe2O9:Er samples decreases with increasing Er3+ ion concentration (Fig. 7b). This is due to that the change of the concentration of Er3+ ions may cause the change of the crystal field surrounding Er3+ ions, and the change of crystal field will cause the change of the optical transition rates of Er3+ ions. Therefore, the variation of radiation transition rate of 2H11/2 and 4S3/2 levels arouse the change of fluorescence intensity ratio FIR in different Er3+ ion doped samples and affect the sensitivity values. In addition, the thermal effects of samples is remarkable when concentration of doped Er3+ ions is higher.28 The temperature of samples has an effect on crystal field. The sensitivities of 20 and 26 mol% Er3+ ions are similar, corresponding to approximately 0.008 and 0.0075 K−1 at 473 K, respectively. When the Er3+ ion concentration reaches 32 mol%, the sensitivity decreases rapidly, and its value is nearly 0.0057 K−1 (Fig. 8b). Therefore, the low Er3+ content should be carefully chosen to obtain the optimal sensitivity performance. The previously reports on temperature sensitivities data were provided in Table S3.† On the basis of the experimental data in Fig. 8b, the K3LaTe2O9:Er material can be used as the luminescent thermometer for temperature sensing. In addition, the above-mentioned results show that the Er3+ ion concentration is important to the thermal quenching and sensitivity of K3LaTe2O9:Er phosphors.
4. Conclusion
A new green up-conversion luminescence material, K3LaTe2O9:Er, was synthesised. The diffraction pattern of the hexagonal K3LaTe2O9 crystal was indexed with Miller indices and the lattice constants a = b = 0.60636 ± 0.00018 nm, c = 1.49543 ± 0.00037 nm, and V = 0.47617 nm3. The DSL and UCL properties of K3LaTe2O9:Er were studied under 380, 980 and 1550 nm excitation. The shortest distance and average distance between Er3+ ions in the K3LaTe2O9 matrix are significantly larger than that in normal phosphors. Therefore, energy migration is significantly suppressed, which is helpful in obtaining heavy Er3+ doping.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 11504039 and 51502031), Liaoning Provincial Natural Science Foundation of China (No. 2015020206), and Research Program of Application Foundation (Main subject) of Ministry of Transport of PR China (No. 2015329225090). Fundamental Research Funds for the Central Universities (Grant Nos. 3132017061, 3132017066, and 3132016349) for their financial support. The authors thank Prof. Chang Liu for the XPS measurements.References
- H. Zheng, B. J. Chen, H. Q. Yu, J. S. Zhang, J. S. Sun, X. P. Li, M. Sun, B. N. Tian, H. Zhong and S. B. Fu, et al., Temperature sensing and optical heating in Er3+ single-doped and Er3+/Yb3+ codoped NaY(WO4)2 particles, RSC Adv., 2014, 4, 47556–47563 RSC.
- Y. H. Zhang, L. X. Zhang, R. R. Deng, J. Tian, Y. Zong, D. Y. Jin and X. G. Liu, Multicolor Barcoding in a Single Upconversion Crystal, J. Am. Chem. Soc., 2014, 136, 4893–4896 CrossRef CAS PubMed.
- H. Dong, L. D. Sun, Y. F. Wang, J. Ke, R. Si, J. W. Xiao, G. M. Lyu, S. Shi and C. H. Yan, Efficient Tailoring of Upconversion Selectivity by Engineering Local Structure of Lanthanides in NaxREF3+x nanocrystals, J. Am. Chem. Soc., 2015, 137, 6569–6576 CrossRef CAS PubMed.
- T. Sasikala, L. Rama Moorthy and A. Mohan Babu, Optical and luminescent properties of Sm3+ doped tellurite glasses, Spectrochim. Acta, Part A, 2013, 104, 445–450 CrossRef CAS PubMed.
- D. D. Yin, Y. W. Qi, S. X. Peng, S. C. Zheng, F. Chen, G. B. Yang, X. S. Wang and Y. X. Zhou, Er3+/Tm3+ codoped tellurite glass for blue upconversion-structure, thermal stability and spectroscopic properties, J. Lumin., 2014, 146, 141–149 CrossRef CAS.
- M. M. Xing, Y. B. Ma, X. X. Luo, Y. Fu, T. Jiang, H. Wang and X. L. Duan, Design and achieving of multicolor upconversion emission based on rare-earth doped tellurite glasses, J. Rare Earths, 2014, 32, 394–398 CrossRef CAS.
- G. Q. Chai, G. P. Dong, J. R. Qiu, Q. Y. Zhang and Z. M. Yang, 2.7 μm emission from transparent Er3+, Tm3+ codoped yttrium aluminum garnet (Y3Al5O12) nanocrystals–tellurate glass composites by novel comelting technology, J. Phys. Chem. C, 2012, 116, 19941–19950 CAS.
- M. Sobczyk and D. Szymanski, Optical properties of Sm3+-doped Y2Te4O11, J. Lumin., 2015, 166, 40–47 CrossRef CAS.
- W. S. Zhang and H. J. Seo, Luminescence and structure of a novel red-emitting phosphor Eu3+-doped tellurate garnet Li3Y3Te2O12, J. Alloys Compd., 2013, 553, 183–187 CrossRef CAS.
- X. Y. Zhang, J. Y. Yao, X. X. Jiang, Y. Fu, Z. S. Lin, C. C. Zhang and Y. C. Wu, K3LaTe2O9: a new alkali-rare earth tellurate with face-sharing TeO6 octahedra, Dalton Trans., 2015, 44, 15576–15582 RSC.
- B. P. Singh, A. K. Parchur and R. K. Singh, Structural and up-conversion properties of Er3+ and Yb3+ co-doped Y2Ti2O7 phosphors, Phys. Chem. Chem. Phys., 2013, 15, 3480–3489 RSC.
- A. N. Radhakrishnan, P. Prabhakar Rao, K. S. Mary Linsa, M. Deepa and P. Koshy, Influence of disorder to order transition on lattice thermal expansion and oxide ion conductivity in (CaxGd1−x)2(Zr1−xMx)2O7 pyrochlore solid solutions, Dalton Trans., 2011, 40, 3839–3848 RSC.
- P. Kubelka, New contributions to the optics of intensely light-scattering materials. Part I, J. Opt. Soc. Am., 1948, 38, 448–457 CrossRef CAS PubMed.
- Y. Tian, B. J. Chen, R. N. Hua, N. S. Yu, B. Q. Liu, J. S. Sun, L. H. Cheng, H. Y. Zhong, X. P. Li and J. S. Zhang, et al., Self-assembled 3D flower-shaped NaY(WO4)2:Eu3+ microarchitectures: microwave-assisted hydrothermal synthesis, growth mechanism and luminescent properties, CrystEngComm, 2012, 14, 1760–1769 RSC.
- L. G. Van Uitert, Characterization of energy transfer interactions between rare earth ions, J. Electrochem. Soc., 1967, 114, 1048–1053 CrossRef CAS.
- J. J. Li, J. S. Sun, J. T. Liu, X. P. Li, J. S. Zhang, Y. Tian, S. B. Fu, L. H. Cheng, H. Y. Zhong and H. P. Xia, et al., Pumping-route-dependent concentration quenching and temperature effect of green up- and down-conversion luminescence in Er3+/Yb3+ co-doped Gd2(WO4)3 phosphors, Mater. Res. Bull., 2013, 48, 2159–2165 CrossRef CAS.
- S. Fischer, B. Fröhlich, K. W. Krämer and J. C. Goldschmidt, Relation between Excitation Power Density and Er3+ Doping Yielding the Highest Absolute Upconversion Quantum Yield, J. Phys. Chem. C, 2014, 118, 30106–30114 CAS.
- G. H. Ju, Y. H. Hu, H. Y. Wu, Z. F. Yang, C. J. Fu, Z. F. Mu and F. W. Kang, A red-emitting heavy doped phosphor Li6Y(BO3)3:Eu3+ for white light-emitting diodes, Opt. Lett., 2011, 33, 1297–1301 CAS.
- X. M. Yin, H. Wang, M. M. Xing, Y. Fu, Y. Tian and X. X. Luo, Upconversion luminescence of Y2Ti2O7:Er3+ under the 1550 and 980 nm excitation, J. Rare Earths, 2017, 35, 230–234 CrossRef CAS.
- R. B. Anderson, S. J. Smith, P. S. May and M. T. Berry, Revisiting the NIR-to-Visible Upconversion Mechanism in β-NaYF4:Yb3+,Er3+, J. Phys. Chem. Lett., 2014, 5, 36–42 CrossRef CAS PubMed.
- M. T. Berry and P. S. May, Disputed Mechanism for NIR-to-Red Upconversion Luminescence in NaYF4:Yb3+,Er3+, J. Phys. Chem. A, 2015, 119, 9805–9811 CrossRef CAS PubMed.
- L. A. Riseberg and H. W. Moos, Multiphonon orbit-lattice relaxation of excited stated states of rare-earth ions in crystals, Phys. Rev., 1968, 174, 429–438 CrossRef CAS.
- J. M. F. van Dijk and M. F. H. Schuurmans, On the nonradiative and radiative decay rates and a modified exponential energy gap law for 4f–4f transitions in rare-earth ions, J. Chem. Phys., 1983, 78, 5317 CrossRef CAS.
- X. L. Shen, M. M. Xing, Y. Tian, Y. Fu, Y. Peng and X. X. Luo, Upconversion photoluminescence properties of SrY2O4:Er3+,Yb3+ under 1550 and 980 nm excitation, J. Rare Earths, 2016, 34, 458–463 CrossRef CAS.
- W. G. Yu, Y. Tian, M. M. Xing, Y. Fu, H. Zhang and X. X. Luo, Up-conversion luminescence of NaY(WO4)2:Yb,Er under 1550 and 980 nm excitation, Mater. Res. Bull., 2016, 80, 223–229 CrossRef CAS.
- B. N. Tian, B. J. Chen, Y. Tian, J. S. Sun, X. P. Li, J. S. Zhang, H. Y. Zhong, L. H. Cheng, Z. L. Wu and R. N. Hua, Ceram. Int., 2012, 38, 3537 CrossRef CAS.
- X. M. Yin, H. Wang, M. M. Xing, Y. Fu, Y. Tian and X. X. Luo, Up-conversion luminescence properties and thermal effects of LaVO4:Er3+ under 1550 nm excitation, Mater. Res. Bull., 2017, 86, 228–233 CrossRef CAS.
- H. Wang, X. M. Yin, M. M. Xing, Y. Fu, Y. Tian, X. Feng, T. Jiang and X. X. Luo, Investigation on the thermal effects of NaYF4:Er under 1550 nm irradiation, Phys. Chem. Chem. Phys., 2017, 19, 8465–8470 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06191a |
| This journal is © The Royal Society of Chemistry 2017 |