Open Access Article
Open Access ArticleGraphene oxide scroll meshes encapsulated Ag nanoparticles for humidity sensing†
Yang Liu,
Lin Wang,
Hao Zhang,
Feirong Ran,
Peng Yang and
Hai Li *
*
Key Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (Nanjing Tech), 30 South PuZhu Road, Nanjing 211816, China. E-mail: iamhli@njtech.edu.cn
First published on 16th August 2017
Abstract
Ag nanoparticles were firstly encapsulated into graphene oxide (GO) scrolls to form GO–Ag scrolls by a molecular combing method. Horizontally and perpendicularly aligned GO–Ag scrolls were then crossly stacked by a dry transfer method to form GO–Ag scroll meshes. After being reduced by hydrazine, the device based on reduced GO–Ag (rGO–Ag) scroll meshes shows 3 orders of magnitude higher response in humidity sensing compared to that of reduced GO (rGO) scroll meshes. In addition, rGO–Ag scroll meshes-based device shows good humidity sensing stability in ambient conditions over a month.
Graphene nanoscroll (GNS), a wrapped graphene sheet with a tubular structure, has been receiving increasing interest in the fields of hydrogen storage, supercapacitors, batteries, and nanodevices.1–12 Several methods have been reported to fabricate GNS, such as scrolling of the mechanically exfoliated graphene sheets with the aid of isopropyl alcohol, sonication of graphite intercalation compounds, microwave-assisted scrolling in ethanol or liquid nitrogen, and Langmuir–Blodgett compression of functionalized graphene oxide single sheets.3,5–7,10,13–15 In order to further improve the performance, various nanomaterials have been encapsulated into GNS, including Pt nanoparticles (NPs), Au NPs, Fe3O4 NPs, Si NPs, TiO2 nanowires (NWs), and phosphor.1,5,7–10,16,17 Due to their highest electrical conductivities, antibacterial properties and excellent catalytic properties, Ag NPs have been extensively explored in various chemical, physical and biological processes.18–21 Therefore it is interesting to encapsulate Ag NPs into GNS to enhance performance while preserving the excellent properties of GNS.
Nowadays, developing highly sensitive sensors for detecting humidity with fast response is of great importance in various fields, including, environmental monitoring, food quality control, and medical industry.22–35 There are increased demands for developing highly sensitive, economical, fast and stable humidity sensors to detect wide range of humidity. Over the past years, various humidity sensors have been developed based on diverse techniques, such as capacitive,22,23 resistive,24–26,34 optical fiber,27,28 field effect transistor,29,30 and surface acoustic wave.31–33 Among them, resistive humidity sensors are most widely used owing to their cost-effective fabrication, simple structures and high compatibility. Currently, many materials have been used for highly sensitive humidity detection, such as WS2,36–38 MoS2,39,40 zinc oxide,41,42 aluminium oxide,23,43 and organic materials.44,45 Recently, graphene oxide and its composite have been widely reported for humidity sensing with good repeatability and response.22,26,46–51 However, the stability and sensitivity of humidity sensors are still in concern for practical applications. Therefore, it is highly desirable to develop sensitive humidity sensors with long term stability.
Recently, GO scroll meshes were fabricated by cross-stacking GO scroll in combination of molecular combing and dry transfer technique.6,11,12,52 We found that GO scroll meshes can be used as transparent and flexible electrodes and show excellent electrical stability after being bent more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In this work, Ag NPs were firstly encapsulated into GO scroll to form GO–Ag scroll by molecular combing. Atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) characterization indicate Ag NPs were uniformly encapsulated into GO scroll. GO–Ag scroll meshes with size up to cm scale were then prepared by dry transfer method. After being reduced by hydrazine, reduced GO–Ag (rGO–Ag) scroll meshes were used for humidity sensing. Compared to rGO scroll meshes, rGO–Ag scroll meshes show much better linear response for humidity.
000 cycles. In this work, Ag NPs were firstly encapsulated into GO scroll to form GO–Ag scroll by molecular combing. Atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) characterization indicate Ag NPs were uniformly encapsulated into GO scroll. GO–Ag scroll meshes with size up to cm scale were then prepared by dry transfer method. After being reduced by hydrazine, reduced GO–Ag (rGO–Ag) scroll meshes were used for humidity sensing. Compared to rGO scroll meshes, rGO–Ag scroll meshes show much better linear response for humidity.
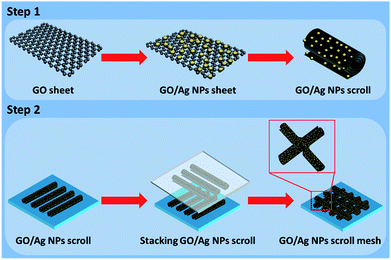
As shown in Scheme 1, Ag NPs were deposited on GO (GO–Ag) sheet by hydrothermal method. Then GO–Ag sheets were transformed into GO–Ag scrolls array by molecular combing (step 1). GO–Ag scroll meshes were fabricated through crossly stacking GO–Ag scrolls array by dry transfer method (step 2).
AFM was firstly used to characterize GO and GO–Ag sheets. As shown in Fig. 1A, the height of GO sheet is 1.08 nm, indicating it is single layer GO. After Ag NPs were deposited on GO sheet, the height of GO–Ag sheet is increased up to ∼6 nm (Fig. 1B). As shown in Fig. 1C, Ag NPs were uniformly distributed on GO sheet. The mean height of Ag NPs is 6.25 ± 0.5 nm (Fig. 1D). UV-vis spectrometer has been utilized to characterize as-prepared GO and GO–Ag sheets. Two characteristic peaks were observed in the UV-vis spectrum of GO sheet (Fig. S1 in ESI†). The peak at 232 nm is attributed to the π–π* transitions of the aromatic C–C bonds and a shoulder at 300 nm is derived from the n–π* transitions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.18,21 The formation of Ag NPs on GO sheet was confirmed by the strong peak around 400 nm, which is attributed to the surface plasmon resonance of Ag NPs, as shown in the UV-vis spectrum of GO–Ag nanocomposite (Fig. S1 in ESI†).
O bonds.18,21 The formation of Ag NPs on GO sheet was confirmed by the strong peak around 400 nm, which is attributed to the surface plasmon resonance of Ag NPs, as shown in the UV-vis spectrum of GO–Ag nanocomposite (Fig. S1 in ESI†).
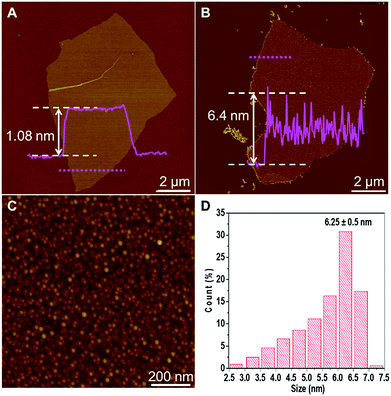 | ||
| Fig. 1 AFM images of (A) GO and (B) GO–Ag sheets, respectively. (C) Magnified image of (B). (D) Height distribution of Ag NPs. | ||
GO–Ag scroll was then prepared by molecular combing GO–Ag sheet on octadecyltrimethoxysilane-modified SiO2 (OTS-SiO2), which is hydrophobic.6 As shown in Fig. 2A, the height of GO–Ag scroll is ∼56 nm, which is a bit higher than that of GO scroll (Fig. S2 in ESI†). In addition, GO–Ag scroll usually has a width less than 1 μm. Ag NPs can be clearly observed in magnified AFM image (Fig. 2B). In order to further investigate the structure of GO–Ag scroll, SEM and TEM were employed. The magnified SEM and TEM images further confirm that dense Ag NPs were homogeneously distributed on GO surface (Fig. 2C–F and S3 in ESI†), which is in good agreement with AFM measurement, indicating successful encapsulation of Ag NPs into GO scroll.
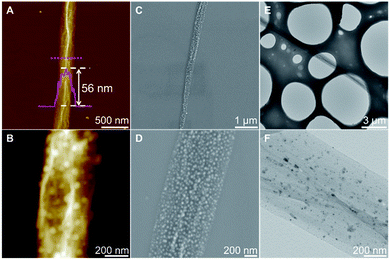 | ||
| Fig. 2 (A) AFM, (C) SEM and (E) TEM images of GO–Ag scroll, respectively. (B, D and F) Magnified images of (A), (C) and (E), respectively. | ||
GO–Ag scroll meshes were fabricated by using dry transfer method as previously reported.11 Firstly, horizontally and perpendicularly aligned GO–Ag scrolls arrays were prepared using molecular combing method (Fig. S4 in ESI†), respectively. We found that the concentration of GO–Ag solution plays an important role in determining the morphology of GO–Ag scroll. Long straight GO–Ag scrolls were observed on OTS-SiO2 when concentration is less than 0.1 mg mL−1, while dendritic GO–Ag scrolls were prepared as the concentration is higher than 0.1 mg mL−1 (Fig. S5 in ESI†). Then horizontally aligned GO–Ag scrolls array was lift off from substrate with the aid of PMMA and PDMS (see Experimental section in ESI†). The lifted horizontally aligned GO–Ag scrolls array was then stacked on perpendicularly aligned GO–Ag scrolls array. After PDMS and PMMA film were removed, GO–Ag scroll meshes with size up to cm scale was formed. Similarly, GO scroll meshes with size up to cm scale was also prepared by this method.
It is known that encapsulating nanomaterials into GNSs can further improve their performance in the fields of supercapacitor, lithium-ion battery and sensor.2–6,53,54 Herein, we tried to investigate the performance of GO–Ag scroll meshes on humidity sensing.
After GO and GO–Ag scroll meshes were reduced by hydrazine at 80 °C for 24 h, Ag paste was deposited on two corners of reduced GO (rGO) and GO–Ag (rGO–Ag) scroll meshes as electrodes to obtain devices. Fig. 3a shows the photograph of rGO–Ag scroll meshes-based device on SiO2/Si substrate. Magnified optical microscopy images show that GO–Ag scrolls were well cross-linked (Fig. 3b and c). The conductivities of GO and GO–Ag scroll meshes-based devices were firstly checked by measuring their I–V characteristics. By applying voltage from 0 to 0.5 V, both rGO and rGO–Ag scroll meshes-based devices showed linear relationship, indicating the well-ohmic contact formed between the electrode and the mesh (Fig. S6 in ESI†). Moreover, the slope of rGO–Ag scroll meshes-based device was larger compared to that of rGO scroll meshes-based device, indicating the encapsulation of Ag NPs can increase the conductivity of GO scroll meshes.
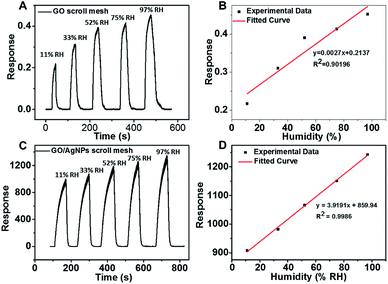
The humidity sensing measurement on rGO and rGO–Ag scroll meshes-based devices was carried out at room temperature. The switching RH test is performed through exposure/recovery cycles at 11%, 33%, 52%, 75% and 97% relative humidity (RH), respectively. The response of the sensors was defined as: response = IRH − IN2/IN2,39,54,55 where IRH and IN2 are the currents through the device in presence of different humidity and dried N2, respectively. The current through the device in presence of ambient air acts as the baseline. Each humidity response is carried out by an exposure interval of 60 s followed by purging with compressed dry N2 to achieve a stable baseline. Fig. 4A shows the real-time response of the rGO scroll meshes-based device exposed to different RH. Although the response keeps increasing as the humidity increased, the response value is too small for practical application. Fig. 4B shows the relationship between the response and relative humidity of rGO scroll meshes-based device. The linear regression coefficient, R2, of rGO scroll meshes-based device is only 0.902. The response time and recovery time of rGO scroll meshes-based device are ∼15 s and ∼18 s, respectively. As shown in Fig. 4C, the rGO–Ag scroll meshes sensor exhibited quite excellent response towards the humidity in a range of 11–97% RH. The response of rGO–Ag scroll meshes sensor reaches 908 even exposed to 11% RH, indicating its high sensitivity for humidity sensing. As the humidity increased from 11% to 97% RH, the response is also increased from 908 to 1243. In addition, the linear regression coefficient of the rGO–Ag scroll meshes sensor, R2, is 0.9986 (Fig. 4D), indicating its good linear behaviour for humidity sensing. The response time and recovery time of rGO–Ag scroll meshes-based device are ∼50 s and ∼13 s, respectively. Based on aforementioned results, it is obvious that the rGO–Ag scroll meshes sensor showed not only excellent response but also good linear behaviour for humidity sensing compared to those of rGO scroll meshes sensor. Table 1 shows the humidity sensing performance of our rGO–Ag scroll meshes sensor in comparison with previously reported works based on graphene oxide and its composite. It indicates that rGO–Ag scroll meshes are promising for humidity sensing.
| Sensing material | Sensor type | Humidity | Response | Ref. |
|---|---|---|---|---|
| Graphene oxide | Capacitive | 0–100% RH | 7680 PF/%RH | 22 |
| GO/PDDA | Resistive | 11–97% RH | 8.69–37.43% | 24 |
| GO/MoS2 | Resistive | 35–85% RH | 3–1600 | 25 |
| GO silicon bi-layer | Stress | 10–98% RH | 28.02 μV/%RH | 46 |
| Defect graphene | Capacitive | 3–30% RH | 0.27–3.3% | 49 |
| Graphene oxide | Impedance | 11–95% RH | 0.9–33.254% | 54 |
| rGO–Ag scroll meshes | Resistive | 11–97% RH | 908–1243 | This work |
In order to demonstrate the different sensitivities of rGO and rGO–Ag scroll meshes, the response curves of rGO and rGO–Ag scroll meshes-based devices towards same relative humidity were measured (Fig. S6 in ESI†). Both rGO and rGO–Ag scroll meshes exhibited immediate response towards humid air. However, rGO–Ag scroll meshes-based device exhibits 3 orders of magnitude higher than that of rGO scroll meshes-based device (Fig. 4A, C and S6 in ESI†). The excellent sensitivity of rGO–Ag scroll meshes can be attributed to the encapsulation of Ag nanoparticles, which enhances the conductivity of rGO–Ag scroll meshes and further contributes to the good current change before and after being exposed to humid air.
Interestingly, our rGO–Ag scroll meshes-based device also shows good long term stability in comparison with previously reported humidity sensors.51,56 After being reduced by hydrazine and exposed to humid air for the first cycle of humidity response measurement, the humidity sensing performance of rGO–Ag scroll meshes-based device was measured after being stored in ambient condition for 1, 5, 10, 20 and 30 days, respectively. As shown in Fig. 5, the response of the sensor almost has no degradation for humidity sensing measurement at 33%, 75% and 97% RH, implying the potential practical application of rGO–Ag scroll meshes in humidity sensing.
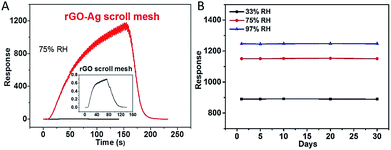 | ||
| Fig. 5 (A) Response contrast between rGO scroll mesh and rGO–Ag scroll mesh at 75% RH. (B) The stability of rGO–Ag scroll mesh at 33%, 75% and 97% RH, respectively. | ||
Conclusions
In conclusion, we have presented a facile one-pot, cost-effective method to synthesize rGO/AgNPs scroll meshes. The rGO/AgNPs scroll meshes, which take advantage of GO or rGO as a template was achieved without any other surfactant or reducing agent. The humidity sensing properties of rGO/AgNPs scroll meshes were investigated when exposed to varying RH at room temperature. Moreover, the sensor show excellent conductivity as current sensor and exhibit notable humidity sensor performance toward different humidity level with outstanding stability and linearity at ambient environment. In addition, the possible mechanism for humidity sensing of rGO/AgNPs was also investigated in this study. It is suggested that the humidity response of the present sensor is ascribed to cooperative effect of both the electron and ion conductivity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21571101 and 51528201), the Natural Science Foundation of Jiangsu Province in China (Grant No. BK20161543), and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 15KJB430016).Notes and references
- J. Zhao, B. Yang, Z. Zheng, J. Yang, Z. Yang, P. Zhang, W. Ren and X. Yan, ACS Appl. Mater. Interfaces, 2014, 6, 9890–9896 CAS.
- G. Mpourmpakis, E. Tylianakis and G. E. Froudakis, Nano Lett., 2007, 7, 1893–1897 CrossRef CAS PubMed.
- X. Xie, L. Ju, X. F. Feng, Y. H. Sun, R. F. Zhou, K. Liu, S. S. Fan, Q. Q. Li and K. L. Jiang, Nano Lett., 2009, 9, 2565–2570 CrossRef CAS PubMed.
- T. Fan, W. Zeng, Q. Niu, S. Tong, K. Cai, Y. Liu, W. Huang, Y. Min and A. J. Epstein, Nanoscale Res. Lett., 2015, 10, 192 CrossRef PubMed.
- M. V. Savoskin, V. N. Mochalin, A. P. Yaroshenko, N. I. Lazareva, T. E. Konstantinova, I. V. Barsukov and I. G. Prokofiev, Carbon, 2007, 45, 2797–2800 CrossRef CAS.
- H. Li, J. Wu, X. Y. Qi, Q. Y. He, C. Liusman, G. Lu, X. Z. Zhou and H. Zhang, Small, 2013, 9, 382–386 CrossRef CAS PubMed.
- Y. Gao, X. Q. Chen, H. Xu, Y. L. Zou, R. P. Gu, M. S. Xu, A. K. Y. Jen and H. Z. Chen, Carbon, 2010, 48, 4475–4482 CrossRef CAS.
- Y. Liu, Y. Xia, H. Yang, Y. Zhang, M. Zhao and G. Pan, Nanotechnology, 2013, 24, 235401 CrossRef PubMed.
- X. L. Li, Y. L. Zhang, T. T. Li, N. Z. Qi, H. Y. Li and J. M. Huang, J. Power Sources, 2014, 268, 372–378 CrossRef CAS.
- B. J. Yang, J. P. Zhao, J. T. Chen, M. He and S. Xu, RSC Adv., 2015, 5, 57906–57911 RSC.
- J. Wu, J. Yang, Y. Huang, H. Li, Z. X. Fan, J. Q. Liu, X. D. Cao, X. Huang, W. Huang and H. Zhang, Adv. Mater. Technol., 2017, 2, 1066231 Search PubMed.
- J. Wu, H. Li, X. Y. Qi, Q. Y. He, B. X. Xu and H. Zhang, Small, 2014, 10, 2239–2244 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- C. Gomez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499–3503 CrossRef CAS PubMed.
- M. S. Zhu, P. L. Chen and M. H. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS PubMed.
- J. Xu, S. Z. Gub and B. Lu, RSC Adv., 2015, 5, 72046–72050 RSC.
- Y. L. Li, X. J. Zhao, P. P. Zhang, J. Ning, J. F. Li, Z. Q. Su and G. Wei, J. Mater. Chem. C, 2015, 3, 4126–4133 RSC.
- X. Z. Zhou, X. Huang, X. Y. Qi, S. X. Wu, C. Xue, F. Y. C. Boey, Q. Y. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CAS.
- B. Adhikari and A. Banerjee, Mater. Chem. Phys., 2013, 139, 450–458 CrossRef CAS.
- K. S. Hui, K. N. Hui, D. A. Dinh, C. H. Tsang, Y. R. Cho, W. Zhou, X. T. Hong and H. H. Chun, Acta Mater., 2014, 64, 326–332 CrossRef CAS.
- R. Guo, W. Tang, C. T. Shen and X. Wang, Comput. Mater. Sci., 2016, 111, 289–293 CrossRef CAS.
- Y. Kim, B. Jung, H. Lee, H. Kim, K. Lee and H. Park, Sens. Actuators, B, 2009, 141, 441–446 CrossRef CAS.
- D. Z. Zhang, J. Tong and B. K. Xia, Sens. Actuators, B, 2014, 197, 66–72 CrossRef CAS.
- D. Burman, R. Ghosh, S. Santra and P. K. Guha, RSC Adv., 2016, 6, 57424–57433 RSC.
- S. Ghosh, R. Ghosh, P. K. Guha and T. K. Bhattacharyya, IEEE Trans. Nanotechnol., 2015, 14, 931–937 CrossRef CAS.
- S. Akita, H. Sasaki, K. Watanabe and A. Seiki, Sens. Actuators, B, 2010, 147, 385–391 CrossRef CAS.
- F. J. Arregui, Y. J. Liu, I. R. Matias and R. O. Claus, Sens. Actuators, B, 1999, 59, 54–59 CrossRef CAS.
- F. X. Liang, L. B. Luo, C. K. Tsang, L. X. Zheng, H. Cheng and Y. Y. Li, Mater. Res. Bull., 2012, 47, 54–58 CrossRef CAS.
- S. H. Song, H. H. Yang, C. H. Han, S. D. Ko, S. H. Lee and J. B. Yoon, Appl. Phys. Lett., 2012, 100, 101603 CrossRef.
- R. Rimeika, D. Čiplys, V. Poderys, R. Rotomskis and M. S. Shur, Sens. Actuators, B, 2009, 137, 592–596 CrossRef CAS.
- Y. Li, P. Li, M. Yang, S. Lei, Y. Chen and X. Guo, Sens. Actuators, B, 2010, 145, 516–520 CrossRef CAS.
- W. P. Xuan, M. He, N. Meng, X. L. He, W. B. Wang, J. K. Chen, T. J. Shi, T. Hasan, Z. Xu, Y. Xu and J. K. Luo, Sci. Rep., 2014, 4, 7206 CrossRef CAS PubMed.
- A. De Luca, S. Santra, R. Ghosh, S. Z. Ali, J. W. Gardner, P. K. Guha and F. Udrea, Nanoscale, 2016, 8, 4565–4572 RSC.
- S. Borini, R. White, D. Wei, M. Astley, S. Haque, E. Spigone, N. Harris, J. Kivioja and T. Ryhanen, ACS Nano, 2013, 7, 11166–11173 CrossRef CAS PubMed.
- Y. H. Luo, C. Y. Chen, K. Xia, S. H. Peng, H. Y. Guan, J. Y. Tang, H. U. Lu, J. H. Yu, J. Zhang, Y. Xiao and Z. Chen, Opt. Express, 2016, 24, 8956–8966 CrossRef CAS PubMed.
- H. Y. Guan, K. Xia, C. Y. Chen, Y. H. Luo, J. Y. Tang, H. H. Lu, J. H. Yu, J. Zhang, Y. C. Zhong and Z. Chen, Opt. Mater. Express, 2017, 7, 1686–1696 CrossRef.
- R. K. Jha and P. K. Guha, Nanotechnology, 2016, 27, 475503 CrossRef PubMed.
- D. Burman, R. Ghosh, S. Santra and P. K. Guha, RSC Adv., 2016, 6, 57424–57433 RSC.
- N. Li, X. D. Chen, X. P. Chen, X. Ding and X. Zhao, IEEE Electron Device Lett., 2017, 38, 806–809 CrossRef.
- Y. S. Zhang, K. Yu, D. S. Jiang, Z. Q. Zhu, H. R. Geng and L. Q. Luo, Appl. Surf. Sci., 2005, 242, 212–217 CrossRef CAS.
- S. P. Yawale, S. S. Yawale and A. T. Lamdhade, Sens. Actuators, A, 2007, 135, 388–393 CrossRef CAS.
- S. W. Chen, O. K. Khor, M. W. Liao and C. K. Chung, Sens. Actuators, B, 2014, 199, 384–388 CrossRef CAS.
- Y. D. Park, B. Kang, H. S. Lim, K. Cho, M. S. Kang and J. H. Cho, ACS Appl. Mater. Interfaces, 2013, 5, 8591–8596 CAS.
- Z. Ahmad, Q. Zafar, K. Sulaiman, R. Akram and K. S. Karimov, Sensors, 2013, 13, 3615–3624 CrossRef CAS PubMed.
- Y. Yao, X. D. Chen, H. H. Guo, Z. Q. Wu and X. Y. Li, Sens. Actuators, B, 2012, 161, 1053–1058 CrossRef CAS.
- N. Weiß, L. Müller-Meskamp, F. Selzer, L. Bormann, A. Eychmüller, K. Leo and N. Gaponik, RSC Adv., 2015, 5, 19659–19665 RSC.
- T. Nakajima, T. Nakamura and T. Tsuchiya, RSC Adv., 2016, 6, 95342–95348 RSC.
- Q. W. Huang, D. W. Zeng, S. Q. Tian and C. S. Xie, Mater. Lett., 2012, 83, 76–79 CrossRef CAS.
- Y. Liu and Y. M. Chen, J. Appl. Phys., 2016, 119, 103301–103306 CrossRef.
- B. H. Wee, W. H. Khoh, A. K. Sarker, C. H. Lee and J. D. Hong, Nanoscale, 2015, 7, 17805–17811 RSC.
- H. Li, J. M. T. Wu, X. Huang, Z. Y. Yin, J. Q. Liu and H. Zhang, ACS Nano, 2014, 8, 6563–6570 CrossRef PubMed.
- X. Chen, L. Li, X. Sun, H. G. Kia and H. Peng, Nanotechnology, 2012, 23, 055603 CrossRef PubMed.
- X. Y. Feng, W. F. Chen and L. F. Yan, Sens. Actuators, B, 2015, 215, 316–322 CrossRef CAS.
- J. F. Feng, X. X. Kang, Q. Y. Zuo, C. Yuan, W. J. Wang, Y. H. Zhao, L. M. Zhu, H. W. Lu and J. Y. Chen, Sensors, 2016, 16, 314 CrossRef PubMed.
- G. H. Lu, L. E. Ocola and J. H. Chen, Appl. Phys. Lett., 2009, 94, 083111 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06177c |
| This journal is © The Royal Society of Chemistry 2017 |