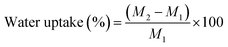Open Access Article
Open Access ArticleNovel benzimidazole-mediated phthalonitrile/epoxy binary blends system with synergistic curing behavior and outstanding thermal properties†
Jianghuai Hu,
Rui Sun,
Yihao Wu,
Jiangbo Lv,
Zhiping Wang,
Ke Zeng * and
Gang Yang*
* and
Gang Yang*
State Key Laboratory of Polymer Materials Engineering, College of Polymer Science and Engineering, Sichuan University, Chengdu 610065, P. R. China. E-mail: zk_ican@sina.com; yanggang65420@163.com; Fax: +86-28-85462736; Tel: +86-28-85462736
First published on 11th September 2017
Abstract
Binary blends composed of benzimidazole-containing phthalonitrile (PNBI) and epoxy resin E51 were prepared. The PNBI/E51 (PE) blends showed good compatibilities and unique synergistic curing behaviors on full consumption of nitrile groups (PE19, PNBI:E51 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in weight ratio) and epoxy groups in the view of DSC, rheology and IR. Curing procedures of the PE blends were determined by DSC and DMA. Studies on the curing procedure showed that the PE blends exhibited good processability and could be fully cured at a temperature lower than 210 °C. Studies on the cured PE blends showed that they have excellent thermal and mechanical properties. The glass transition temperatures (Tg) of cured PNBI/E51 blends were higher than 207 °C (obtained by DMA). The 5% weight loss (T5) and char yield at 800 °C (CR) were higher than 360 °C and 14% in nitrogen, respectively (obtained by TGA). Consequently, this study shows a novel modification in the method for the preparation of epoxy or phthalonitrile blend systems.
9 in weight ratio) and epoxy groups in the view of DSC, rheology and IR. Curing procedures of the PE blends were determined by DSC and DMA. Studies on the curing procedure showed that the PE blends exhibited good processability and could be fully cured at a temperature lower than 210 °C. Studies on the cured PE blends showed that they have excellent thermal and mechanical properties. The glass transition temperatures (Tg) of cured PNBI/E51 blends were higher than 207 °C (obtained by DMA). The 5% weight loss (T5) and char yield at 800 °C (CR) were higher than 360 °C and 14% in nitrogen, respectively (obtained by TGA). Consequently, this study shows a novel modification in the method for the preparation of epoxy or phthalonitrile blend systems.
1 Introduction
Because of their excellent chemical and corrosion resistance, good dimensional stability, high modulus and tensile strength, epoxy resins have been widely used in various fields such as electronic industries, high-performance composites and aerospace applications.1,2 However, conventional epoxy resins still have numerous defects such as low thermal properties and low Tg, which are unable to meet the requirements of advanced materials. Therefore, studies on improving thermal and mechanical performances of epoxy resins have become an important need.3,4 The approach to improve the thermal properties of epoxy resins could be divided into three categories: modifying skeletons of epoxy monomers or polymers, changing the curing agents and blending with other materials.2,4–6Phthalonitrile resins have been studied for over 30 years as a class of high temperature/performance polymers. Because of their excellent thermal and thermal oxidative stabilities, outstanding mechanical properties, and superior flame resistance, phthalonitrile resins have been used in numerous fields, such as electronic packaging applications, marine applications, and aerospace applications.7–9 However, high curing temperatures (>300 °C) and long curing durations limit their widespread applications.5,7–9
As discussed above, the study on the combination of the characteristics of these two types of resins will be a valuable research.5,10–13 Researchers have conducted various studies on this topic. Dominguez et al. reported a series of binary blends composed of phthalonitrile monomer and oligomer along with epoxy resin. The blends exhibited attractive combination of processability and high-temperature properties.10 Liu et al. reported a 4-aminophenoxyphthalonitrile/epoxy resin blend system that exhibited an attractive self-promoted curing reaction, desirable processing features, excellent thermal and thermal oxidative stabilities, and high char yields.13 Although these reports have inspired us to study the blend systems of phthalonitrile and epoxy, there are a few problems to be solved. Most of the reported curing agents of phthalonitrile/epoxy resin blends were aromatic amines, which are well-known as an important class of curing agents for epoxy resins. However, a large number of amino groups was consumed in the low-temperature curing stage. Thus, a low content of amino groups and low activity of curing agents are not sufficient to initiate the reaction of nitrile groups at low temperatures (lower than 200 °C). Therefore, the reaction of nitrile groups should be carried out at a higher temperature (usually higher than 300 °C). However, due to the relatively low thermal stability of epoxy resins,3,4 their curing reaction is usually carried out at temperatures below 200 °C. Thus, the reduction in curing temperatures of epoxy/phthalonitrile blend systems becomes a basic problem to be solved. The conversion of nitrile groups and the compatibility of the blends should also be taken into consideration.
In our previous study, a self-promoted benzimidazole-containing phthalonitrile (PNBI) was synthesized.14,15 However, the curing reaction of PNBI is a high temperature and sluggish process, which may be induced by the weak basicity of benzimidazole group.16 Simultaneously, imidazoles are an important class of medium or high-temperature curing agents for epoxy resins.17–19 It is well-known that the curing reactions of epoxy monomers with imidazole groups are exothermic producing a large amount of active intermediates (e.g. benzimidazolium cation and oxygen anion). If the acidity and basicity are considered to be important factors in determining the activity of the curing agents of phthalonitrile, the active intermediates may have higher catalytic activities for phthalonitrile, which would reduce the curing temperature and speed up the curing rate.16–19 Thus, high activity curing agents, which derive from the reaction of epoxy monomer and benzimidazole, could initiate the curing reaction of phthalonitrile at low temperatures. As discussed above, the study on PNBI/epoxy binary system may be significant considering its thermal properties and the reactivity of phthalonitrile.
In this study, to explore new solutions for phthalonitrile/epoxy blend systems, the novel PNBI/epoxy resin E51 (PE) binary blend systems were constructed. The PE binary blend systems exhibited a unique self-promoted synergistic curing behavior and showed good compatibility. The curing procedures of PE blends were determined by DSC and DMA. Studies on the curing procedure showed that the PE blends exhibited good processability and could be fully cured at a temperature lower than 210 °C. The systematic studies showed that the epoxy and phthalonitrile groups in the PE blend systems could be fully consumed at lower temperatures (≤210 °C) during the curing process, and the cured PNBI/EP blend system showed good processability, homogeneous structure, outstanding thermal properties and high glass transition temperatures (Tg).
2 Experimental
2.1 Materials
Low molecular weight bisphenol A liquid epoxy resin YN1828LA (E51, epoxy equivalent: 186–189 g mol−1) was bought from Jiangsu Yangnong Chemical Group Co., Ltd. Furthermore, 4-nitrophthalonitrile was purchased from Ji'nan Weido Chemical Co. Ltd. and recrystallized from ethanol. Dimethyl sulfoxide (DMSO), dicyclohexylcarbodiimide (DCC), potassium carbonate and tetrahydrofuran (THF) were purchased from Tianjin BoDi Co. Ltd. o-Phenylenediamine, 4-hydroxybenzoic acid and other chemicals were purchased from Chengdu Kelong Chemical Reagent Co. Ltd. and used as received.2.2 Synthesis of 4-(4-(1H-benzo[d]imidazol-2-yl)phenoxy)phthalonitrile (PNBI)
PNBI was synthesized according to reported literature.14,152.3 Preparation of the PNBI/E51 blends
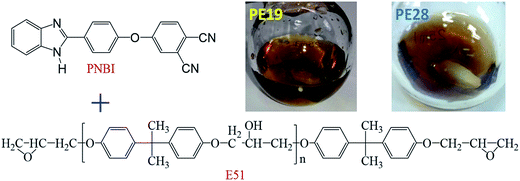
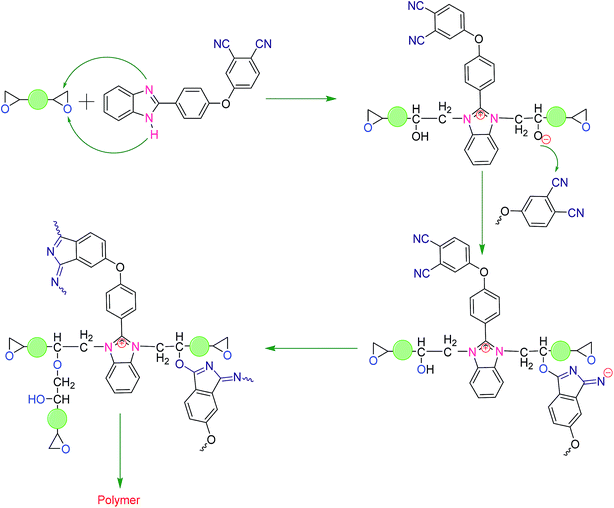
The PNBI/E51 blend (Scheme 1) was prepared by mixing a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 with PE19 and 2
9 with PE19 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 with PE28 in a flask. The mixture was stirred until it became homogeneous.
8 with PE28 in a flask. The mixture was stirred until it became homogeneous.
2.4 Preparation of the PNBI/E51 blend resins
Each PNBI/E51 blend (PE19 and PE28) was added into a single-neck bottle and heated at 95–100 °C until the mixture became a transparent brown solution (Scheme 1 and Fig. S1† (the mixture exhibited good storage stability)). After defoaming in vacuum for 10 min, the solution was poured into a hot mold (100 °C) and treated using a heating program in an oven under a nitrogen atmosphere.Heating program:
PE19: 100 °C/0.5 h, 130 °C/1 h, 150 °C/2 h, 180 °C/2 h, 210 °C/2 h.
PE28: 100 °C/0.5 h, 130 °C/1 h, 150 °C/2 h, 180 °C/2 h.
The abbreviation of cured products:
PE19-150: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h,
PE19-180: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h,
PE19-210: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h and 210 °C/2 h,
PE28-150: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h,
PE28-180: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h,
PE28-210: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h and 210 °C/2 h,
PE28-250: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h and 210 °C/2 h and 250 °C/2 h,
PE28-300: 100 °C/30 min and 130 °C/1 h and 150 °C/2 h and 180 °C/2 h and 210 °C/2 h and 250 °C/2 h and 280 °C/1 h.
2.5 Measurements
1H NMR (300 MHz) spectra were obtained with a Bruker Avance-300 NMR spectrometer, using DMSO-d6 as the solvent and tetramethylsilane (TMS) as the internal standard. FTIR spectra were obtained with a Nicolet FTIR-380 Fourier transform infrared spectrometer using KBr pellets. Thermogravimetric analysis (TGA) was carried out in the temperature range from 40 °C to 800 °C using a TA instrument Q500 thermogravimetric analyzer in nitrogen or air (60 mL min−1) at a heating rate of 10 °C min−1. Differential scanning calorimetry (DSC) was carried out using a TA Instrument Q200 Differential Scanning Calorimeter using nitrogen as the purge gas. Conventional DSC measurements were performed at a heating rate of 2 °C min−1, 5 °C min−1, 10 °C min−1, and 15 °C min−1. The rheological behaviors of the powder mixture of the PNBI/E51 blends were studied in dynamic oscillation mode using a TA Instruments AR-2000ex rheometer with a 25 mm diameter parallel plate in conjunction with an environmental testing chamber for temperature control. The tests were performed at a low strain value (2.5 × 10−4) and a frequency of 1 Hz. The samples were prepared by press-molding the powder mixture (approximately 0.45 g) at room temperature. The compacted sample disk was subsequently loaded into the rheometer fixture. The dynamic storage modulus of the mixture was also monitored at 100, 130 and 150 °C in air as a function of time, with a temperature ramp to acquire values at a rapid heating rate of 50 °C min−1, and equilibrated for 1 min. Dynamic mechanical analysis (DMA) was recorded on a TA instrument DMA Q800 at a heating rate of 5 °C min−1 with a load frequency of 1 Hz in air (specifications of blends: 2.5 mm thick, 12 mm wide and 30 mm long). The morphologies of PNBI/E51 were determined by a JEOL JSM-7500F field-emission scanning electron microscopy (SEM) system. The specimens were coated with Au prior to observation. For water uptake, samples of the cured resins were immersed in boiling distilled water at 96 °C for 45 hours. Then, the samples were removed from the boiling water, dried with filter paper, and weighed periodically to determine the amount of water absorbed.3 Results and discussion
3.1 Curing behaviors
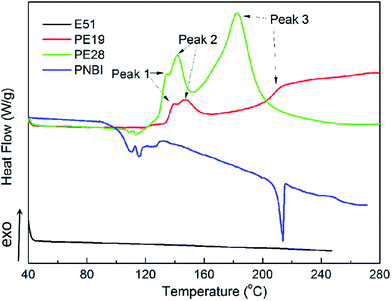
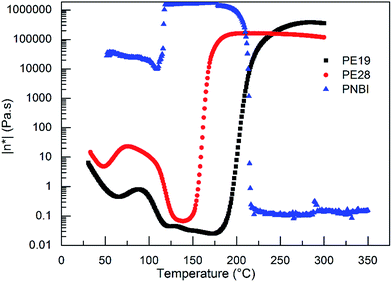
The TGA curves of PNBI, E51 and their blends (PE19 and PE28) are shown in Fig. 1. As shown in Fig. 1, the temperature at 1% weight loss (T1), 5% weight loss (T5), and at 10% weight loss (T10) are 211, 245 and 259 °C, respectively, indicating that the curing temperature of phthalonitrile should be limited to 210 °C. The thermal stabilities (T5, T10 and CR at 800 °C) of E51 significantly improved when a small amount PNBI was added. The thermal properties of E51 improved with the increase in content of PNBI. Furthermore, the T5, T10, and CR of PE28 improved by 110 °C, 116 °C, and 15%, respectively, compared with those of pure E51. TGA studies indicate that PNBI could promote the curing reaction and increase the thermal stability of E51 (see ESI Fig. S2†). In order to further study the interactions and reactions between PNBI and E51, the DSC, rheology and IR analyses were carried out to study the curing behaviors of PE19 and P28.The curing and rheological behaviors of PNBI, E51, PE19 and PE28 were studied using DSC and rheology techniques, as shown in Fig. 2 and 3, respectively. As found from Fig. 2, the melt peaks of PNBI could not be observed in the PNBI/E51 blends (PE19 and PE28), suggesting the good compatibility between PNBI and E51. Both PNBI/E51 blends exhibit three exothermic peaks around 140 °C (peak 1), 150 °C (peak 2) and above 180 °C (peak 3), which may be induced due to the polymerization of epoxy and phthalonitrile groups. All of the peaks shift to lower temperatures with increasing PNBI content, indicating that PNBI can efficiently promote the curing reaction of the PNBI/epoxy blends.
In order to further study the curing behaviors of the blends, a rheology measurement was carried out. As shown in Fig. 3, the viscosities of PE28 and PE19 increase rapidly when temperature is higher than 150 and 175 °C, respectively. This further confirms the occurrence of the curing reactions. The curing temperatures decrease with PNBI contents, confirming that PNBI could promote the curing reaction of the PNBI/epoxy blends. However, the reactive groups involved in the reaction are still uncertain. Therefore, the products obtained after the rheology tests were used to study their structures using IR analysis. The IR spectra of PE28 and the products obtained after the rheology tests carried out up to 300 °C (PE19 R and PE28 R) are presented in Fig. 4. As shown in Fig. 4, both of the peaks of nitrile (around 2230 cm−1) and epoxy group (915 cm−1) disappear in PE19 R and PE28 R. Moreover, the characteristic peaks around 1630 cm−1 are observed in PE19 R and PE28 R, which correspond to the formation of isoindoline. The complete consumption of nitrile group, which may be induced by the formation of isoindoline other than triazine ring, was rarely reported before.14,16,20,21 The complete consumption of nitrile group inspired us to study the preparation and the properties of PNBI/epoxy resins. In addition, whether the entire curing process can be controlled within 210 °C is a challenge worthy of study. Thus, the determination of curing procedures and the studies on the cured resins are presented in the following section.
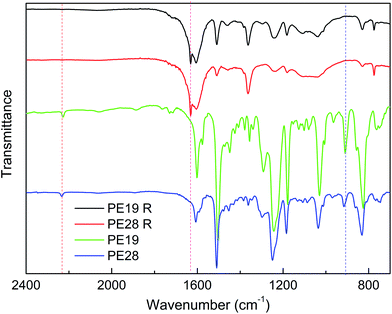 | ||
| Fig. 4 The IR spectra of PE19, PE28 and their cured products (PE19 R and PE28 R) obtained after the rheology tests. | ||
3.2 Determination of curing procedures
Curing temperature and curing time are key parameters, which affect the properties of the cured products. Thus, initially, DSC was introduced to determine the curing temperatures of PE19 and PE28. The DSC curves at different heating rates (2, 5, 10, and 15 °C min−1, β) of PE19 and PE28 are shown in Fig. 5a and b, respectively. From Fig. 5a and b, it can be clearly observed that the initial and the exothermic peak temperatures increase with the increase in heating rate. The initial curing temperatures (Ti) and the exothermic peak temperatures (peak 2, Tp) at different heating rates of PE19 and PE28 were determined and plotted in Fig. 5c. The plots of T–β in Fig. 5c were used to calculate the initial curing temperatures and the curing exothermic peak temperatures at β = 0 °C min−1 using linear fitting method.22 The Ti and Tp of PE19 and PE28 at β = 0 °C min−1 are obtained from Fig. 5c, which are 102.4 °C (PE19 Ti), 127.2 °C (PE19 Tp), 95.3 °C (PE28 Ti) and 115.1 °C (PE28 Tp). Based on the Ti and Tp obtained from Fig. 5c, 100 °C and 130 °C were preliminarily determined as the temperatures for the pre-polymerization stages. In order to further confirm the feasibility of these two pre-polymerization temperatures, the isothermal rheological measurements were applied to study the curing behaviors of the PNBI/E51 blends at 100 and 130 °C (Fig. 5d). From the plots in Fig. 5d, it is evident that the low melt viscosities of PE19 and PE28 could be kept for 125 min and 60 min, respectively, at 100 °C, which indicate the outstanding processability of the PE blends at this temperature. Simultaneously, the melt viscosity of PE19 increases rapidly after 25 min dwell-time at 130 °C, indicating its high activity at 130 °C. Thus, using 100 and 130 °C as the pre-polymerization temperatures is feasible. According to the rheological curves in Fig. 5d, the pre-polymerization procedures are divided into two isothermal steps: isothermal process at 100 °C for 30 min, and then an isothermal process at 130 °C for 1 h.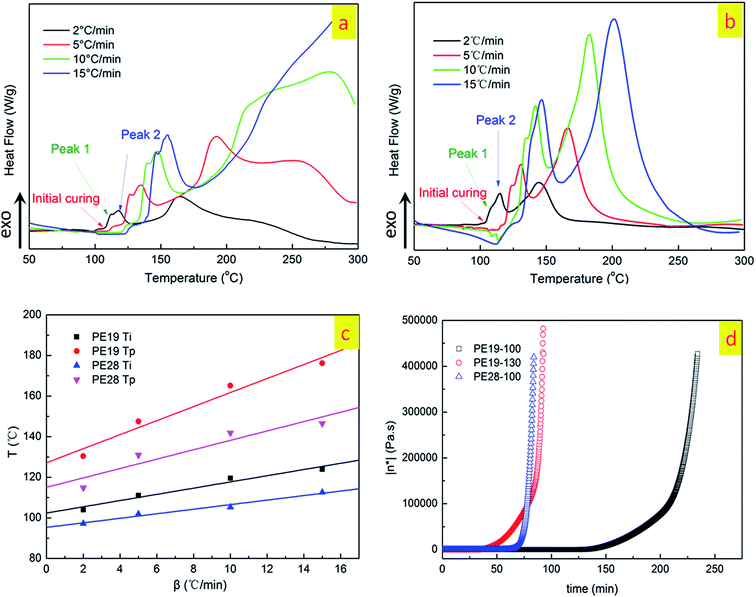 | ||
| Fig. 5 The DSC curves of PE19 (a) and PE28 (b) at different heating rates, the relationships of initial and peak temperatures and β (c), and isothermal rheology curves of PE19 and PE28 (d). | ||
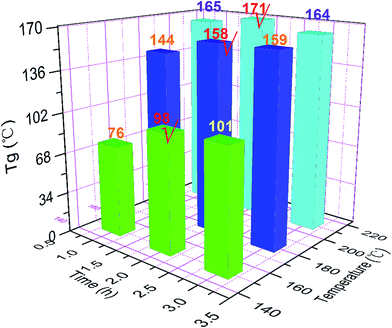
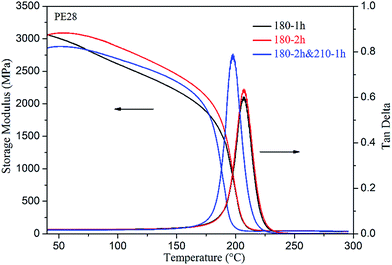
According to the temperature–viscosity curves of the PE blends in Fig. 3, we conclude that the PE blend can be cured at around 200 °C. Moreover, if the stability of epoxy groups is also considered, curing temperatures of the PE blends should be limited to the maximum temperature at 210 °C. Therefore, the subsequent curing temperatures are preliminarily determined as 150, 180 and 210 °C. In order to further determine the isothermal time for each curing temperature, DSC and DMA were applied to monitor the change in the glass transition temperatures of the PE blends. The studies on isothermal time for each temperature point will be presented for PE19 as an example. The pre-polymers of PE19, obtained by curing at 100 °C for 30 min and 130 °C for 1 h, were cured at 150 °C for 1, 2 and 3 hours, and the corresponding Tg (obtained by DSC, see ESI Fig. S3†) at each curing time was 76, 98 and 101 °C, respectively (Fig. 6). Considering that the Tg values are close to each other when the curing time is 2 h and 3 h, 2 h was determined as the curing time of PE19 at 150 °C. Likewise, the sample of 150 °C/2 h was applied to determine the curing time at 180 °C, and 2 h was determined as the curing time of PE19 at 180 °C (obtained by DSC, see Fig. 6 and ESI Fig. S4†). The pre-polymers of PE19, obtained by curing at 100 °C for 30 min and 130 °C for 1 h along with those obtained at 150 °C for 2 h and 180 °C for 2 h, were cured at 210 °C for 1, 2 and 3 hours and the Tg at each curing time was recorded as 165, 171 and 164 °C, respectively (obtained by DSC, see Fig. 6 and ESI Fig. S5†). The variation of Tg for different curing times at 210 °C indicates that the curing reaction and the degradation reaction may occur at the same time. This phenomenon further confirms that the curing temperatures of epoxy resins should be limited to the maximum temperature of about 210 °C. Therefore, 210 °C was determined as the final curing temperature for the curing reaction, and 2 h was selected as the curing time at 210 °C. The determination of curing procedure for PE28 is similar to that for PE19. However, because of the fact that Tg of PE28-180, cured at 210 °C, was lower than that of PE28-180 cured at 180 °C (obtained by DMA, see Fig. 7), 180 °C was determined as the final temperature of the curing reaction, and 2 h was determined as the curing time at 180 °C. As discussed above, the curing procedures of PE19 and PE28 were determined as follows: 100 °C/0.5 h, 130 °C/1 h, 150 °C/2 h, 180 °C/2 h, and 210 °C/2 h and 100 °C/0.5 h, 130 °C/1 h, 150 °C/2 h, and 180 °C/2 h, respectively.
The cured PE blends (PE19-210 and PE28-180) used for the structure and property characterizations were obtained by the curing procedures determined above.
3.3 Structures and morphologies of cured resins
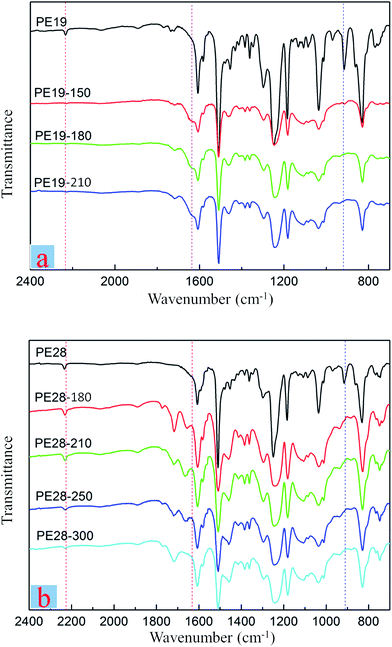
The FTIR spectra of PE19 and PE28 of each curing stage are shown in Fig. 8a and b. As shown in Fig. 8a, the nitrile peak around 2230 cm−1 of PE19 could be observed when the blends were heated at 150 °C (PE19-150). To the best of our knowledge, this is a very low temperature in the view of the reaction of nitrile groups in the resin systems, and the full consumption of nitrile groups in phthalonitriles is also rarely reported.5,10–14,20,21 This unique phenomenon indicates that few new high activity curing agents (e.g. oxygen anion) for phthalonitriles were generated during the curing reaction between epoxy monomer and benzimidazole. Moreover, the peak attributed to epoxy group (915 cm−1) disappeared in the spectra of cured PE blends, and a characteristic peak around 1630 cm−1 was observed, which corresponded to the formation of isoindoline.16,20,21 These results are consistent with the rheology studies (Fig. 3 and 4). Significantly, the nitrile peak is still evident in PE28-180, which indicates that the nitrile groups did not participate in the curing reaction on a large scale at a temperature below 180 °C. This phenomenon may be induced by the faster curing rates and the higher crosslinking density (higher Tg), which inhibited the further reaction of nitrile groups (the DSC curves in ESI Fig. S2–S6† further confirm this speculation. The Tg of PE28 of each curing stage is higher than the corresponding curing temperature, indicating that the curing reaction at 150 °C and 180 °C may occur at glass transition state. To further confirm this speculation, PE28-180 was cured at temperatures higher than the Tg of PE28-180 (210 °C, 250 °C and 300 °C). The obtained IR spectra are shown in Fig. 8b (PE28-210, PE28-250 and PE28-300). As shown in Fig. 8b, the peaks of nitrile groups began to weaken when the samples were cured at 250 °C. However, the peaks of nitrile groups did not completely disappear when the samples were cured at 300 °C, which may be due to the limited molecular motion. Despite the relatively high reactive temperatures of nitrile groups in PE28 (compared with PE19), the nitrile groups in PE28 possessed higher activities compared with those in pure PNBI,14,15 which further confirmed that some high activity curing agents for phthalonitriles were generated during the curing reaction between the epoxy monomer and benzimidazole.As discussed above, we believe that a synergistic curing between PNBI and epoxy resin occurred as follows: PNBI promoted the curing reaction of epoxy, and the products of the curing reaction of the epoxy monomer promoted the curing reaction of phthalonitrile. Based on the theory available and comparing with our obtained data,16,19 a synergistic curing mechanism between PNBI and epoxy group is proposed and presented in Scheme 2. Through the synergistic curing mechanism proposed, it is observed that the curing reaction of epoxy was initiated by benzimidazole, and then the products of epoxy ring opening (e.g. oxygen anion) further promoted the curing reaction of epoxy. Meanwhile, the active groups released by the reaction promoted the curing reaction of phthalonitrile. Due to the insoluble and infusible nature of thermoset resins, the study on curing mechanism of PNBI/epoxy resins is still a challenge. However, this study shows a novel modification in the method for the preparation of epoxy or phthalonitrile blend systems.
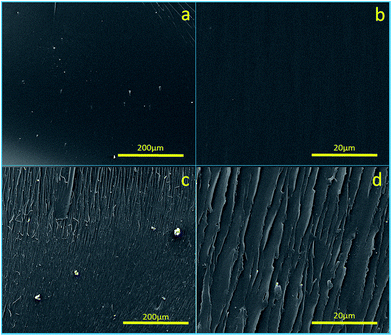
The cross-sectional morphologies of the cured PE19 (Fig. 9a and b) and PE28 (Fig. 9c and d) are shown in Fig. 9. No significant phase separation could be observed, which indicates the good compatibility between PNBI and E51. In addition, no voids are observed in the cured PE blends at different magnifications, which confirms the void-free structures of the cured PE blends. The void-free structures indicate that no evident decomposition occurred during the curing processes and also guarantee the excellent thermal and mechanical properties. As shown in Fig. 9c and d, the cross-section of the cured PE28 comprises long fracture lines, while the cured PE19 exhibits a smooth and featureless fractured cross-section. This observation indicates that the epoxy resin could be toughened using PNBI13,23 and this phenomenon may be correlated with the un-reacted nitrile groups.
3.4 Thermal and mechanical properties
The thermal and mechanical properties of the cured PE blends are shown in Fig. 10, 11 and Table 1. The TGA curves of PE19-210 and PE28-180 in nitrogen and air atmosphere are shown in Fig. 11, and the specific parameters are tabulated in Table 1. The cured PE19 and PE28 exhibit good thermal properties with 14.2 and 17.7% CR at 800 °C in nitrogen atmosphere, respectively. They show a weighted residual at T5 at 369 °C and 360 °C in nitrogen atmosphere and at 347 °C and 352 °C in air atmosphere. CR could be used as a criterion for evaluating the limiting oxygen index (LOI) by Van Krevelen and Hoftyzer equation below.22,24| LOI = 17.5 + 0.4CR |
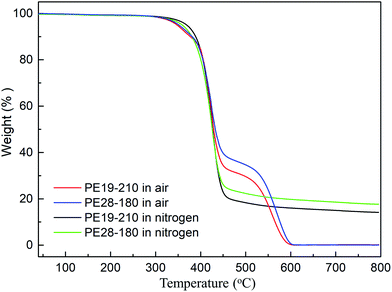 | ||
| Fig. 10 The TGA curves of the cured phthalonitrile/epoxy blends in nitrogen and air atmosphere (PE19-210 and PE28-180). | ||
| Sample name | T5 (°C) | T10 (°C) | CR (%) | Tg (°C) | Initial G′ (MPa) |
|---|---|---|---|---|---|
| PE19 | 318 | 362 | 13.6 | — | — |
| PE19-210 (N2) | 369 | 389 | 14.2 | — | — |
| PE19-210 (air) | 347 | 377 | 0 | 210 | 3076 |
| PE28 | 254 | 376 | 16.9 | — | — |
| PE28-180 (N2) | 360 | 380 | 17.7 | — | — |
| PE28-180 (air) | 352 | 381 | 0 | 207 | 3046 |
The LOI values of the cured PE19 and PE28 are 23.2 and 24.6, respectively. On the basis of LOI values, the cured PE19 and PE28 exhibit good flame-retardant properties,25 which indicate that the flame-retardant properties of epoxy resin can be promoted by PNBI.
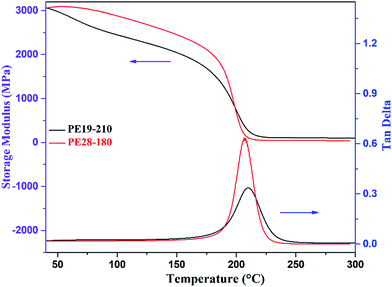
The dynamic mechanical properties of the cured PE blends were evaluated by DMA testing, and the plots of storage modulus (G′) and damping factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) are shown in Fig. 11. It is evident from the plots that PE19-210 and PE28-180 exhibited outstanding thermal and mechanical properties. The G′ of the cured PE19 and PE28 at 40 °C are up to 3076 and 3046 MPa, respectively. The Tg obtained from the peak of tan
δ) are shown in Fig. 11. It is evident from the plots that PE19-210 and PE28-180 exhibited outstanding thermal and mechanical properties. The G′ of the cured PE19 and PE28 at 40 °C are up to 3076 and 3046 MPa, respectively. The Tg obtained from the peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of PE19-210 and PE28-180 are 210 and 207 °C, respectively, which indicates that when compared with other E51 epoxy resin systems the thermal properties of E51 could be significantly improved by the addition of PNBI. From the G′ and Tg data, it could be determined that the dynamic mechanical properties of PE28-180 are lower than those of PE19-210, which may be due to a lower degree of the consumption of nitrile groups in PE28-180.
δ of PE19-210 and PE28-180 are 210 and 207 °C, respectively, which indicates that when compared with other E51 epoxy resin systems the thermal properties of E51 could be significantly improved by the addition of PNBI. From the G′ and Tg data, it could be determined that the dynamic mechanical properties of PE28-180 are lower than those of PE19-210, which may be due to a lower degree of the consumption of nitrile groups in PE28-180.
3.5 Water uptakes
The water uptakes of PE19-210 and PE28-180 were calculated using the following equation:where M1 and M2 is the weight of the cured PE19 and PE28 at the dry state and after immersion in boiling distilled water, respectively.
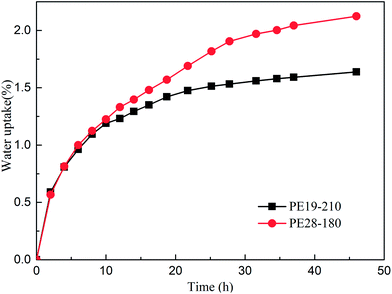
Fig. 12 shows the water uptake properties of the cured PE blends in boiling distilled water. As shown in Fig. 12, the water uptake increases rapidly during the initial stage, and it appears to level off after approximately 48 hours in boiling distilled water. As shown in Fig. 12, the water uptake of PE28-180 (2.2%) is higher than that of PE19-210 (1.6%), which could be related to the water absorption of benzimidazole. The water uptake of the cured PE19 and PE28 is higher than those of other E51 resins,26,27 which could be due to water absorption nature of benzimidazole.28 Moreover, the water uptakes of the cured PE blends are lower than those of the other phthalonitrile resins.16,29,30
4 Conclusions
A benzimidazole-containing phthalonitrile (PNBI) was introduced to promote the curing reactions and modify the thermal properties of epoxy resins (E51). The results obtained show that PNBI and E51 exhibit good compatibility. The curing behaviors, structures and thermal properties of the PNBI/E51 blends (PE19 and PE28) were monitored by IR, SEM, DSC, rheology, TGA and DMA. The IR spectra of the cured PNBI/E51 blends show that nitriles would be fully consumed at a temperature lower than 150 °C in the PNBI/E51 blend system (PE19). The Tg of the cured PNBI/E51 blends were higher than 207 °C, and the T5 and CR values were higher than 360 °C and 14% in nitrogen atmosphere, respectively. This study shows a novel modification method for inhibiting the phase separation in the blend systems, preparation of phthalonitrile or epoxy systems and modification thermal properties of epoxy resins.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 51173114) for the financial support.Notes and references
- G. Yang, S. Y. Fu and J. P. Yang, Polymer, 2007, 48, 302–310 CrossRef CAS.
- J. Qin, G. Zhang, R. Sun and C. Wong, J. Therm. Anal. Calorim., 2014, 117, 831–843 CrossRef CAS.
- P. Jain, V. Choudhary and I. K. Varma, J. Macromol. Sci., Polym. Rev., 2002, 42, 139–183 CrossRef.
- R. Jain, V. Choudhary and A. K. Narula, J. Appl. Polym. Sci., 2007, 106, 2593–2598 CrossRef CAS.
- D. Augustine, K. P. Vijayalakshmi, R. Sadhana, D. Mathew and C. P. R. Nair, Polymer, 2014, 55, 6006–6016 CrossRef CAS.
- Z. Gao, Y. Yu, Y. Xu and S. Li, J. Appl. Polym. Sci., 2007, 105, 1861–1868 CrossRef CAS.
- K. Zeng and G. Yang, Phthalonitrile Matrix Resins and Composites, Wiley Online Library, 2012 Search PubMed.
- T. M. Keller and J. R. Griffith, Resins for aerospace, ACS Symp. Ser., 1980, 25–34 CrossRef CAS.
- G. Yu, C. Liu, X. Li, J. Wang, X. Jian and C. Pan, Polym. Chem., 2012, 3, 1024–1032 RSC.
- D. D. Dominguez and T. M. Keller, J. Appl. Polym. Sci., 2008, 110, 2504–2515 CrossRef CAS.
- X. Zhao, H. Guo, Y. Lei, R. Zhao, J. Zhong and X. Liu, J. Appl. Polym. Sci., 2013, 127, 4873–4878 CrossRef CAS.
- H. Guo, Y. Zou, Z. Chen, J. Zhang, Y. Zhan, J. Yang and X. Liu, High Perform. Polym., 2012, 24, 571–579 CrossRef CAS.
- X. Zhao, Y. Lei, R. Zhao, J. Zhong and X. Liu, J. Appl. Polym. Sci., 2012, 123, 3580–3586 CrossRef CAS.
- D. Wu, Y. Zhao, K. Zeng and G. Yang, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4977–4982 CrossRef CAS.
- Y. Zhao, J. Zhu, X. Shen, J. Hu, K. Zeng and G. Yang, Thermosetting Resin, 2012, 27, 1–4 CAS.
- J. Hu, Y. Liu, Y. Jiao, S. Ji, R. Sun, P. Yuan, K. Zeng, X. Pu and G. Yang, RSC Adv., 2015, 5, 16199–16206 RSC.
- L. Liu and M. Li, J. Appl. Polym. Sci., 2010, 117, 3220–3227 CAS.
- K. Kudo, M. Furutani and K. Arimitsu, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 3411–3414 CrossRef CAS.
- T. Vidil, F. Tournilhac, S. Musso, A. Robissonb and L. Leibler, Prog. Polym. Sci., 2016, 62, 126–179 CrossRef CAS.
- P. Yuan, S. Ji, J. Hu, X. Hu, K. Zeng and G. Yang, Polymer, 2016, 102, 266–280 CrossRef CAS.
- S. Ji, P. Yuan, J. Hu, R. Sun, K. Zeng and G. Yang, Polymer, 2016, 84, 365–370 CrossRef CAS.
- F. Zhao, R. Liu, X. Yu, K. Naito, X. Qu and Q. Zhang, J. Appl. Polym. Sci., 2015, 132, 42606 Search PubMed.
- Y. Huang, Y. Luo, M. Xu, Y. Lei and X. Liu, Composites, Part B, 2016, 106, 294–299 CrossRef CAS.
- D. W. van Krevelen, Polymer, 1975, 16, 615–620 CrossRef CAS.
- C. H. Lin and C. S. Wang, Polymer, 2001, 42, 1869–1878 CrossRef CAS.
- P. Ren, G. Liang and Z. Zhang, Polym. Compos., 2006, 27, 591–598 CrossRef CAS.
- S. G. Prolongoa, G. del Rosariob and A. Urena, Int. J. Adhes. Adhes., 2006, 26, 125–132 CrossRef.
- J. Hu, P. Yuan, K. Zeng and G. Yang, Thermochim. Acta, 2014, 590, 30–39 CrossRef CAS.
- S. B. Sastri and T. M. Keller, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2105–2111 CrossRef CAS.
- H. Sheng, X. Peng, H. Guo, X. Yu, C. Tang, X. Qu and Q. Zhang, Mater. Chem. Phys., 2013, 142, 740–747 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06162e |
| This journal is © The Royal Society of Chemistry 2017 |