Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A multicomponent bicyclization reaction of isocyanide, allenoate, imine and water to synthesize pyrrolidine-fused rings†
Hui Jiang*ab,
Yaming Tianc,
Lumin Tianc and
Jian Li *c
*c
aState Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, No.4 Second Section Jianshe North Road, Chengdu 610054, P. R. China. E-mail: jianghui@uestc.edu.cn
bDepartment of Applied Chemistry, University of Electronic Science and Technology of China, No.4 Second Section Jianshe North Road, Chengdu 610054, P. R. China
cDepartment of Chemistry, Shanghai University, 99 Shangda Road, Shanghai 200444, P. R. China. E-mail: lijian@shu.edu.cn
First published on 23rd June 2017
Abstract
A multicomponent bicyclization of isocyanide, allenoate, imine, and water has been disclosed. This protocol involves the formation of five chemical bonds (two C–C, two C–N, and one C–O), thus providing a new pathway to structurally unusual fused rings.
Isocyanide is a fascinating one-carbon synthon that is widely used in a variety of carbon–carbon and carbon–heteroatom bond-forming reactions.1,2 In particular, the reactivity of isocyanide to undergo an α-addition with both an electrophile and a nucleophile has made isocyanide a particularly significant reaction partner in multicomponent reactions (IMCRs)3 ever since the classical Ugi and Passerini reactions. To date, the isocyanide-based multicomponent reaction has become a powerful synthetic tool in organic synthesis.4,5 Recently, the multicomponent bicyclization reactions (MBRs) have enjoyed considerable attention from the organic chemistry community.6 Notably, the isocyanide-based bicyclization reactions (IMBRs) as multiple-bond forming processes allow the rapid synthesis of structurally complex drug-like molecules, which make them superior to traditional methods.7
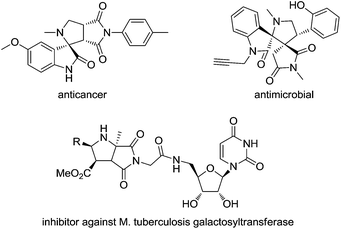
Pyrrolidines are important heterocycles that are frequently found in numerous natural products,8 bioactive molecules,9 as well as organocatalysts10 in organic synthesis. In this regard, much effort has been devoted to the synthesis of such scaffolds. Among these structures, bicyclic pyrrolidine frameworks bearing pyrrolidine-2,5-dione are found to shown significant biological activities, including anticancer and antimicrobial activities.11 A structurally unique pyrrolidine containing diester linkage to the 5′-position of uridine was proven to be inhibitor against M. tuberculosis galactosyltransferase enzyme (Scheme 1).12 Traditionally, these compounds were prepared using [3 + 2]1,3-dipolar cycloadditions of azomethine ylides with a variety of alkenes or alkynes, thus offering a good tool for the construction of chiral pyrrolidine scaffolds.13 Recently, Zhao and co-workers has reported that organocatalytic [3 + 2] cycloaddition of isatin-derived azomethine ylides with alkenes or alkynes to the enantioselective synthesis of structurally complex, and potentially bioactive pyrrolidine-fused spirooxindoles.14 Although much progress has been made in the past decades, most of the methods seemed to suffer from insufficient synthetic efficiency since only one ring can be formed in these reactions. As a consequence, there is continues demand to development new strategies to synthesize such scaffolds in an efficient manner.
 | ||
| Scheme 1 Representative reactivity mode of isocyanide-based multicomponent reactions involving allenoate. | ||
In the past several years, we have spent much time in the synthesis of heterocycles using isocyanides as versatile building blocks.7a–7d,15 As such, many interesting and valuable results were developed. Of late, we have established that the multiple and double isocyanide insertion reaction could serve as efficient tool for the construction of spirooxindole and indole-fused polycyclic rings, respectively.15a,15b Before that, we have initiated a very interesting research program aimed at exploiting the application of isocyanide-based multicomponent reactions involving isocyanide since our first example was developed in 2011.15c–15e In particular, we have also proven that this protocol provided a new opportunity for the generation of structurally complex bicyclic derivatives with high synthetic efficiency when substituted allenoates were used.7a–7d The experimental outcome revealed that carbon carbon double bond and carbon oxygen double bond could both compatible in the above-mentioned cycloaddition. However, no successful example using carbon nitrogen double bond as electron-deficient system was ever reported. As a continuation of our previous work, we report the four-component reaction of isocyanide, allenoate, imine, and water to synthesize structurally complex pyrrolidine-fused rings. This strategy also represents the first example using carbon nitrogen double bond electron-deficient system in isocyanide-based multicomponent reaction involving allenoate.
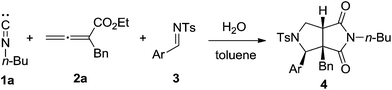
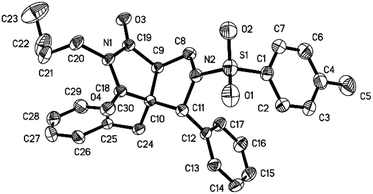
Initially, we began the experiment investigation by selecting n-butyl isocyanide 1a, allenoate 2a, and imine 3a as model substrate. In the presence of water, heating the mixture in toluene solution under reflux essentially led to the formation of cycloadduct 4a in 65% yield (Table 1, entry 1). Furthermore, the structure of compound 4a was unambiguously confirmed by single-crystal X-ray analysis (Fig. 1).16 The replacement of toluene with other solvents such as CH3CN, THF, and DMF only led to decreased yield. The experimental outcome revealed that 50% yield of product 4a was generated when one equivalent water was added. Poor performance was also observed when the reaction was conducted under lower temperature. The optimization experiment also showed that a slightly excessive amount of allenoate 2a (1.2 equiv.) could facilitate the formation of product 4a (65%), while the employment of excessive isocyanide 1a only led to lower yield (57%). With the optimized conditions in hand, we then focused our attention to investigate the substrate scope with regard to different imine 3. As shown in Table 1, various substituted imines 3 with electron-withdrawing groups (entries 2–6) and electron-donating substituents (entries 7–10) on the aromatic ring were firstly used to react under optimized conditions and all new compounds 4 were characterized by 1H NMR, 13C NMR, and HRMS spectra.17 Our experimental findings showed that halide and methoxy group substitution on the aromatic ring was well tolerated, which was potentially useful for further functionalization. Furthermore, the present reaction was not limited to simple substrates bearing mono-substituent on the aromatic ring, dimethyl-substituted imines 3k and 3l were also proven to be good reaction components in the present transformation to produce 4k and 4l (entries 11–12).
| Entry | Ar | Product | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 0.5 mmol isocyanide 1a, 0.6 mmol allenoate 2a, 0.5 mmol imine 3 in 5 mL solvent (toluene/H2O (v/v) = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reflux, 12 hours.b Yields of product after silica gel chromatography. 1), reflux, 12 hours.b Yields of product after silica gel chromatography. |
|||
| 1 | C6H5 | 4a | 65 |
| 2 | 2-ClC6H4 | 4b | 81 |
| 3 | 4-ClC6H4 | 4c | 79 |
| 4 | 2-BrC6H4 | 4d | 82 |
| 5 | 3-BrC6H4 | 4e | 71 |
| 6 | 4-BrC6H4 | 4f | 85 |
| 7 | 2-MeC6H4 | 4g | 73 |
| 8 | 3-MeC6H4 | 4h | 75 |
| 9 | 4-MeC6H4 | 4i | 76 |
| 10 | 4-MeOC6H4 | 4j | 61 |
| 11 | 2,3-Me, MeC6H4 | 4k | 78 |
| 12 | 2,4-Me, MeC6H4 | 4l | 79 |
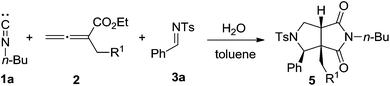
After a broad imine scope was established, changing substituent on the aromatic ring at α-position of substrate 2 was subsequently carried out. As shown in Table 2, a series of substituted allenoates 2 were employed to react with isocyanide 1a, imine 3a, and water under the optimized conditions. Gratifyingly, all reactions proceeded smoothly to give the desired products 5. It was also worthy to note that many substituents, including halide, methyl, methoxy, and cyano groups at ortho, meta, and para positions of the aromatic ring were well-tolerated (Table 2, entries 1–8) and the representative results were summarized in Table 2. Moreover, experiments with substrate 2j containing naphthyl group substitution at β-position was also conducted (Table 2, entry 9). In such cases, product 5j was afforded in good yield, thus further expanded the substrate scope. Furthermore, the present method show high stereoselectivity in all cases and only one isomer was detected during our investigation. Remarkably, the allenoate was also fully incorporated into the final product as a four carbon building block, which was quite rare in previous reports.
| Entry | R1 | Product | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 0.5 mmol isocyanide 1a, 0.6 mmol allenoate 2, 0.5 mmol imine 3a in 5 mL solvent (toluene/H2O (v/v) = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reflux, 12 hours.b Yields of product after silica gel chromatography. 1), reflux, 12 hours.b Yields of product after silica gel chromatography. |
|||
| 1 | 3-ClC6H4 | 5a | 70 |
| 2 | 4-ClC6H4 | 5b | 82 |
| 3 | 3-BrC6H4 | 5c | 74 |
| 4 | 4-BrC6H4 | 5d | 78 |
| 5 | 4-FC6H4 | 5e | 85 |
| 6 | 2-MeC6H4 | 5f | 66 |
| 7 | 3-MeC6H4 | 5g | 68 |
| 8 | 4-MeOC6H4 | 5h | 60 |
| 9 |  |
5i | 80 |
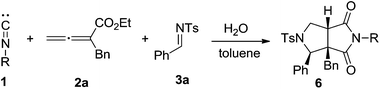
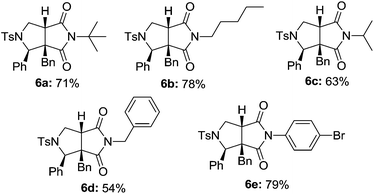
To further explore the utility of the present reaction, the possibility of substituted isocyanides 2 were then performed. As shown in Table 3, a series of aliphatic and aromatic isocyanides 1 were subjected to the optimal conditions. To our delight, all the reactions proceeded smoothly to produce the desired products 6a–6e in good performance. Notably, the present reaction seemed to be not sensitive to sterical hindrance since tert-butyl and admantyl groups were all proven to be compatible. In addition, reactions with the less-reactive para-bromophenyl isocyanide 1e also worked well to yield the desired product 6e, which was quite interesting.
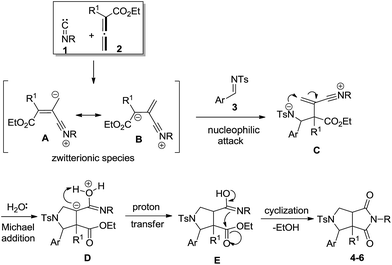
The mechanism of the aforementioned multicomponent cycloaddition reaction has not been unequivocally established, but one reasonable mechanistic proposal is outlined to explain the formation of products 4–6. As shown in Scheme 2, the present reaction starts from the nucleophilic attack between isocyanide 1 and allenoate 2, thus leading to the formation of zwitterionic species, which exist as a resonance-stabilized form A ↔ B.15 The in situ generated species are then trapped by imine substrate 3 to produce intermediate C. After that, the resultant nitrene cation C reacts with water to yield intermediate D. Further proton transfer and cyclization gave rise to the final products 4–6.
Conclusions
In conclusion, we have described a novel multicomponent reaction of isocyanide, allenoate, imine, and water to generate pyrrolidine-fused heterocycles in an efficient manner. Furthermore, two rings and five chemical bonds (two C–C, two C–N, and one C–O) were formed in one operation, which represents high synthetic efficiency. And the resultant structurally unusual compounds are difficult to be synthesized by other methods. Four carbon atoms in allenoate were incorporated into the ring formation, which is quite rare. As a consequence, the above-mentioned advantages and the excellent stereoselectivity make the present strategy be further applied.Acknowledgements
We thank the Fundamental Research Funds for the Central Universities (ZYGX2015J026) and the National Natural Science Foundation of China (No. 21272148) for financial support.Notes and references
- For reviews, see:
(a) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC
; (b) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed
; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed
.
- For recent examples:
(a) G. C. Senadi, T. Y. Lu, G. K. Dhandabani and J.-J. Wang, Org. Lett., 2017, 19, 1172 CrossRef CAS PubMed
; (b) G. Qiu, M. Mamboury, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2016, 55, 15377 CrossRef CAS PubMed
; (c) Y. Kobiki, S. Kawaguchi and A. Ogawa, Org. Lett., 2015, 17, 3490 CrossRef CAS PubMed
; (d) T.-H. Zhu, S.-Y. Wang, T.-Q. Wei and S.-J. Ji, Adv. Synth. Catal., 2015, 358, 823 CrossRef
; (e) J. Y. Liao, P. L. Shao and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 628 CrossRef CAS PubMed
; (f) V. Estévez, G. V. Baelen, B. H. Lentferink, T. Vlaar, E. Janssen, B. U. W. Maes, R. V. A. Orru and E. Ruijter, ACS Catal., 2014, 4, 40 CrossRef
; (g) U. M. V. Basavanag, A. Santos, L. El Kaim, R. Gámez-Montaño and L. Grimaud, Angew. Chem., Int. Ed., 2013, 52, 7194 CrossRef CAS PubMed
.
- For reviews, see:
(a) Multicomponent Reactions in Organic Synthesis, ed. J. Zhu, Q. Wang and M.-X. Wang, Wiley-VCH, Weinheim, Germany, 2014 Search PubMed
; (b) B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323 CrossRef CAS PubMed
; (c) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed
; (d) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef PubMed
; (e) T. Zarganes-Tzitzikas, A. L. Chandgude and A. Dömling, Chem. Rec., 2015, 15, 981 CrossRef CAS PubMed
.
-
(a) A. L. Chandgude and A. Dömling, Org. Lett., 2017, 19, 1228 CrossRef CAS PubMed
; (b) A. Żądło-Dobrowolska, S. Kłossowski, D. Koszelewski, D. Paprocki and R. Ostaszewski, Chem.–Eur. J., 2016, 22, 16684 CrossRef PubMed
; (c) Y. Zhang, Y.-F. Ao, Z.-T. Huang, D.-X. Wang, M.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2016, 55, 5282 CrossRef CAS PubMed
; (d) Q. Gao, W.-J. Hao, F. Liu, S.-J. Tu, S.-L. Wang, G. Li and B. Jiang, Chem. Commun., 2016, 52, 900 RSC
; (e) G. Qiu, Y. He and J. Wu, Chem. Commun., 2012, 48, 3836 RSC
; (f) R. Riva, L. Banfi, A. Basso, V. Cerulli, G. Guanti and M. Pani, J. Org. Chem., 2010, 75, 5134 CrossRef CAS PubMed
.
- For examples on the synthesis of structurally complex molecules, see:
(a) S. Santra and P. R. Andreana, Angew. Chem., Int. Ed., 2011, 50, 9418 CrossRef CAS PubMed
; (b) O. Pando, S. Stark, A. Denkert, A. Porzel, R. Preusentanz and L. A. Wessjohann, J. Am. Chem. Soc., 2011, 133, 7692 CrossRef CAS PubMed
; (c) P. Janvier, H. Bienaymé and J. Zhu, Angew. Chem., Int. Ed., 2002, 41, 4291 CrossRef CAS
; (d) A. Endo, A. Yanagisawa, M. Abe, S. Tohma, T. Kan and T. Fukuyama, J. Am. Chem. Soc., 2002, 124, 6552 CrossRef CAS PubMed
.
-
(a) L. Wang, L.-X. Shi, L. Liu, Z.-X. Li, T. Xu, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, J. Org. Chem., 2017, 82, 3605 CrossRef CAS PubMed
; (b) J.-K. Qiu, B. Jiang, Y.-L. Zhu, W.-J. Hao, D.-C. Wang, J. Sun, P. Wei, S.-J. Tu and G. Li, J. Am. Chem. Soc., 2015, 137, 8928 CrossRef CAS PubMed
; (c) W. Fan, Q. Ye, H.-W. Xu, B. Jiang, S.-L. Wang and S.-J. Tu, Org. Lett., 2013, 15, 2258 CrossRef CAS PubMed
; (d) L. K. Ransborg, M. Overgaard, J. Hejmanowska, S. Barfüsser, K. A. Jørgensen and Ł. Albrecht, Org. Lett., 2014, 16, 4182 CrossRef CAS PubMed
; (e) M. Li, P. Shao, S.-W. Wang, W. Kong and L.-R. Wen, J. Org. Chem., 2012, 77, 8956 CrossRef CAS PubMed
; (f) M. Li, H. Cao, Y. Wang, X.-L. Lv and L.-R. Wen, Org. Lett., 2012, 14, 3470 CrossRef CAS PubMed
.
- For examples on isocyanide-based multicomponent bicyclization, see:
(a) Z. Z. Tang, Z. Liu, Y. An, R. L. Jiang, X. L. Zhang, C. J. Li, X. S. Jia and J. Li, J. Org. Chem., 2016, 81, 9158 CrossRef CAS PubMed
; (b) S. B. Xu, S. K. Su, H. T. Zhang, L. M. Tian, P. C. Liang, J. W. Chen, Y. Zhang, C. J. Li, X. S. Jia and J. Li, Synthesis, 2015, 47, 2414 CrossRef CAS
; (c) S. K. Su, C. J. Li, X. S. Jia and J. Li, Chem.–Eur. J., 2014, 20, 5905 CrossRef CAS PubMed
; (d) S. L. Jia, S. K. Su, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2014, 16, 5604 CrossRef CAS PubMed
; (e) L. Moni, C. F. Gers-Panther, M. M. Sc. Anselmo, T. J. J. Müller and R. Riva, Chem.–Eur. J., 2016, 22, 2020 CrossRef CAS PubMed
; (f) Q. Gao, P. Zhou, F. Liu, W.-J. Hao, C. Yao, B. Jiang and S.-J. Tu, Chem. Commun., 2015, 51, 9519 RSC
; (g) Z.-G. Xu, F. D. Moline, A. P. Cappelli and C. Hulme, Org. Lett., 2013, 15, 2738 CrossRef CAS PubMed
; (h) D. Zheng, S. Li and J. Wu, Chem. Commun., 2012, 48, 8568 RSC
.
-
(a) J. P. Michael, Nat. Prod. Rep., 2005, 22, 603 RSC
; (b) Y. Cheng, Z. T. Huang and M. X. Wang, Curr. Org. Chem., 2004, 8, 325 CrossRef CAS
.
-
(a) C. Alvarez-Ibarra, A. G. Csaky, G. A. Lopez de Silanes and M. L. Quiroga, J. Org. Chem., 1997, 62, 479 CrossRef CAS PubMed
; (b) A. Bianco, M. Maggini, G. Scorrano, C. Toniolo, G. Marconi, C. Villani and M. Prato, J. Am. Chem. Soc., 1996, 118, 4072 CrossRef CAS
; (c) R. Murugan, S. Anbazhagan and S. Sriman Narayanan, Eur. J. Med. Chem., 2009, 44, 3272 CrossRef CAS PubMed
; (d) T.-H. Kang, Y. Murakami, K. Matsumoto, H. Takayama, M. Kitajima, N. Aimi and H. Watanabe, Eur. J. Pharmacol., 2002, 455, 27 CrossRef CAS PubMed
.
-
(a) J. Seayad and B. List, Org. Biomol. Chem., 2005, 3, 719 RSC
; (b) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed
.
-
(a) K. Karthikeyan, P. M. Sivakumar, M. Doble and P. T. Perumal, Eur. J. Med. Chem., 2010, 45, 3446 CrossRef CAS PubMed
; (b) A. S. Girgis, J. Stawinski, N. S. M. Ismail and H. Farag, Eur. J. Med. Chem., 2012, 47, 312 CrossRef CAS PubMed
; (c) R. Ranjith Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 3821 CrossRef CAS PubMed
.
- A. E. Trunkfield, S. S. Gurcha, G. S. Besra and T. D. H. Bugg, Bioorg. Med. Chem., 2010, 18, 2651 CrossRef CAS PubMed
.
-
(a) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed
; (b) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef CAS PubMed
.
- H. W. Zhao, Z. Yang, W. Meng, T. Tian, B. Li, X.-Q. Song, X.-Q. Chen and H. L. Pang, Adv. Synth. Catal., 2015, 357, 2492 CrossRef CAS
.
-
(a) Y. M. Tian, L. M. Tian, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2016, 18, 840 CrossRef CAS PubMed
; (b) Y. M. Tian, L. M. Tian, X. He, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2015, 17, 4874 CrossRef CAS PubMed
; (c) J. Li, Y. J. Liu, C. J. Li and X. S. Jia, Chem.–Eur. J., 2011, 17, 7409 CrossRef CAS PubMed
; (d) J. Li, Y. J. Liu, C. J. Li and X. S. Jia, Adv. Synth. Catal., 2011, 353, 913 CrossRef CAS
; (e) J. Li, N. Wang, C. J. Li and X. S. Jia, Chem.–Eur. J., 2012, 18, 9645 CrossRef CAS PubMed
; (f) Z. Liu, X. Zhang, J. X. Li, F. Li, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2016, 18, 4052 CrossRef CAS PubMed
; (g) J. Li, S. K. Su, M. Y. Huang, B. Y. Song, C. J. Li and X. S. Jia, Chem. Commun., 2013, 49, 10694 RSC
; (h) C. L. Li, T. Jin, X. Zhang, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2016, 18, 1916 CrossRef CAS PubMed
.
- CCDC 1544098 for compound 4a contains the supplementary crystallographic data for this paper.†.
- See ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1544098. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra05701f |
| This journal is © The Royal Society of Chemistry 2017 |