Open Access Article
Open Access ArticleA two-stage pretreatment using acidic dioxane followed by dilute hydrochloric acid on sugar production from corn stover
Shengxin Anab,
Wenzhi Li *a,
Qiyu Liua,
Minghao Lia,
Qiaozhi Maa,
Longlong Ma*c and
Hou-min Changd
*a,
Qiyu Liua,
Minghao Lia,
Qiaozhi Maa,
Longlong Ma*c and
Hou-min Changd
aLaboratory of Basic Research in Biomass Conversion and Utilization, Department of Thermal Science and Energy Engineering, University of Science and Technology of China, Hefei 230026, PR China. E-mail: liwenzhi@ustc.edu.cn; Tel: +86-0551-63600786
bInstitute of Chemical Engineering, Anhui University of Science and Technology, Huainan 232001, PR China
cGuangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510650, PR China. E-mail: mall@ms.giec.ac.cn
dDepartment of Forest Biomaterials, North Carolina State University, Raleigh, NC 27695-8005, USA
First published on 26th June 2017
Abstract
A two-stage pretreatment method was developed to improve sugar recovery in this study. Firstly, the corn stover was pretreated with acidic dioxane to remove lignin, then the residue was subjected to dilute hydrochloric acid to eliminate the negative effects of hemicelluloses on enzymatic hydrolysis as well as increasing xylose yield. The optimal condition was 90 °C, 20 min, and 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the first stage followed by 120 °C and 40 min for 1.0 wt% dilute hydrochloric acid in the second stage. The total yields of glucose and xylose were 91.5% and 79.7%, respectively, with a low cellulase dosage of 3 FPU g−1 of substrate. This two-stage pretreatment was effective due to the removal of lignin in the first stage and the hydrolysis of hemicelluloses in the second stage, resulting in a very high sugar recovery with a low enzyme loading.
1 Introduction
The shortage of fossil energy and environmental pollution have aroused increasing interest in the utilization of lignocellulosic biomass,1,2 a kind of abundant renewable resource, containing plenty of carbohydrates (cellulose and hemicelluloses). The carbohydrate in biomass could be hydrolyzed into monosaccharides, which can be further converted into diverse biofuels and chemicals.3–5 However, the utilization of lignocellulose is hindered by its natural tight three-dimensional structure mainly composed of lignin, hemicellulose and cellulose,6,7 and as a consequence pretreatment is compulsory before hydrolysis. The objective of pretreatment is to destroy its complex structure and to produce a cellulose-rich substrate which is sensitive to enzymatic hydrolysis (i.e., to supply fast conversion of cellulose into glucose at low enzyme dosage).For this purpose, various pretreatment methods have been investigated during the past decades. Commonly reported methods include physical, chemical, biological and physicochemical treatments, such as milling,8,9 dilute acid,10,11 alkaline,12,13 hot water,14,15 organic solvent,16,17 ionic liquid,18,19 biological pretreatment20,21 and so on. No matter which method was used, the fundamental objective was to reduce hemicelluloses and lignin contents, increase porosity and surface area, and decrease crystallinity and fibre size.22
Although the pretreatment methods mentioned above have demonstrated positive effects on lignocelluloses hydrolysis, it is still difficult to maximize the utilization of lignocelluloses via one-stage pretreatment due to its natural recalcitrant structure composed of cellulose, hemicelluloses and lignin.23,24 Therefore, a two-stage pretreatment method has been developed. The main purpose of two-stage pretreatment method was to destroy the compact structure of lignocelluloses by the removal of lignin and hemicelluloses,25–27 which allow cellulose to be more accessible to cellulase, resulting in a high glucose recovery with a low enzyme dosage. In this way, hemicelluloses and cellulose could be recovered under optimum conditions respectively, reaching a target of integrated utilization of lignocelluloses.
During two-step process, dilute acid pretreatment was frequently used to hydrolyze hemicelluloses and recover pentose.8,28 The removal of hemicelluloses could increase the porosity and facilitate the accessibility of enzyme to cellulose. However, dilute acid pretreatment only shows negligible impact on lignin removal. Lignin existing in biomass has an unfavorable effect on enzymatic hydrolysis for glucose because of its adsorption for cellulases and physical barrier around cellulose.29–31 In order to further destroy lignocellulosic structure and obtain high glucose yield, it is necessary to implement a delignification process.
Organic solvent pretreatments were extensively applied in view of the significant efficacy on lignin removal. However, most of organic solvent pretreatment process such as ethanol pretreatments (with or without catalyst) required relatively high temperature, long reaction time and high enzyme dosage,17,27,32 which went against industrial applications. Compared with those solvents, 1,4-dioxane could be a splendid organic solvent being used in pretreatment on account of low boiling point, excellent solubility of lignin, easy recovery and mild reaction condition. As high as 74.14% lignin could be obtained from bioethanol production residue by dioxane extraction,33 which implies 1,4-dioxane/water solution plays an effective role in lignin extraction and could be used in the pretreatment process. However, the delignification effect was poor in pure dioxane solution. This condition could be improved if acid catalysts (organic or mineral acids) were added into 1,4-dioxane solution (named acidic dioxane solution), H+ could cleave intermolecular bonds existed in lignin, hemicelluloses and lignin–carbohydrate complex (LCC), causing plenty of lignin and small amount of hemicelluloses degraded and dissolved into acidic dioxane solution. Therefore, acidic dioxane pretreatment can deconstruct the compact structure of biomass, and facilitate enzymatic hydrolysis for glucose recovery.
With the aim to recover glucose and xylose as much as possible with a lower cellulase loading and less energy input, a novel two-stage pretreatment process using acidic dioxane followed by dilute acid pretreatment was developed in this research. During the first stage, a large proportion of lignin and part of hemicelluloses were removed under optimal conditions by acidic dioxane pretreatment (dioxane–water solution mixed with certain amount of HCl). The lignin and hemicelluloses solubilized in dioxane solution could be recovered directly or further converted into other high-value chemicals or liquid fuels in this system, which enabled acidic dioxane solution recyclable. Despite of partial degradation of hemicellulose in the first stage, the remained hemicelluloses in the residue still had an unfavorable effect on glucose enzymatic hydrolysis, leading to the decrease of glucose yield.34,35 A subsequent dilute hydrochloric acid pretreatment was designed to eliminate this negative effect. After that process, the compact structure of biomass was broken, exposing the active sites of cellulose, increasing accessible surface area and making the enzymatic hydrolysis more effective. At the same time, the xylose could be recovered respectively through two stage, reaching to the target of sufficient utilization of lignocelluloses. To achieve a high sugar yield, reaction conditions were investigated and optimized in this study. The structural change before and after pretreatment were characterized by a series of analytical methods, including scanning electron microscopy (SEM) images and Brunner–Emmet–Teller (BET) in order to evaluate the impacts of two-stage pretreatment on corn stover.
2 Material and methods
2.1 Materials
The corn stover was obtained from the northern Anhui province of China. The biomass was washed, dried, milled and stored using the methods in our previous work.8 1,4-dioxane and HCl were provided by Sinopharm Group Chemical Reagent Co., Ltd (Shanghai, China). Enzymes were donated by Novozymes (China) Investment Co., Ltd. All chemical reagents had no further purification before use.2.2 Two stage pretreatment of corn stover
In the first stage, 4 g dried corn stover and 40 mL different acid concentrations (0.2, 0.4, 0.6, 1.0, 1.2 and 1.5 wt%) in dioxane solution were mixed in 70 mL batch reactor with agitation. Different temperature (70, 80 and 90 °C), reaction time (10, 20, 30, 40 and 50 min), dioxane–water ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9
1, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 12
1, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) have been investigated. After pretreatment, the reactors was removed from the heating jacket and directly cooled to room temperature with circulating water. Then the obtained hydrolysate was separated from the residues by filtration. The hydrolysate was divided into halves, one half was used to analyze the lignin recovery and the other half was analyzed to determine its glucose, xylose and lignin contents. The optimal condition was chosen according to the consumption of energy and acid as well as the recovery of glucose, xylose and lignin. All the reactions were conducted in duplicated. The solid residue was used for the second stage pretreatment.
1, v/v) have been investigated. After pretreatment, the reactors was removed from the heating jacket and directly cooled to room temperature with circulating water. Then the obtained hydrolysate was separated from the residues by filtration. The hydrolysate was divided into halves, one half was used to analyze the lignin recovery and the other half was analyzed to determine its glucose, xylose and lignin contents. The optimal condition was chosen according to the consumption of energy and acid as well as the recovery of glucose, xylose and lignin. All the reactions were conducted in duplicated. The solid residue was used for the second stage pretreatment.
In the second dilute acid stage, the wet residue from the first stage was further pretreated under the optimal conditions (1 wt% HCl, 120 °C, 40 min) which were investigated in our previous work.36 After this process, the residual solid was collected by filtration, washed with deionized water until it was free of acid and then stored for enzyme hydrolysis stock. The hydrolysate was collected to determine the glucose and xylose content with the same method in the first stage.
2.3 Enzymatic hydrolysis
Enzymatic hydrolysis was performed by using a cellulase concentrate (Cellulast 1.5 L) supplemented with β-glucosidase (Novozym 188), both from Novozymes (China) Investment Co., Ltd. The enzyme activities was 67.8 filter paper units (FPU) per mL (expressed as micromoles of glucose produced per minute, with filter paper as a substrate) and 210.5 cellobiose units (CBU) per mL (expressed as micromoles of cellobiose that is converted to glucose per minute, with cellobiose as a substrate) for Celluclast 1.5 L and Novozyme 188, respectively.37 Enzymes were directly used without further purification.The enzymatic hydrolysis of untreated and wet pretreated samples (0.2 g dry substrate) were conducted in a centrifuge tube containing 50 mM sodium acetate buffer (pH 4.8). The mass concentration was set at 5% (w/v). Sodium azide (0.3%, w/v) was also added for inhibiting the microbial infection. Cellulase (3, 5 and 10 FPU per gram substrate) and β-glucosidase (20 CBU per gram substrate) were added into the tube as well. The enzyme hydrolysis experiments were performed in a shaking incubator at 50 °C, 120 rpm. After 72 h, the hydrolysate was separated from the mixture by centrifugation at 8000 rpm for 1 min. Glucose yield released in the hydrolysate were determined by high performance liquid chromatography (HPLC). Each reaction was carried out in duplicates.
2.4 Analysis methods
| Glucose yield = [(grams of glucose produced in the hydrolyzate) × 0.9]/(grams of glucan in raw biomass) × 100% |
| Xylose yield = [(grams of xylose produced in the hydrolyzate) × 0.88]/(grams of xylan in raw biomass) × 100% |
| DP = [(1.65η − 116H)/G]1.11 |
3 Results and discussion
3.1 Effect of pretreatment methods on sugar recovery
The chemical compositions of raw material were as follows: 32.6% glucan, 22.5% xylan, 2.9% arabinan, 1.3% galactan, 17.6% acid-insoluble lignin (Klasen lignin), 3.1% acid-soluble lignin, 9.8% extractive, 4.1% ash and 6.1% other components.In this study, three pretreatment approaches were performed to find a method leading to the higher glucose yield. These methods included: dilute hydrochloric acid pretreatment (HP), acidic dioxane pretreatment (ADP), and acidic dioxane followed by dilute hydrochloric acid pretreatment (ADPH). HP was proceeded at 120 °C for 40 min. ADP was proceeded at 90 °C for 20 min in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
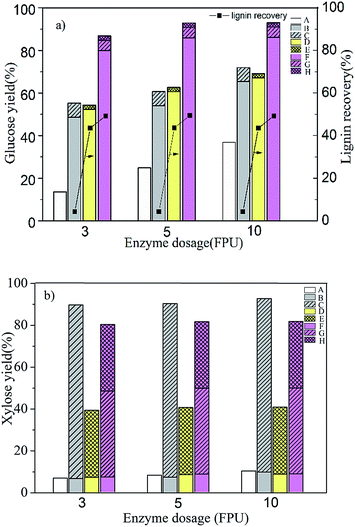
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (V/V) dioxane-to-water solution containing 1.0 wt% HCl. ADPH was proceeded at 90 °C, 20 min, 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the first stage followed by 120 °C and 40 min for 1.0 wt% dilute hydrochloric acid in the second stage. The results of the effect of different processes on enzymatic hydrolysis were shown in Fig. 1.
1 (V/V) dioxane-to-water solution containing 1.0 wt% HCl. ADPH was proceeded at 90 °C, 20 min, 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the first stage followed by 120 °C and 40 min for 1.0 wt% dilute hydrochloric acid in the second stage. The results of the effect of different processes on enzymatic hydrolysis were shown in Fig. 1.
From Fig. 1a, the sum of glucose yield obtained from the first stage and the second stage during ADPH process was less than 8.0%, which suggested that most of cellulose remained in the pretreated materials because cellulose is hard to be depolymerized due to its semi-crystalline structures. Compared with HP, ADP and ADPH, the glucose yield of untreated material after enzymatic hydrolysis was very low (17.2% with a cellulase dosage of 3 FPU g−1 substrate), indicating that natural compact structure of corn stover was detrimental to enzymatic hydrolysis. After HP and ADP process, glucose yields were increased by 31.5% and 37.6% respectively at 3 FPU g−1 substrate, which was ascribed to the fact that HP and ADP could increase the dissolution of hemicelluloses (up to 83.9% in HP process and 32.1% in ADP process, Fig. 1b). In addition, ADP process had a great advantage on lignin solubilization (up to 49.3%, Fig. 1a). Both hemicelluloses hydrolysis and delignification could contribute a lot to enzymatic hydrolysis.44 But the weak effect on delignification in HP process (4.2% lignin removed, Fig. 1a) and the finite effect on hemicelluloses hydrolysis in ADP process seemed to have a limited impact for increasing the glucose yield during enzymatic hydrolysis. Then ADPH was investigated. After ADPH, the yield of xylose and lignin was 72.5% and 49.5% respectively (Fig. 1a and b). That removal of a large amount of lignin and hemicelluloses in corn stover destroyed its tight structure (Fig. 3d), reduced the absorption amount of lignin to cellulose enzyme, improved the accessibility of carbohydrates to enzyme, resulting in a high yield of glucose in enzymatic hydrolysis under a low enzyme loading (91.5% glucose yield with a cellulase dosage of 3 FPU g−1 substrate). Besides an acceptable xylose yield of 79.7% was obtained after ADPH (31.5% obtained in the first stage, 41.0% obtained in second stage and 7.2% obtained in enzymatic stage with a cellulase dosage of 3 FPU g−1 substrate, Fig. 1b), which demonstrated that hemicelluloses presented in biomass were easier to be hydrolyzed. The results indicated that ADPH was a more effective method than single-step pretreatment using either HP or ADP to improve sugars especially glucose recovery.
3.2 Effect of the order of acidic dioxane process used in the two-stage pretreatment method on sugar recovery
The effect of the order of acidic dioxane process used in the two-stage pretreatment method was also studied. HADP was also a two-stage pretreatment method using dilute hydrochloric acid followed by acidic dioxane pretreatment, which was conducted with 1.0 wt% HCl at 120 °C for 40 min in the first stage followed by 80 °C, 30 min, 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the second stage. The only difference between ADPH and HADP was the order of acidic dioxane process used in the two-stage pretreatment.Fig. 2a clearly indicated that ADHP had a larger impact on glucose recovery than HADP with a yield of 80.3% at 3 FPU enzyme dosage (80 °C, 30 min, 1 wt% acidic dioxane, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 liquid–solid ratio). The results may be explained as follows: after ADPH, a large amount of lignin (44.9%) and hemicelluloses (77.6% xylose yield) were removed from the biomass, which enhanced enzymatic accessibility and increase the glucose recovery. During HADP process, due to most of hemicelluloses (83.9% xylose yield, Fig. 2b) had been hydrolyzed in the first stage, H+, as a catalyst, catalyzed lignin repolymerization on the surface of cellulose,45 enhancing the physical barrier for enzymatic hydrolysis and resulting in a lower glucose yield than ADPH. Besides, it was worth noting that xylose yield obtained from ADPH was lower than that from HADP (77.6% in ADPH and 83.9% in HADP, excluding the yield of enzymatic process, Fig. 2b). This could be ascribed to the fact that partial of xylose converted into furfural and organic acid in the first stage at set reaction condition. These results were in agreement with previous study that the organosolv pretreatment with acid catalysts might cause the degradation of pentose generating new compounds chemical compounds.46,47 To achieve maximum utilization of lignocellulosic biomass, the yield of both xylose and glucose must be simultaneously considered in this study. Compared with HADP, the yield of glucose and xylose gained from ADPH 80.1% and 84.9% respectively (59.1% and 90.1% in HADP), which demonstrated ADPH was a better pretreatment method.
1 liquid–solid ratio). The results may be explained as follows: after ADPH, a large amount of lignin (44.9%) and hemicelluloses (77.6% xylose yield) were removed from the biomass, which enhanced enzymatic accessibility and increase the glucose recovery. During HADP process, due to most of hemicelluloses (83.9% xylose yield, Fig. 2b) had been hydrolyzed in the first stage, H+, as a catalyst, catalyzed lignin repolymerization on the surface of cellulose,45 enhancing the physical barrier for enzymatic hydrolysis and resulting in a lower glucose yield than ADPH. Besides, it was worth noting that xylose yield obtained from ADPH was lower than that from HADP (77.6% in ADPH and 83.9% in HADP, excluding the yield of enzymatic process, Fig. 2b). This could be ascribed to the fact that partial of xylose converted into furfural and organic acid in the first stage at set reaction condition. These results were in agreement with previous study that the organosolv pretreatment with acid catalysts might cause the degradation of pentose generating new compounds chemical compounds.46,47 To achieve maximum utilization of lignocellulosic biomass, the yield of both xylose and glucose must be simultaneously considered in this study. Compared with HADP, the yield of glucose and xylose gained from ADPH 80.1% and 84.9% respectively (59.1% and 90.1% in HADP), which demonstrated ADPH was a better pretreatment method.
3.3 Effect of dioxane-to-water ratio on sugar recovery
The effect of different dioxane-to-water ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9
1, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 12
1, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in 1 wt% HCl dioxane solution (v/v) in 1 wt% HCl dioxane solution n enzymatic hydrolysis was investigated and the results were illustrated in Table 1.
1, v/v) in 1 wt% HCl dioxane solution (v/v) in 1 wt% HCl dioxane solution n enzymatic hydrolysis was investigated and the results were illustrated in Table 1.
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (%, v/v) H2O (%, v/v) |
Lignin removal (%) | Glucose yield (%) | Xylose yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Total | A | B | C | Total | ||
a A: glucose yield obtained in the first stage (90 °C, 20 min, 1 wt% acidic dioxane, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C, 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis process (3 FPU enzyme dosage). 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C, 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis process (3 FPU enzyme dosage). |
|||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.9 | 0.6 | 5.7 | 64.4 | 70.7 | 5.9 | 54.8 | 8.1 | 68.8 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45.9 | 1.9 | 5.4 | 76.4 | 83.7 | 27.5 | 33.3 | 8.3 | 69.1 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
49.8 | 1.5 | 5.3 | 85.3 | 91.5 | 31.5 | 41.0 | 7.2 | 79.7 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52.3 | 1.4 | 5.2 | 85.2 | 91.8 | 11.1 | 45.0 | 5.9 | 64.0 |
As shown in Table 1, it was clear that glucose yield in enzymatic hydrolysis process increased with an increase of dioxane-to-water ratio. However, there was almost no further improvement on glucose yield while dioxane-to-water ratio increased from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the preset research. The reason was that the dioxane–water ratio used in organosolv pretreatment process was an influential parameter for the delignification of corn stover. At the ratio of 1
1 in the preset research. The reason was that the dioxane–water ratio used in organosolv pretreatment process was an influential parameter for the delignification of corn stover. At the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, only 18.9% lignin was achieved because high proportion of water in acidic dioxane solution was adverse to the solubility of lignin. With the increase of dioxane content, a large amount of lignin dissolved into dioxane–water mixture, and the removal of lignin was beneficial for the exposure of cellulose micro-fibris.48 Meanwhile, when the ratio ranging from 1
1, only 18.9% lignin was achieved because high proportion of water in acidic dioxane solution was adverse to the solubility of lignin. With the increase of dioxane content, a large amount of lignin dissolved into dioxane–water mixture, and the removal of lignin was beneficial for the exposure of cellulose micro-fibris.48 Meanwhile, when the ratio ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of xylose increased from 5.9% to 31.5%, then reduced to 11.1%. A supposed reason was that hemicelluloses were hard to be hydrolyzed at such mild conditions as 90 °C, 20 min, 1
1, the yield of xylose increased from 5.9% to 31.5%, then reduced to 11.1%. A supposed reason was that hemicelluloses were hard to be hydrolyzed at such mild conditions as 90 °C, 20 min, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane-to-water ratio with 1 wt% HCl. When dioxane-to-water ratio increased, much more lignin fell off from lignocellulose, resulting in more LCC bands broken, so hemicelluloses could be easy hydrolyzed into xylose. However, the degradation of xylose became serious when further increased dioxane-to-water ratio from 9
1 dioxane-to-water ratio with 1 wt% HCl. When dioxane-to-water ratio increased, much more lignin fell off from lignocellulose, resulting in more LCC bands broken, so hemicelluloses could be easy hydrolyzed into xylose. However, the degradation of xylose became serious when further increased dioxane-to-water ratio from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Together with the xylose yield obtained from the second stage, a large amount of hemicelluloses were removed and recovered from lignocelluloses during two-stage pretreatment process. The removal of lignin and hemicelluloses could improve the cellulose accessibility for enzymatic hydrolysis and increase the glucose yield obviously. 91.5% glucose was obtained at 90 °C, 20 min, 9
1. Together with the xylose yield obtained from the second stage, a large amount of hemicelluloses were removed and recovered from lignocelluloses during two-stage pretreatment process. The removal of lignin and hemicelluloses could improve the cellulose accessibility for enzymatic hydrolysis and increase the glucose yield obviously. 91.5% glucose was obtained at 90 °C, 20 min, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane-to-water ratio with 1 wt% HCl. With a view of xylose yield and consumption of chemical reagent, 9
1 dioxane-to-water ratio with 1 wt% HCl. With a view of xylose yield and consumption of chemical reagent, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was chosen as the appropriate dioxane-to-water ratio in ADPH process.
1 (v/v) was chosen as the appropriate dioxane-to-water ratio in ADPH process.
3.4 Effect of acidic dioxane solution acidity on sugar recovery
The effect of acidic dioxane solution acidity (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.5 wt% hydrochloric acid) on glucose yield was investigated. Results are shown in Table 2.| HCl concentration (wt%) | Lignin removal (%) | Glucose yield (%) | Xylose yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Total | A | B | C | Total | ||
a A: glucose yield obtained in the first stage (90 °C, 20 min, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane-to-water ratio, 10 1 dioxane-to-water ratio, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C, 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis process (3 FPU enzyme dosage).b HCl concentration in acidic dioxane solution represented acidic dioxane solution acidity in this research. 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C, 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis process (3 FPU enzyme dosage).b HCl concentration in acidic dioxane solution represented acidic dioxane solution acidity in this research. |
|||||||||
| 0.2 | 18.6 | 0.6 | 5.6 | 61.3 | 67.5 | 1.8 | 79.1 | 6.5 | 87.4 |
| 0.4 | 24.6 | 1.1 | 5.4 | 68.6 | 75.1 | 19.7 | 58.1 | 7.1 | 84.9 |
| 0.6 | 44.5 | 1.3 | 5.3 | 72.7 | 79.3 | 29.3 | 47.5 | 6.9 | 82.7 |
| 0.8 | 46.6 | 1.5 | 5.2 | 78.3 | 85.0 | 33.6 | 40.3 | 7.4 | 81.3 |
| 1.0 | 49.8 | 1.5 | 5.3 | 85.3 | 91.5 | 31.5 | 41.0 | 7.2 | 79.7 |
| 1.2 | 51.4 | 1.8 | 6.1 | 86.2 | 94.1 | 28.4 | 40.5 | 6.6 | 75.4 |
| 1.5 | 52.1 | 2.0 | 6.5 | 86.4 | 94.9 | 26.4 | 40.1 | 6.5 | 73.0 |
As depicted in Table 2, after the two-stage pretreatment, the glucose yield obviously increased from 61.3% to 85.3%, when the acid concentration was increased from 0.2 wt% to 1.0 wt%, whereas further increase of acid concentration from 1.0 to 1.5 wt% only a little increase from 85.3% to 86.4% was observed. The results could be explained as follows: with the increase of acid concentration, more H+ would get involved in the cleavage of bonds between lignin–carbohydrates or lignin–lignin, leading to much more dissolution of lignin as well as hydrolysis of hemicellulose. Table 2 also shows that the recovery of lignin increased from 18.6% to 52.1% when the acid concentration inclined from 0.2 wt% to 1.5 wt%. However, further increasing acid concentration from 0.8% to 1.5%, the xylose yield obtained in the first stage obviously decreased from 33.6% to 22.4% although the glucose yield and the lignin recovery were still improved, which suggested partial xylose in dioxane–water mixture was converted into other by-products. Together with the xylose yield obtained in the second stage, a large proportion of hemicelluloses were hydrolyzed and recovery from lignocellulose. The removal of lignin and hemicelluloses made the cellulose more accessible to enzymes, achieving a higher efficiency of enzyme hydrolysis and glucose recovery. 79.7% xylose and 49.5% lignin were gained at 90 °C, 20 min, 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the first stage by 120 °C and 40 min for 1.0 wt% HCl in the second stage. To ensure both sugars recovery and equipment corrosion, a relatively low acid concentration of HCl (1 wt%) in dioxane–water solution was recommended, giving satisfactory xylose and glucose yields of 79.7%, 91.5% respectively with a cellulase dosage of 3 FPU g−1 of the substrate.
3.5 Effect of reaction time and temperature on sugar recovery
Effect of the time and temperature in the first stage on enzymatic hydrolysis was also evaluated in this research. The experiments were conducted at given temperatures (70 °C, 80 °C, 90 °C) and times (10 min, 20 min, 30 min, 40 min, 50 min) in the first stage.As depicted in Table 3, the sum yield of glucose obtained in the first stage and the second stage was less than 8.0%, which demonstrated that this pretreatment method had little impact on cellulose solubilization due to its tight structure and most of cellulose remained in the pretreated materials. It was worth to notice that the glucose yield in the first stage (acidic dioxane system) was especially low (0.5–1.8%). A possible reason was that only glycosidic bonds in amorphous region of cellulose in corn stover were cleaved in acidic dioxane system under such mild conditions (70–90 °C, 20–50 min). However the glucose yield had an increasing trend with the increasing time and prolonging time, which was agreement with the results of J. Zhang.49 In his report, they found that the glycosidic bonds could be effectively cleaved in acidic dioxane–water system and 48.3% D-glucosamine was obtained at 175 °C for 1 h during the depolymerization of chitin in acidic dixoane–water system. It was also observed that both reaction temperature and time had positive effects on glucose yield in enzymatic hydrolysis within the time scope of 10–40 min and temperature range of 70–90 °C. The reason was that the accessibility of enzyme to cellulose was improved as much more hemicellulose and lignin were hydrolyzed and removed with increasing temperature and prolonging time. Specifically, a high glucose yield of 91.5% was obtained with 3 FPU g−1 cellulose at 90 °C for 20 min but no significant increase was discovered prolonging time from 20–40 min. Also, reaction condition, whereas, it exhibited a decreasing tendency further prolonging time mainly because of the propensity of xylose into furfural or other by-products in acidic environment.46,47 Comprehensively considering glucose and xylose yield as well as energy consumption, 90 °C, 20 min was employed as the appropriate reaction condition for ADPH.
| Time and temperature | Lignin removal (%) | Glucose yield (%) | Xylose yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Total | A | B | C | Total | ||
a A: glucose yield obtained in the first stage (1 wt% HCl in dioxane/water solution, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane-to-water ratio, 10 1 dioxane-to-water ratio, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis (3 FPU enzyme dosage). 1 liquid–solid ratio); B: glucose yield obtained in the second stage (120 °C 40 min 1 wt% HCl); C: glucose yield obtained in enzymatic hydrolysis (3 FPU enzyme dosage). |
|||||||||
| 70 °C 10 min | 20.3 | 0.5 | 5.2 | 64.1 | 69.8 | 4.1 | 74.6 | 7.4 | 86.1 |
| 70 °C 20 min | 22.8 | 0.6 | 5.6 | 69.8 | 76.0 | 4.6 | 72.2 | 7.5 | 84.3 |
| 70 °C 30 min | 25.5 | 0.6 | 5.5 | 70.7 | 76.8 | 5.7 | 73.7 | 7.7 | 86.1 |
| 70 °C 40 min | 27.0 | 0.7 | 5.8 | 71.9 | 78.4 | 8.9 | 69.0 | 7.7 | 85.6 |
| 70 °C 50 min | 34.6 | 0.8 | 5.8 | 73.6 | 80.2 | 11.2 | 63.0 | 7.8 | 82.0 |
| 80 °C 10 min | 35.6 | 0.9 | 5.5 | 69.5 | 75.9 | 10.6 | 55.6 | 7.0 | 73.2 |
| 80 °C 20 min | 43.7 | 0.9 | 5.6 | 70.3 | 76.8 | 19.2 | 55.5 | 7.2 | 81.9 |
| 80 °C 30 min | 44.9 | 1.0 | 5.7 | 73.4 | 80.1 | 26.2 | 46.8 | 7.4 | 80.4 |
| 80 °C 40 min | 46.8 | 1.1 | 5.8 | 77.5 | 84.4 | 24.1 | 50.3 | 7.4 | 81.8 |
| 80 °C 50 min | 48.1 | 1.3 | 5.9 | 81.2 | 88.4 | 30.7 | 43.1 | 7.4 | 81.2 |
| 90 °C 10 min | 47.6 | 1.5 | 5.2 | 80.3 | 87.0 | 32.3 | 41.3 | 7.1 | 80.7 |
| 90 °C 20 min | 49.8 | 1.5 | 5.3 | 85.3 | 91.5 | 31.5 | 41.0 | 7.2 | 79.7 |
| 90 °C 30 min | 51.0 | 1.5 | 5.4 | 85.9 | 92.8 | 29.1 | 42.5 | 7.4 | 79.0 |
| 90 °C 40 min | 52.1 | 1.7 | 5.5 | 86.1 | 93.3 | 27.3 | 40.0 | 7.3 | 73.6 |
| 90 °C 50 min | 52.7 | 1.8 | 6.0 | 86.3 | 94.1 | 28.1 | 35.8 | 6.6 | 70.5 |
3.6 Characterization of corn stover pretreated and untreated
As discussed above, the enhancement of carbohydrates accessibility to enzyme by pretreatment had a strong association with the physical structure or altered chemical composition of biomass. To further understand ADPH pretreatment, the specific surface area, pore volume and morphology feature of biomass before and after pretreatment were characterized by BET and SEM.Porosity is an important factor that can influence the efficiency of enzymatic hydrolysis. The specific surface area (SSA) and pore volume of untreated, HP, ADP and ADHP materials were determined, with the results listed in Table 4. As depicted in Table 4, the SSA and pore volume of untreated samples was small, which demonstrated that the structure of raw material was compact and it was difficult to be hydrolyzed by enzyme. However, both SSA and pore volume increased no matter which pretreatment method was adopted because of the hemicelluloses and lignin removal. As shown in Table 4, after HP, SSA increased from 1.5444 to 3.8816 m2 g−1 while pore volume increased from 0.0022 to 0.0174 cm3 g−1. This could be triggered by the removal of hemicelluloses. Compared to HP, ADP process caused SAA increased to 4.281 m2 g−1 and the pore volume decreased to 0.0080 cm3 g−1 (Table 4). A possible reason was that the removal of the lignin (43.9% lignin) during ADP process caused the structural collapse and disruption of corn stover particles. And this collapse and disruption of particles could improve the specific surface area of biomass and reduce the pore volume. In the case of the ADPH process, the impact was prominently strengthened, which led to further increases of total pore volume and specific surface area (SAA 3.8816 m2 g−1, pore volume 0.0108 cm3 g−1, Table 4) because plenty of lignin and hemicellulose was removed. This helped to explain the reason why ADPH enable biomass to achieve a high enzymatic hydrolysis yield.
| Untreated | APa | ADPb | ADPHc | |
|---|---|---|---|---|
| a Treated only with 1 wt% HCl at 120 °C for 40 min.b Treated only with 9/1 (v/v) 1 wt% HCl dioxane–water solution at 90 °C for 20 min.c Treated with 9/1 (v/v) 1 wt% HCl dioxane–water solution at 90 °C for 20 min and followed by 1 wt% HCl at 120 °C for 40 min.d SSA represents specific surface area measured by N2 absorption method. | ||||
| SSAd (m2 g−1) | 1.5444 | 3.8816 | 4.2841 | 5.1265 |
| Pore volumed (cm3 g−1) | 0.0022 | 0.0174 | 0.0080 | 0.0108 |
Fig. 3 showed the SEM images of untreated corn stover, samples after HP, ADP and ADPH process. Fig. 3a illustrated that untreated corn stover exhibited a non-porous, tight and glossy structure, which enabled cellulose to possess a natural shield against enzymatic attack. Fig. 3b showed that the compact structure was partially damaged after HP pretreatment. Comparing with raw material, the fibers became fluff and some pores were founded on the surface of corn fiber, which improved the accessibility between cellulose and enzyme. The possible reason was that dilute hydrochloric acid, as a catalyst, could break the bonds existed in hemicelluloses and LCC,50 resulting in most of hemicelluloses degraded into xylose (83.9% xylose obtained). From Fig. 3c, it seemed that the surface architecture of corn stover after ADP process was damaged to a larger extent than that of HP process. It was due to the fact that H+ in acidic dioxane solution cleaved the same bonds as well as it did in HP process, but caused segmental hemicelluloses and a large amount of lignin dissolved into acidic dioxane solution (49.8% lignin and 31.5% xylose obtained). After ADPH, much lignin and hemicelluloses (49.8% lignin recovery and 72.5% xylose yield) was removed from the biomass, which severely destroyed the surface of samples, and resulted in a highly rough and irregular morphology (Fig. 3d), causing a significant increase of surface area which had a beneficial influence on the enzymatic hydrolysis. This result suggested that ADPH was an available method to improve enzymatic hydrolysis of cellulose by removing lignin and hemicelluloses.
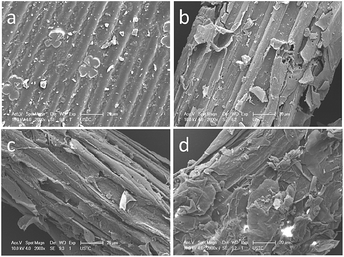 | ||
| Fig. 3 The surface appearance of (a) untreated, (b) HCl pretreated, (c) acidic dioxane pretreated and (d) two stage pretreated with acidic dioxane used in the first stage. | ||
Degree of polymerization (DP) could represent glucan chain length, which was deemed as one of the factors affecting enzymatic hydrolysis. The DPv changes could reflect the effect of pretreatment on corn stover and it was determined according the method mentioned in 2.4.6. Through determination, the DPv of untreated, HP-pretreated, ADP-pretreated and ADPH pretreated corn stover was 6498, 2332, 2153, 1446, respectively. After HP, ADP and ADPH pretreatment, the DPv was reduced by 64%, 67%, 78% as compared with that of untreated corn stover. Lower DPv value demonstrated shorter glucan chain length, which would improve the glucose yield in enzyme hydrolysis by increasing the specific surface area of material and the binding sites of cellulase.
4 Conclusions
The two-stage pretreatment using acidic dioxane followed by dilute hydrochloric acid could efficiently recover glucose from enzymatic hydrolysis. The impact of temperature, time, dioxane-to-water ratio, acid concentration in the first stage were investigated to determine the optimum pretreatment conditions. Under optimal conditions (90 °C, 20 min, 9/1 (v/v) dioxane–water including 1.0 wt% HCl solution in the first stage followed by 120 °C and 40 min for 1.0 wt% dilute hydrochloric acid in the second stage), 91.5% glucose and 79.7% xylose (including 7.2% from enzymatic hydrolysis) could be recovered with an enzyme dosage of 3 FPU g−1 substrate. The two-stage pretreatment showed the importance of both hemicelluloses and lignin removal on overall sugar recovery. After pretreatment, the specific surface area and pore volume increased, which improved the accessibility of enzymes to the cellulose.Acknowledgements
This study was financially supported by the State Key Program of National Natural Science Foundation of China (51536009), Science and Technological Fund of Anhui Province for Outstanding Youth (1508085J01), the National Key Technology R&D Program of China (No. 2015BAD15B06) and the international technology cooperation plan of Anhui (No. 1503062030).Notes and references
- Y. Kuang, Y. Zhang, B. Zhou, C. Li, Y. Cao, L. Li and L. Zeng, Renewable Sustainable Energy Rev., 2016, 59, 504–513 CrossRef.
- S. M. Shafie, Renewable Sustainable Energy Rev., 2016, 59, 1089–1100 CrossRef.
- S. Alipour and H. Omidvarborna, RSC Adv., 2016, 6, 111616–111621 RSC.
- B. M. Upton and A. M. Kasko, Chem. Rev., 2015, 11, 2275–2306 Search PubMed.
- J. N. Putro, F. E. Soetaredjo, S. Y. Lin, Y. H. Ju and S. Ismadji, RSC Adv., 2016, 6, 46834–46852 RSC.
- M. E. Himmel, S. Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804–807 CrossRef CAS PubMed.
- R. Ravindran and A. K. Jaiswal, Bioresour. Technol., 2016, 199, 92–102 CrossRef CAS PubMed.
- Q. Liu, W. Li, Q. Ma, S. An, M. Li, H. Jameel and H. M. Chang, Bioresour. Technol., 2016, 211, 435–442 CrossRef CAS PubMed.
- S. M. Kim, M. E. Tumbleson, K. D. Rausch and V. Singh, Bioresour. Technol., 2017, 232, 297–303 CrossRef CAS PubMed.
- A. Djioleu and D. J. Carrier, ACS Sustainable Chem. Eng., 2016, 4, 4124–4130 CrossRef CAS.
- J. Zhang, S. Shao and J. Bao, Bioresour. Technol., 2016, 201, 355–359 CrossRef CAS PubMed.
- G. D. Saratale and M. K. Oh, RSC Adv., 2015, 5, 97171–97179 RSC.
- M. C. Coimbra, A. Duque, F. Saéz, P. Manzanares, C. H. Garcia-Cruz and M. Ballesteros, Renewable Energy, 2016, 86, 1060–1068 CrossRef CAS.
- M. Michelin and J. A. Teixeira, Bioresour. Technol., 2016, 216, 862–869 CrossRef CAS PubMed.
- X. Zhuang, W. Wang, Q. Yu, W. Qi, Q. Wang, X. Tan and Z. Yuan, Bioresour. Technol., 2016, 199, 68–75 CrossRef CAS PubMed.
- K. Zhang, Z. Pei and D. Wang, Bioresour. Technol., 2016, 199, 21–33 CrossRef CAS PubMed.
- K. Zhang, Z. Pei and D. Wang, Bioresour. Technol., 2016, 21–33 CrossRef PubMed.
- X. Sun, X. Sun and F. Zhang, RSC Adv., 2016, 6, 99455–99466 RSC.
- R. Financie, M. Moniruzzaman and Y. Uemura, J. Chem. Eng., 2016, 110, 1–7 CAS.
- R. Sindhu, P. Binod and A. Pandey, Bioresour. Technol., 2016, 199, 76–82 CrossRef CAS PubMed.
- M. García-Torreiro, M. López-Abelairas, T. A. Lu-Chau and M. Lema, Ind. Crops Prod., 2016, 89, 486–492 CrossRef.
- V. Chang and M. Holtzapple, Appl. Biochem. Biotechnol., 2000, 84–86, 5–37 CrossRef CAS PubMed.
- P. Phitsuwan, K. Sakka and K. Ratanakhanokchai, Biomass Bioenergy, 2013, 58, 390–405 CrossRef CAS.
- R. Ravindran and A. K. Jaiswal, Bioresour. Technol., 2016, 199, 92–102 CrossRef CAS PubMed.
- S. M. Kim, B. S. Dien, M. E. Tumbleson, K. D. Rausch and V. Singh, Bioresour. Technol., 2016, 216, 706–713 CrossRef CAS PubMed.
- G. Brodeur, J. Telotte, J. J. Stickel and S. Ramakrishnan, Bioresour. Technol., 2016, 220, 621–628 CrossRef CAS PubMed.
- L. Mesa, E. González, C. Cara, M. González, E. Castro and S. I. Mussatto, J. Chem. Eng., 2011, 168, 1157–1162 CrossRef CAS.
- W. Li, Q. Liu, Q. Ma, T. Zhang, L. Ma, H. Jameel and H. M. Chang, Bioresour. Technol., 2016, 219, 753–756 CrossRef CAS PubMed.
- F. A. Perras, H. Luo, X. Zhang, N. S. Mosier, M. Pruski and M. M. Abu-Omar, J. Phys. Chem. A, 2016, 121, 623–630 CrossRef PubMed.
- A. Berlin, M. Balakshin, N. Gilkes, J. Kadla, V. Maximenko, S. Kubo and J. Saddler, J. Biotechnol., 2006, 125, 198–209 CrossRef CAS PubMed.
- J. K. Saini, A. K. Patel, M. Adsul and R. R. Singhania, Renewable Energy, 2016, 98, 29–42 CrossRef CAS.
- B. B. Hewetson, X. Zhang and N. S. Mosier, Energy Fuels, 2016, 30, 9975–9977 CrossRef CAS.
- G. Guo, S. Li, L. Wang, S. Ren and G. Fang, Bioresour. Technol., 2013, 135, 738–741 CrossRef CAS PubMed.
- Q. Qing, B. Yang and C. E. Wyman, Bioresour. Technol., 2010, 101, 9624–9630 CrossRef CAS PubMed.
- X. Wang, K. Li, M. Yang and J. Zhang, Carbohydr. Polym., 2016, 148, 362–370 CrossRef CAS PubMed.
- S. Zu, W. Z. Li, M. Zhang, Z. Li, Z. Wang, H. Jameel and H. M. Chang, Bioresour. Technol., 2014, 152, 364–370 CrossRef CAS PubMed.
- T. Ghose, Pure Appl. Chem., 1987, 59, 257–268 CAS.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of structuralcarbohydrates and lignin in biomass, NREL, 2008 Search PubMed.
- A. Sluiter, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, Determination of Extractives in Biomass, Laboratory Analytical Procedure (LAP), NREL, 2005 Search PubMed.
- A. Sluiter, B. Hames, D. Hyman, C. Payne, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and J. Wolfe, Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples, NREL, 2008 Search PubMed.
- W. J. Huijgen, J. H. Reith and H. D. Uil, Ind. Eng. Chem. Res., 2010, 49, 10132–10140 CrossRef CAS.
- ASTM, Standard test method for intrinsic viscosity of cellulose (D 1795), Am. Soc. Test. Master, 1986, vol. 15, pp. 360–366 Search PubMed.
- R. Kumar, G. Mago, V. Balan and C. E. Wyman, Bioresour. Technol., 2009, 100, 3948–3962 CrossRef CAS PubMed.
- H. Y. Li, X. Chen and C. Z. Wang, Biotechnol. Biofuels, 2016, 9, 166–179 CrossRef PubMed.
- T. Renders, W. Schutyser, S. V. Bosch and S. F. Koelewijn, ACS Catal., 2016, 6, 2055–2066 CrossRef CAS.
- D. E. Kim and X. Pan, Ind. Eng. Chem. Res., 2010, 49, 12156–12163 CrossRef CAS.
- W. J. Huijgen, A. T. Smit, J. H. Reith and H. D. Uil, J. Chem. Technol. Biotechnol., 2011, 86, 1428–1438 CrossRef CAS.
- Y. L. Loow, T. Y. Wu, J. M. Jahim, A. W. Mohammad and W. H. Teoh, Cellulose, 2016, 23, 1491–1520 CrossRef CAS.
- J. Zhang and N. Yan, ChemcatChem, 2017 DOI:10.1002/cctc.201601715.
- X. Ye, Z. Zhang, Y. Chen, J. Cheng, Z. Tang and Y. Hu, Ind. Crops Prod., 2016, 87, 280–286 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |