Open Access Article
Open Access ArticleFate of nitrogen-15 in the subsequent growing season of greenhouse tomato plants (Lycopersicon esculentum Mill) as influenced by alternate partial root-zone irrigation
Maomao Hou *a,
Fenglin Zhonga,
Qiu Jinb,
Enjiang Liuc,
Jie Fenga,
Tengyun Wanga and
Yue Gaoc
*a,
Fenglin Zhonga,
Qiu Jinb,
Enjiang Liuc,
Jie Fenga,
Tengyun Wanga and
Yue Gaoc
aCollege of Horticulture, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China. E-mail: njhoumaomao@126.com
bInstitute of Water Conservancy Science of Jiangsu Province, Nanjing 210000, Jiangsu, China
cDevelopment and Reform Commission of Suihua, Suihua, Heilongjiang, China
First published on 10th July 2017
Abstract
Alternate partial root-zone irrigation (APRI) has profound impacts on the distribution of nitrogen fertilizer applied in-season. However, the fate of previous residual nitrogen in the subsequent crop growing season under APRI has seldom been studied. Our objective in this study was to investigate the effects of APRI on the reutilization, redistribution and loss of residual nitrogen in the subsequent season. To achieve this objective, in the previous season, greenhouse tomatoes (Lycopersicon esculentum Mill) were chosen as a plant material, and were treated with two irrigation patterns (APRI and conventional irrigation (CI)), two 15N labeling depths (K15NO3 with a 15N abundance of 10.57%, labeled in the 0–20 cm and 40–60 cm soil layers, respectively) and two transplant times (early and late summer). In the subsequent season, we adopted the same irrigation patterns, but with no 15N labeled in the soil. Our results showed that 81.3–90.7% of the residual 15N from the previous season still remained in the 0–100 cm soil layer, 4.1–7.3% was absorbed by the subsequent-season tomatoes, and 2.9–14.6% was lost. The 15N reutilization rates (defined as the ratio of 15N uptake by the subsequent tomatoes to the total applied 15N) were 2.20–4.73% under the different treatments (the 15N utilization rates of the in-season tomatoes were 18.8–27.9%). Compared to CI, APRI significantly (p < 0.05) increased the plant 15N uptake and 15N reutilization rate, and APRI also contributed to a greater mineral and organic 15N amount in shallower soil layers. Overall, the tomato 15N reutilization rate was found to be significantly (p < 0.05) higher when 15N labeling was performed in the 0–20 cm soil layer compared to that in the 40–60 cm layer. Moreover, the 15N reutilization rate had a significant positive relationship with the root dry weight (R = 0.74*), root length density (R = 0.72*), soil mineral 15N (R = 0.91**) and total residual 15N amount (R = 0.88**).
Introduction
Chinese farmers have been using inorganic fertilizers extensively since the 1980s due to increasing labor costs and the relatively lower efficiency of organic fertilizers.1 Inorganic fertilizer application is one of the important factors of the so-called “Miracle in China”, using 7% of the arable land to feed 22% of the population of the world. The high residues of inorganic fertilizers, particularly nitrogen fertilizers, have induced a series of ecological and environmental problems, such as soil acidification,2 salinization3,4 and crop nitrate/nitrite and ammonia poisoning.5,6 These problems are of great concern, not only in China, but also in many other countries.7–9 For greenhouse-covered arable fields, the situation of nitrogen residues is worsening. China can apply 569–2000 kg ha−1 of pure nitrogen during one season of production of greenhouse crops, which is several times or even dozens of times over that applied to ordinary field crops, leading to a large quantity of nitrogen residues10 and losses.11 Among different forms of residual nitrogen, nitrate-nitrogen (NO3−–N) is characterized as easily leached;12 NO3−–N is hard to convert to other forms in deeper soil layers, and therefore it not only pollutes surface water through runoffs, but also poses a serious threat to underground water environments.13Greenhouse agriculture in northern China suffers from high NO3−–N residues, as well as severe water shortages. The distribution of water resources in China is geographically uneven, with 81% of the total water resources being intensively distributed in the Yangtze River basin and southern regions. Specifically, there is ten times more fresh water per capita in the south than the north.14 Thus, for agricultural production in northern China, it is of great importance to utilize water resources efficiently and control the outputs of agricultural contaminants.
Efforts have been made to employ innovative irrigation methods to promote the growth of crop roots, and to recover the residual nitrogen fertilizer in the soil.15,16 A study using 15N labeling has revealed that water-saving irrigation is conducive to winter wheat recovering nitrogen fertilizer in the deeper soil layer at 100–150 cm.17 Alternate partial root-zone irrigation (APRI) is one component of partial root-zone irrigation (PRI). As a relatively new water-saving irrigation technique, APRI has now been applied in the production of soybean, peppers, apples, potatoes, tomatoes, cotton, grapes, etc.18 In APRI, half of the root-zone is irrigated while the other half is allowed to dry, and then the previously well-watered side of the root system is allowed to dry while the previously dried side is irrigated when the next irrigation occurs.15 Earlier results have demonstrated that APRI can significantly save irrigation water without significantly decreasing the yield.19,20 APRI has also been proved to promote dry matter accumulation in the roots and increase the root length density.21 Although many studies have investigated the impact of APRI on crop yield and water use, few studies have focused on how APRI influences the crop NO3−–N uptake, and no research has studied the fate and balance of applied nitrogen fertilizers in the subsequent growing season of the crop under the influence of APRI.
In our previous study in 2014, we labeled K15NO3 (abundance of 10.57%) in different soil layers at 0–20 and 40–60 cm, and studied the impact of alternate partial root-zone irrigation on the 15N uptake of greenhouse tomatoes. We found that APRI had a profound impact on the distribution of in-season applied fertilizer nitrogen.22 The objective of this study (conducted in 2015) was to investigate the fate and balance of previous residual 15N in the subsequent growing season of greenhouse tomatoes, as influenced by APRI. Details included: (1) the reutilization of 15N by the subsequent tomatoes; (2) the distribution of 15N in different soil layers; (3) the balance of 15N in the subsequent growing season. The study conclusions are expected to provide useful information for those in areas suffering from an agricultural water shortage and excessive nitrogen residues.
Materials and methods
Experimental site description and the previous experiment
The experiments were conducted in 2015 at the Production Base of Greenhouse Vegetables (longitude 126°22′E, latitude 46°12′N) of Lanxi county, Suihua city, Heilongjiang province. Suihua belongs to the northern hemisphere temperate zone; it has four clear seasons, with snow cover in winter, while being warm and humid in summer. The annual average temperature from 2000 to 2013 in Suihua ranged from 1.3 °C to 4.0 °C. There is an annual duration of 120–140 days in the frost-free season, and a sunshine duration of 2600–2900 h. The annual average precipitation in Suihua is 483 mm. Precipitation occurs intensively in July and August. The experiment was carried out in a solar greenhouse. The span of the greenhouse is 10 m, the length is 8 m and the height of the back wall is 3 m. For ventilation and cooling in summer, several vents were installed in the back wall with 1 m height above the ground; for details, see Fig. 1. Crop seedlings were transplanted separately in early and late summer. The day/night average temperature was 24.7/20.2 °C in early summer, and 21.3/18.5 °C in late summer, during the whole growth stage of the crop. | ||
| Fig. 1 The solar greenhouse used in this experiment (the day/night average temperature was 24.7/20.2 °C in early summer, and 21.3/18.5 °C in late summer, during the whole growth stage of the crop).22 | ||
Since this experiment constitutes a second part of our previous work, here we briefly introduce the previous experiment:22
The previous experiment was carried out in 2014 in the above-mentioned greenhouse. The physical and chemical attributes of the original soil were determined as shown in Table 1. The tomato (Lycopersicon esculentum Mill) cultivar “Red Ruby” was used as the plant material. The experiment included two irrigation patterns (APRI and conventional irrigation (CI)), two 15N labeling locations in the soil layers (0–20 and 20–40 cm), and two transplant times (early and late summer). The transplant dates in 2014 for early and late summer were June 18 and August 22, respectively. For details of the experimental design, see Table 2. As was recorded, the total irrigation amount of CI and APRI was 498 and 324 mm, respectively, at the transplant time of early summer, and 476 and 310 mm at that of late summer.
| Soil depth (cm) | pH | Bulk density (g cm−3) | Organic matter (g kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Total N (g kg−1) |
|---|---|---|---|---|---|---|---|
| 0–10 | 7.37 | 1.39 | 14.71 | 122.4 | 18.81 | 121.3 | 1.40 |
| 10–20 | 7.44 | 1.42 | 10.93 | 105.7 | 14.92 | 106.2 | 1.25 |
| 20–60 | 7.65 | 1.55 | 8.62 | 91.6 | 5.33 | 63.4 | 0.78 |
| 60–100 | 7.91 | 1.51 | 5.36 | 61.3 | 3.21 | 35.5 | 0.39 |
| Transplant time | Treatment | Transplant date | Irrigation pattern | Depth of 15N labeling (cm) |
|---|---|---|---|---|
| a Note: APRI represents alternate partial root-zone irrigation, and CI represents conventional irrigation. | ||||
| Early summer | APRI10 | June 18 | APRI | 0–20 |
| CI10 | June 18 | CI | 0–20 | |
| APRI50 | June 18 | APRI | 40–60 | |
| CI50 | June 18 | CI | 40–60 | |
| Late summer | APRI10 | August 22 | APRI | 0–20 |
| CI10 | August 22 | CI | 0–20 | |
| APRI50 | August 22 | APRI | 40–60 | |
| CI50 | August 22 | CI | 40–60 | |
Several soil columns that were pre-buried in the soil were used for the experiment. The soil columns were prepared using a PVC cylindrical mold. The height of the PVC mold was 1 m and the diameter was 40 cm, with the bottom unsealed. Plastic film was employed and kept close to the inner side of the mold. The soils were dug out by 20 cm in each layer and then filled back into the mold as the field's original soil layers. The backfilled soils inside and outside of the mold were kept at the same height during the filling process in order to avoid the deflection of the mold. To provide the nutrients that are needed by the tomato plants, the 0–20 cm layer of the soil was mixed with 100 mg kg−1 N, 150 mg kg−1 P2O5 and 150 mg kg−1 K2O. These pure nutrients came from NH4NO3, Ca(H2PO4)2 and K2SO4, respectively. The 15N used for labeling was K15NO3 (abundance of 10.57%), and the 15N was labeled to a thickness of 10 cm, as is displayed in Fig. 2. The dosage of 15N was 466 mg for each soil column. After finishing the soil backfilling and 15N labeling, the molds were taken out from the field, only leaving the plastic film to separate the soils inside and outside the columns. At 55 and 76 DAT, dissolved urea was applied two times as additional fertilizer, and each time the application amount was 60 mg kg−1 N. For each APRI treatment, a film separator (20 cm height) was buried in the middle of each soil column, leaving 5 cm height out of the soil surface (Fig. 2). The film separator had a gap in its center for transplanting the tomato seedlings. Fig. 3 displays the arrangement of the soil columns. The distance between two adjacent columns was 20 cm, and the distance between two plots for different transplant times was 40 cm. Fig. 1–3 can also be seen in our previous study.22
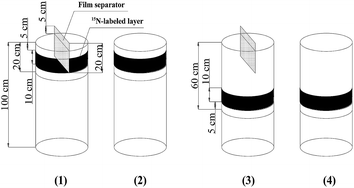 | ||
| Fig. 2 Diagrammatic sketch of 15N labeling in the soil columns in 2014 (soil columns (1) and (3) are for the plants with alternate partial root-zone irrigation, and columns (2) and (4) are for the plants with conventional irrigation).22 | ||
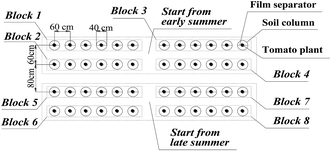 | ||
| Fig. 3 The arrangement of soil columns (in blocks 1 and 5, the plants are treated with alternate partial root-zone irrigation, and 15N is labeled at 10 cm depth in the soil; in blocks 3 and 7, the plants are treated with alternate partial root-zone irrigation, and 15N is labeled at 50 cm depth in the soil; in blocks 2 and 6, the plants are treated with conventional irrigation, and 15N is labeled at 10 cm depth in the soil; in blocks 4 and 8, the plants are treated with conventional irrigation, and 15N is labeled at 50 cm depth in the soil).22 | ||
After the experiment, the total amount of 15N remaining in the soil at 0–100 cm was 251.4–309.6 mg per column; for details, see ref. 22.
Experimental design
This study (2015) was conducted to investigate the fate of residual 15N from the previous experiment (2014), therefore this experiment was carried out in situ. After the experiment in 2014, no crops were planted in the soil columns until starting this experiment. The soils in the 0–20 cm layer for each soil column were ploughed before the tomato planting of this season.This experiment adopted the same irrigation patterns as our previous study in 2014, namely APRI and CI. An earlier study conducted in a solar greenhouse in northern China proved that the tomato water use efficiency, yield and quality could reach an optimal compromise when controlling the lower limit of soil moisture at 70% θf (field capacity) and the upper limit at 90% θf.23 Thus, during the whole growth stage of the tomato plants, the soil moisture of CI in this study was controlled at a lower limit of 70% θf, and an upper limit of 90% θf. Moreover, earlier results demonstrated that APRI saved 40% irrigation water, while not significantly reducing the crop yield.21,24 According to this information, the total irrigation amount of APRI in our study was designed as 60% of the amount of CI. An irrigation amount of 62 mm was applied for the survival of the seedlings during 0–28 DAT (days after transplant), for the transplant times of both early and late summer. After that, the tomatoes were irrigated with different patterns of APRI and CI.
Moreover, the fertilization and transplant times in this experiment were also the same as those in 2014, while in this study no 15N was labeled in the soil. The transplant date was June 15 for the transplant time of early summer, and August 17 for that of late summer. The total irrigation amount of CI and APRI was recorded as 501 and 325 mm for the tomatoes transplanted in early summer, and 486 and 316 mm for those transplanted in late summer.
Sampling and measurement
For both transplant times in 2015, plant samples were collected separately as root, stem, fruit and leaf (including ordinary leaves and fallen leaves) samples after the last harvest; meanwhile, soil samples were collected using a diminutive soil auger at 10 cm per layer. Ten samples were collected in the 0–100 cm soil layer for each soil column.(1) 15N atom percentage excess: air-dried soil samples were ground and passed through a 0.15 mm sieve for measuring the 15N atom percentage excess. Mineral nitrogen in fresh soil samples was extracted using 2 M KCl and distilled using micro Kjeldahl apparatus, in the presence of MgO and Devarda alloy.25 The 15N atom percentage excess in the sample was determined using a mass spectrometer (Finniga-Mat-251, Mass-Spectrometers, Finnigan, Germany) at Nanjing Institute of Soil Science, CAS.
(2) Root length density: root samples from the tomato plants were cleaned and scanned using an EPSON EXPRESSION 1680 scanner, and then analyzed using WinRHIZO software to obtain the data on root length density.
(3) Root dry matter: fresh root samples from tomato plants were placed in an oven, and dried firstly at 105 °C for 30 min, and then at 70 °C until a constant weight was achieved.
Calculations and statistical analysis
(1) Reutilization rate of 15N (15NUE, %). 15NUE was calculated as:1
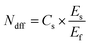 | (1) |
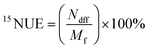 | (2) |
(2) Organic 15N. The organic 15N content in each soil layer was calculated as the difference between the total 15N minus the mineral 15N in the respective soil layer.25
(3) 15N recovery amount (mg per soil column). The amount of 15N recovery was the sum of the residual 15N in the 0–100 cm soil layer and the 15N uptake by the tomato plants.26
(4) 15N loss (mg per soil column). The amount of 15N loss is calculated using the applied 15N amount minus the 15N recovery amount.11
The data were compared statistically using SPSS software Version 17.0.27
Results
15N uptakes in different plant parts
APRI significantly (p < 0.05) increased the 15N accumulation in all plant parts except for the stem in the plants transplanted in early summer (Table 3). The 15N amount under APRI was increased by 37.9–53.4%, 10.1–38.3%, 36.6–73.1% and 47.1–61.9%, respectively in the leaves, stems, roots and fruits of the tomato plants, when compared to those under CI.| Transplant time | Treatment | Leaf (mg per plant) | Stem (mg per plant) | Root (mg per plant) | Fruit (mg per plant) |
|---|---|---|---|---|---|
| a Note: APRI10 and APRI50 represent that 15N was labeled at 10 and 50 cm soil depths, respectively, under alternate partial root-zone irrigation, and CI10 and CI50 represent that 15N was labeled at 10 and 50 cm soil depths, respectively, under conventional irrigation in the 2014 season. In the same column, means followed by the same letter (a, b, c, d, or e) do not differ significantly at the 5% level, according to Duncan's multiple range test. Each value is the mean ± SD. | |||||
| Early summer | APRI10 | 7.68 ± 0.34a | 2.14 ± 0.10a | 1.23 ± 0.09a | 10.99 ± 1.40a |
| CI10 | 5.00 ± 0.45b | 1.94 ± 0.07ab | 0.82 ± 0.07bc | 6.89 ± 1.28c | |
| APRI50 | 5.40 ± 0.35b | 1.99 ± 0.11ab | 0.93 ± 0.03b | 8.95 ± 0.36b | |
| CI50 | 3.75 ± 0.28c | 1.54 ± 0.20d | 0.68 ± 0.03cd | 6.08 ± 0.74cd | |
| Late summer | APRI10 | 6.99 ± 0.41a | 2.05 ± 0.13a | 1.26 ± 0.04a | 10.78 ± 0.73a |
| CI10 | 4.59 ± 0.26bc | 1.80 ± 0.04bc | 0.73 ± 0.06c | 6.78 ± 0.70c | |
| APRI50 | 5.51 ± 0.90b | 1.59 ± 0.04cd | 0.79 ± 0.04c | 7.37 ± 0.37bc | |
| CI50 | 3.99 ± 0.15c | 1.15 ± 0.06e | 0.58 ± 0.09d | 4.55 ± 0.59d | |
For each plant part, the difference in 15N amount at the different labeling depths but under the same irrigation pattern was significant (p < 0.05), except for some unrepresentative cases. In treatments with 15N labeled at the soil depth of 10 cm, the 15N amount in the leaves, stems, roots and fruits of the tomato plants was 15.0–42.3%, 7.7–56.2%, 20.5–59.2% and 13.2–44.8% higher than that labeled at a depth of 50 cm.
Although a delay in the transplant time slightly decreased the 15N accumulative amount in the respective plant parts, the decrease was not significant (p > 0.05) overall. A noticeable decrease in 15N caused by transplant time was found in the stem under APRI50 and CI50, and in the roots under APRI50.
Total 15N, mineral 15N and organic 15N in the soil layers
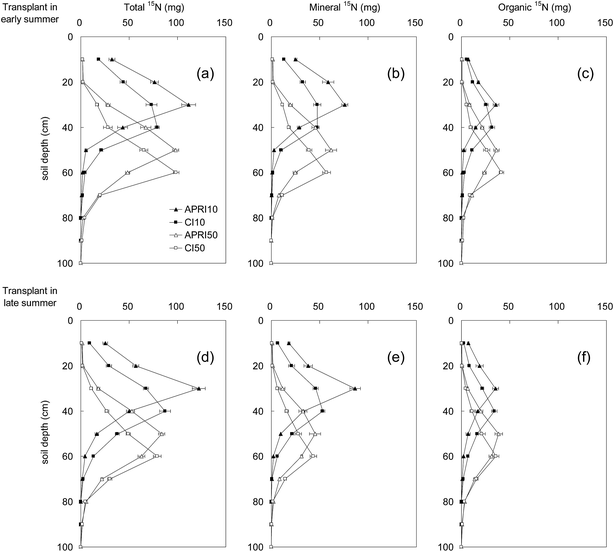
Fig. 4 displays the profiles of total 15N, mineral 15N and organic 15N in the 0–100 cm soil layer under different treatments. From the distribution of total 15N, it was found that the peak value under APRI10, CI10, APRI50 and CI50 appeared at a soil depth of 30, 40, 50 and 60 cm, respectively. Similar trends were obtained with the transplant time of both early and late summer. Compared to CI, APRI reserved 48.6–176.3% more 15N in the respective labeled layer. Under the same treatment (irrigation and labeling), the distribution of total 15N was similar between the different transplant times, while the amount of total 15N was found to be lower with the transplant time of late summer.Mineral 15N and organic 15N were distributed analogously in the soil layers to the total 15N. As is shown in Fig. 4(b–e), the 15N in the soil was mainly present in the mineral form. When the 15N was labeled at 10 cm depth, the mineral 15N in the 0–30 cm layer under APRI was significantly (p < 0.05) higher than that under CI. And when the 15N was labeled at 50 cm depth, the mineral 15N in the 0–50 cm layer under APRI was significantly (p < 0.05) higher than that under CI.
Overall, the transplant time in our study did not significantly change the distribution of total 15N, mineral 15N or organic 15N in the 0–100 cm soil layer. However, the irrigation pattern had clear effects on the 15N accumulation and distribution in the soil layers. APRI contributed greatly to the preservation of 15N in a shallower soil layer relative to CI, for the 15N labeling depths of both 10 and 50 cm.
Distribution and balance of 15N
The distribution and balance of 15N is shown in Table 4. After the experiment in 2015, the accumulation amount of 15N in the 0–100 cm soil layer was in the range of 204.5–276.4 mg per soil column, accounting for 81.3–90.7% of the original 15N amount. The greatest 15N accumulation amount in the soil was achieved by APRI10, and the difference between the two transplant times was not significant (p > 0.05). The irrigation pattern had an obvious influence on the accumulation amount of 15N in the soil, as APRI increased it significantly (p < 0.05) by 10.8–18.0%; the greatest increment was found between APRI50 and CI50 when the tomato plants were transplanted in late summer. Otherwise, a deeper 15N labeling depth resulted in a lower accumulation amount of 15N in the soil; this was particularly obvious for the transplant time of late summer. Overall, the plant 15N uptake was significantly (p < 0.05) increased by APRI in comparison to CI, and was also significantly (p < 0.05) increased by a 10 cm labeling depth in comparison to 50 cm. Although a slight increase in 15N uptake was also detected in the tomatoes transplanted in early summer compared to those transplanted in late summer, the increase was not significant (p > 0.05).| Transplant time | Treatment | Residual 15N from the 2014 season (mg) | Recovery (mg) | Loss (mg) | |
|---|---|---|---|---|---|
| Soil residual (mg) | Plant uptake (mg) | ||||
| a Note: APRI10 and APRI50 represent that 15N was labeled at 10 and 50 cm soil depths, respectively, under alternate partial root-zone irrigation, and CI10 and CI50 represent that 15N was labeled at 10 and 50 cm soil depths, respectively, under conventional irrigation in the 2014 season. In the same column, means followed by the same letter (a, b, c, d, or e) do not differ significantly at the 5% level, according to Duncan's multiple range test. Each value is the mean ± SD. The amount of 15N refers to the amount in each soil column. The amount of soil residual 15N includes that in the 0–100 cm soil layer. | |||||
| Early summer | APRI10 | 302.3 | 271.5 ± 6.8a | 22.0 ± 1.6a | 8.7 |
| CI10 | 267.0 | 242.2 ± 5.6b | 14.7 ± 1.9bcd | 10.2 | |
| APRI50 | 304.0 | 267.5 ± 9.9a | 17.3 ± 0.8b | 19.2 | |
| CI50 | 278.4 | 238.1 ± 11.3b | 12.1 ± 1.2de | 28.2 | |
| Late summer | APRI10 | 309.6 | 276.4 ± 9.0a | 21.1 ± 0.7a | 12.1 |
| CI10 | 271.4 | 246.2 ± 8.9b | 13.9 ± 0.8cd | 11.3 | |
| APRI50 | 286.3 | 249.2 ± 5.8b | 15.3 ± 1.3bc | 21.8 | |
| CI50 | 251.4 | 204.5 ± 7.2c | 10.3 ± 0.9e | 36.7 | |
After the experiment in the 2015 season, the amount of 15N recovery under the different treatments was found to range from 214.7 to 297.5 mg, and the amount of 15N loss ranged from 8.7 to 36.7 mg. The recovery and loss amount of 15N in the 2015 season accounted for 85.4–97.1% and 2.9–14.6%, respectively, of the total residual 15N from 2014. Among the different treatments, APRI10 gave the greatest 15N recovery and the lowest 15N loss. With the same irrigation pattern and labeling depth, the 15N loss was lower when the plants were transplanted in early summer, relative to late summer.
15N reutilization rate and its influencing factors
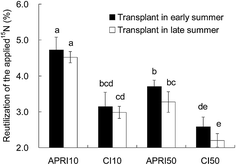
The 15NUE ranged from 2.20 to 4.73% under the different treatments (Fig. 5). The highest 15NUE of 4.73% was obtained for the tomato plants transplanted in early summer under the APRI10 treatment. Compared to CI, APRI significantly (p < 0.05) increased the 15NUE by 43.2–51.7%. However, the transplant time had no significant (p > 0.05) effect on 15NUE, although a slight decrease in 15NUE was observed for the transplant time of late summer. In addition, it should be noticed that the 15NUE was higher when the 15N was labeled in a shallower soil layer. Compared to APRI10, a significant (p < 0.05) reduction in 15NUE was found for APRI50, for both transplant times. A similar trend was also detected between CI10 and CI50.The relationship between 15NUE and its influencing factors is shown in Table 5. 15NUE had a significant positive relationship with the root dry matter amount (R = 0.74*), root length density (R = 0.72*), total 15N (R = 0.90**), mineral 15N (R = 0.91**) and total residual 15N amount from the 2014 season (R = 0.88**). This indicated that the 15NUE was closely related to the plant roots, as well as the 15N amount and its availability.
| 15N reutilization rate | Root dry matter | Root length density | Total 15N | Mineral 15N | 15N residual by 2014 | |
|---|---|---|---|---|---|---|
| a Represents significant correlation at the 0.01 level.b Represents significant correlation at the 0.05 level. Total 15N, mineral 15N and 15N residual from 2014 are that of the 0–60 cm soil layer. | ||||||
| 15N reutilization rate | 1.00 | 0.74b | 0.72b | 0.90a | 0.91a | 0.88a |
| Root dry matter | 1.00 | 0.68 | 0.66 | 0.65 | 0.54 | |
| Root length density | 1.00 | 0.48 | 0.45 | 0.60 | ||
| Total 15N | 1.00 | 0.98a | 0.81b | |||
| Mineral 15N | 1.00 | 0.77b | ||||
| 15N residual by 2014 | 1.00 | |||||
Discussion
Much research has been done on the reuse of applied nitrogen by succeeding crops. Liang1 observed a reutilization rate of fertilizer nitrogen of 2–9%. Macdonald28 reported that approximately 6% of residual nitrogen was taken up by the succeeding crop. Bhogal29 found that equivalent to 8–20% of the fertilizer nitrogen applied in the previous season was absorbed by the crops in the succeeding season. A four-year case study in eastern China also demonstrated that the total reutilization of fertilizer nitrogen applied in the first season was 11–15% during the later three seasons, almost reaching half of that in the first season.30 Presently, many earlier studies have evaluated the reuse and the redistribution of previous applied nitrogen in the subsequent season. The main difference between our study and those earlier works is that we investigated the fate of previous residual nitrogen under the influence of APRI, a promising irrigation pattern for areas suffering from a water shortage.We observed a significantly higher 15N amount in the tomato leaves under APRI when compared to CI; this might be explained as follows: (1) APRI enhanced the availability of soil 15N,31 and (2) before this experiment, the soil 15N amounts in the original soil under different treatments were different, and more 15N remained in the shallow soil layer under APRI in the previous season (2014). In the previous season, the soil total 15N amount under APRI treatment was 34.3% higher compared to that under CI treatment.22 The higher 15N amount in other plant parts, including the stem, roots and fruits, also proved the advantages of APRI in promoting crop nitrogen reuptake. The high plant nitrogen uptake under APRI might also be explained by the higher microbial biomass and nitrogen immobilization in the soil.32 An earlier study indicated that the plant nitrogen uptake decreased as the temperature decreased,33 possibly due to a reduction in soil mineral nitrogen under lower temperatures.34 We found a slight 15N decrease in the plants transplanted in late summer compared to those transplanted in early summer, though the decrease was not significant (p > 0.05).
Besides the 15N residual effects from the previous season, the redistribution of total 15N in the 0–100 cm soil layer may have been affected by the irrigation pattern and 15N labeling location. Under APRI, the position of the 15N peak in the soil profile was approximately 10 cm shallower than that under CI, indicating that nitrogen leaching was weakened by APRI. This confirmed the result of Wang's21 study. Besides, Wang also reported that 61.3% of the 15N labeled at a 45 cm depth was moved upwards under APRI. However, in our study, the rate was 26.0–36.8%, possibly due to the fact that we labeled the 15N in a deeper soil layer, and that our experiment was carried out in the subsequent season when some of the 15N had been lost in the previous season.
The form of residual nitrogen in the soil greatly affects its bioavailability.35,36 Bhogal29 pointed out that large amounts of residual fertilizer nitrogen were in the mineral form, while the study by Macdonald37 found that major amounts of residual nitrogen were in the organic form, and only small amounts were in the mineral form. These differences primarily related to the amount and type of the applied nitrogen, as well as the soil attributes.38,39 After the experiment in the 2015 season, more than 50% of the residual 15N in our study was in the mineral form, remaining available in the soil for utilization by the succeeding crop. The higher mineral 15N under APRI could possibly be explained by the fact that the dry and wet cycles stimulate the mineralization of soil nitrogen.40 It was also observed in our study that the mineral 15N at the transplant time of late summer was lower than that of early summer, which might be attributed to a relatively lower temperature in late summer. Early findings by Tian41 indicated that soil temperature had the greatest contribution to the mineralization of total nitrogen compared to other environmental factors, presenting a positive relationship with the amount of mineral nitrogen.
In our study, APRI significantly (p < 0.05) increased 15N recovery. Namely, APRI contributed to a higher recovery of residual nitrogen, which remained in the soil from the previous season. The reason might be that the relatively lower irrigation amount under APRI limited the amount of 15N that could leach into the deeper soil layer when compared to CI, thus reducing the risk of 15N loss. Previous studies by Vázquez42 and Sims43 reported that nitrogen losses from the soil occurred primarily when excessive irrigation occurred, leading to variations in the residual nitrogen in different soil layers. Our result was similar to that obtained by Wang;21 the main difference was that his experiment was on nitrogen recovery by the in-season crops under APRI.
Nitrogen use efficiency (NUE) is one of the key indicators for evaluating irrigation regimes, together with the water use efficiency, crop yield, quality, etc.44,45 This study observed the nitrogen reuse efficiency as influenced by different irrigation patterns and transplant times, as well as different residual nitrogen amounts. In our study, the transplant time appeared to have little effect on the 15NUE; however, the 15NUE was significantly (p < 0.05) increased by APRI in comparison to CI, for both 15N labeling depths. It may be that the dry and wet alternate conditions caused by APRI promote pre-stored carbon remobilization, and in most situations, increases in carbon remobilization from vegetative tissues are closely associated with a higher NUE.46,47 Except for the mineral 15N mentioned earlier, it was found that 15NUE correlated significantly with the root dry matter (R = 0.74*), as well as the root length density (R = 0.72*). The higher 15NUE under APRI might also be explained by the fact that APRI could cause alternate water stress in the root-zone and promote compensatory root growth, thereby regulating the functioning of the crop root system.15 Significantly higher root dry weights under APRI or dry-wet cycling have been reported by many previous studies.45,48,49 It cannot be ignored that the 15NUE was also closely related to the total 15N residual amount from the previous season (R = 0.88**), which is in line with the findings in tobacco.30
Our study revealed the impact of APRI on the fate of 15N in the subsequent growing season. However, caution should be taken as this experiment was conducted under greenhouse conditions, and the environments might be different under field conditions; thus more research under various growth conditions needs to be carried out in the future.
Conclusion
After the experiment in the 2015 season, it was found that 81.3–90.7% of the residual 15N from the 2014 season remained in the 0–100 cm soil layer, 4.1–7.3% was absorbed by the 2015-season tomato plants, and 2.9–14.6% was lost. The 15N reutilization rates (defined as the ratio of plant 15N uptake to total 15N applied in 2014) were 2.20–4.73% under the different treatments. Compared to CI, APRI significantly (p < 0.05) increased the accumulation amount of 15N in the 0–100 cm soil layer, as well as the plant 15N uptake and reutilization rate, and APRI also contributed to a greater 15N distribution in the shallower soil layers. Overall, the tomato 15N reutilization rate was found to be significantly (p < 0.05) higher with 15N labeled in the 0–20 cm soil layer in comparison to the 40–60 cm layer, and insignificantly higher when transplanting in early summer compared to late summer. Furthermore, the 15N reutilization rate had a significant positive relationship with the root dry matter (R = 0.74*), root length density (R = 0.72*), mineral 15N (R = 0.91**) and total residual 15N from 2014 (R = 0.88**). It was concluded from our study that an enlarged root system and a high nitrogen availability under APRI might have contributed to the higher 15N reutilization rate.Acknowledgements
The work was financed by Natural Science Foundation of Fujian Province (2016J05069), Young Talent Foundation of Horticultural College of Fujian Agriculture and Forest University (61201400705), Postdoctoral Funds of Fujian A&F University (132300198), Key Project on Poor Supporting of Fujian Province (K1516035A), as well as New Technology Project on Irrigation and Drainage of Tiaozini (SF-201724).References
- B. Liang, W. Zhao, X. Yang and J. Zhou, Field Crops Res., 2013, 144, 126–134 CrossRef.
- S. Zhang, P. Gao, Y. Tong, D. Norse, Y. Lu and D. Powlson, Agric., Ecosyst. Environ., 2015, 209, 89–99 CrossRef CAS.
- T. Darwish, T. Atallah, M. El Moujabber and N. Khatib, Agric Water Manag., 2005, 78, 152–164 CrossRef.
- J. Zhang, X. H. Shao and T. T. Chang, J. Food, Agric. Environ., 2012, 10, 1409–1412 Search PubMed.
- A. Cockburn, C. W. Heppner and J. L. C. M. Dorne, in Encyclopedia of Food Safety, Academic Press, Waltham, 2014, pp. 332–336 Search PubMed.
- M. J. Wright and K. L. Davison, in Advances in Agronomy, ed. A. G. Norman, Academic Press, 1964, vol. 16, pp. 197–247 Search PubMed.
- J. Y. Yang, C. F. Drury, R. De Jong, E. C. Huffman, X. M. Yang and K. Reid, Ecol. Modell., 2013, 267, 26–38 CrossRef CAS.
- A. M. Davis, M. Tink, K. Rohde and J. E. Brodie, Agric., Ecosyst. Environ., 2016, 223, 190–196 CrossRef CAS.
- K. Granlund, K. Rankinen, R. Etheridge, P. Seuri and J. Lehtoranta, Agric. Sys., 2015, 133, 167–176 CrossRef.
- H. Du, Doctor, Chinese Academy of Sciences, 2007 Search PubMed.
- M. Hou, X. Shao, Q. Jin and X. Gao, Arch. Agron. Soil Sci., 2016, 1–10 Search PubMed.
- G. Ojeda, D. Tarrasón, O. Ortiz and J. M. Alcañiz, Agric., Ecosyst. Environ., 2006, 117, 49–56 CrossRef CAS.
- D. Stefanelli, I. Goodwin and R. Jones, Food Res. Int., 2010, 43, 1833–1843 CrossRef CAS.
- P. Wu, C. Xia, F. Liu, Y. Wu and Y. He, Appl. Math. Model., 2016, 40, 8108–8124 CrossRef.
- Q. Zhang, S. Wu, C. Chen, L.-Z. Shu, X.-J. Zhou and S.-N. Zhu, Agric Water Manag., 2014, 142, 56–65 CrossRef.
- L. I. N. Yechun, Z. Zhaohai, R. E. N. Changzhong and H. U. Yuegao, Procedia Eng., 2012, 28, 33–42 CrossRef.
- Y. Wu, S. Zhou, Z. Wang and X. Zhang, Acta Ecol. Sin., 2005, 25, 1869–1873 CAS.
- S. Z. Kang and J. H. Zhang, J. Exp. Bot., 2004, 55, 2437–2446 CrossRef CAS PubMed.
- S. Du, S. Kang, F. Li and T. Du, Agric Water Manag., 2017, 179, 178–182 CrossRef.
- Z. Wei, T. Du, J. Zhang, S. Xu, P. J. Cambre and W. J. Davies, Agric Water Manag., 2016, 165, 33–43 CrossRef.
- C. Wang, P. Zhu, L. Shu, J. Zhu, H. Yu, Y. Zhan and M. Yuan, Nongye Gongcheng Xuebao, 2014, 30, 92–101 Search PubMed.
- M. Hou, Q. Jin, X. Lu, J. Li, H. Zhong and Y. Gao, Front. Plant Sci., 2017, 8, 00666 CrossRef PubMed.
- J. Lv, J. Gansu Agric. Univ., 2013, 2, 37–41 Search PubMed.
- T. S. Du, S. Z. Kang, J. H. Zhang, F. S. Li and X. T. Hu, Agric Water Manag., 2006, 84, 41–52 CrossRef.
- X.-E. Liu, X. G. Li, R.-Y. Guo, Y. Kuzyakov and F.-M. Li, Eur. J. Agron., 2015, 70, 71–77 CrossRef CAS.
- C. Carranca, A. de Varennes and D. E. Rolston, Eur. J. Agron., 1999, 11, 145–155 CrossRef.
- M. Hou and X. Shao, Zemdirbyste-Agriculture, 2016, 103, 221–228 CrossRef.
- A. J. Macdonald, P. R. Poulton, E. A. Stockdale, D. S. Powlson and D. S. Jenkinson, Plant Soil, 2002, 246, 123–137 CrossRef CAS.
- A. Bhogal, A. D. Rochford and R. Sylvester-Bradley, J. Agric. Sci., 2000, 135, 139–149 CrossRef.
- M. Hou, X. Shao, Y. Zhai, Y. Yuan and F. Ding, Nongye Gongcheng Xuebao, 2016, 32, 118–123 Search PubMed.
- Z. Wang, F. Liu, S. Kang and C. R. Jensen, Environ. Exp. Bot., 2012, 75, 36–40 CrossRef CAS.
- C. Liu, G. H. Rubæk, F. Liu and M. N. Andersen, Agric Water Manag., 2015, 159, 66–76 CrossRef.
- C. E. Demurtas, G. Seddaiu, L. Ledda, C. Cappai, L. Doro, A. Carletti and P. P. Roggero, Agric., Ecosyst. Environ., 2016, 219, 83–92 CrossRef CAS.
- L. Ma, Acta Pedol. Sin., 2010, 47, 286–294 Search PubMed.
- G. Agegnehu, P. N. Nelson and M. I. Bird, Soil Tillage Res., 2016, 160, 1–13 CrossRef.
- R. H. Masunga, V. N. Uzokwe, P. D. Mlay, I. Odeh, A. Singh, D. Buchan and S. De Neve, Appl. Soil Ecol., 2016, 101, 185–193 CrossRef.
- A. J. Macdonald, P. R. Poulton, D. S. Powlson and D. S. Jenkinson, J. Agric. Sci., 1997, 129, 125–154 CrossRef.
- X. H. He, M. G. Xu, G. Y. Qiu and J. B. Zhou, J. Plant Ecol., 2009, 2, 107–118 CrossRef.
- B. Liang, X. Y. Yang, X. H. He and J. B. Zhou, Biol. Fertil. Soils, 2011, 47, 121–128 CrossRef CAS.
- Y. Sun, F. Yan and F. Liu, Agric Water Manag., 2013, 128, 85–91 CrossRef.
- M. Tian, Nat. Sci., 2004, 25, 299–303 Search PubMed.
- N. Vázquez, A. Pardo, M. L. Suso and M. Quemada, Agric., Ecosyst. Environ., 2006, 112, 313–323 CrossRef.
- J. T. Sims, R. R. Simard and B. C. Joern, J. Environ. Qual., 1998, 27, 277–293 CrossRef CAS.
- M. H. Shahrokhnia and A. R. Sepaskhah, Agric Water Manag., 2016, 172, 18–30 CrossRef.
- Z. Wang, W. Zhang, S. S. Beebout, H. Zhang, L. Liu, J. Yang and J. Zhang, Field Crops Res., 2016, 193, 54–69 CrossRef.
- J. C. Yang and J. H. Zhang, New Phytol., 2006, 169, 223–236 CrossRef CAS PubMed.
- B. Ehdaie, Crop Sci., 1995, 35, 1617–1626 CrossRef.
- G. Chu, Z. Wang, H. Zhang, L. Liu, J. Yang and J. Zhang, Food Energy Secur., 2015, 4, 238–254 CrossRef.
- H. Zhang, Y. G. Xue, Z. Q. Wang, J. C. Yang and J. H. Zhang, Crop Sci., 2009, 49, 2246–2260 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |