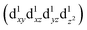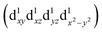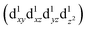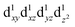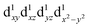Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Significance of the electron-density of molecular fragments on the properties of manganese(III) β-diketonato complexes: an XPS and DFT study
Roxanne Gostynski,
Jeanet Conradie * and
Elizabeth Erasmus
* and
Elizabeth Erasmus *
*
Department of Chemistry, University of the Free State, PO Box 339, Bloemfontein, 9300, South Africa. E-mail: erasmuse@ufs.ac.za; conradj@ufs.ac.za; Fax: +27-51-4017295; Fax: +27-51-4019656; Tel: +27-51-4019656 Tel: +27-51-4012194
First published on 24th May 2017
Abstract
DFT and XPS studies were conducted on a series of nine manganese(III) complexes of the general formula [Mn(β-diketonato)3], with the ligand β-diketonato = dipivaloylmethanato (1), acetylacetonato (2), benzoylacetonato (3), dibenzoylmethanato (4), trifluoroacetylacetonato (5), trifluorothenoylacetonato (6), trifluorofuroylacetonato (7), trifluorobenzoylacetonato (8) and hexafluoroacetylacetonato (9). The binding energy position of the main and satellite structures of the Mn 2p3/2 photoelectron line, as well as the spin–orbit splitting, gave insight into the electronic structure of these manganese(III) complexes. DFT calculations showed that an experimental sample of the d4 [Mn(β-diketonato)3] complex can contain a mixture of different bond stretch isomers and different electronic states, in dynamic equilibrium with one other. The presence of more than one isomer in the experimental sample, as well as interaction between an unpaired 2p electron (originating after photoemission) and an unpaired 3d electron, which aligned anti-parallel to the unpaired 2p electron, caused broadening of the Mn 2p photoelectron lines. Multiplet splitting simulations of these photoelectron lines, similar to those calculated by Gupta and Sen for the free Mn(III) ion, gave good fits with the observed Mn 2p3/2 photoelectron lines. The XPS spectra of complexes with unsymmetrical β-diketonato ligands were simulated with two sets of multiplet splitting peaks, representing both the mer and fac isomers. The satellite structures obtained in both the Mn 2p3/2 photoelectron line (shake-up peaks) and the ligand F 1s photoelectron line (shake-down peaks), are representative of the ligand-to-metal charge transfer during photoionisation. The binding energies of the Mn 2p, F 1s and S 2p electrons, as well as the amount of charge transfer from ligand-to-metal, are both dependent on the electronegativity of the different groups attached to the β-diketonato ligand.
1 Introduction
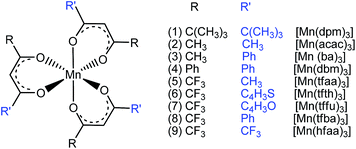
Knowledge of the electronic structure and properties of transition metal complexes is important, due to their role in a variety of chemical and biological processes. High spin manganese(III) complexes have various applications, for example as building blocks in molecular magnets,1–8 catalysis,9–14 redox mediators in dye-sensitised solar cells,15,16 and in biochemical reaction cycles.17–21 Due to the four unpaired d electrons of high spin Mn(III) complexes, their geometry displays the Jahn–Teller effect and also the X-ray photoelectron spectroscopy (XPS) lines of these complexes split into many peaks, due to a coupling interaction between the unpaired core p-electrons (originating after photoemission) and the unpaired valence d-electrons.22 Recently an X-ray photoelectron spectroscopy study on [MnIII(ferrocenyl-β-diketonato)3] complexes, where the β-diketonato ligand contained at least one ferrocenyl group,23 showed that a charge-transfer process from the β-diketonato ligand to the Mn(III) metal centre is responsible for prominent shake-up satellite peaks of the main Mn 2p photoelectron lines. These results further showed that the binding energies of the Mn 2p photoelectron lines are linearly influenced by the sum of the Gordy scale group electronegativities24 of the different R groups attached to the β-diketonato ligands (RCOCHCOFc)− (Fc = ferrocenyl group), varying over a binding energy range of 0.55 eV. By adding the XPS data of [MnIII(β-diketonato)3] complexes, where the β-diketonato ligand does not contain an electron donating ferrocenyl group, we hereby enlarge the range of binding energies of the Mn 2p photoelectron lines by nearly 100%, to a range of 1.07 eV. Thus, in continuation of our interest in the geometrical25,26 and electronic structure27 of [Mn(β-diketonato)3] complexes containing β-diketonato ligands with different electrondonating and -withdrawing properties, we hereby present a combined study of theoretical quantum chemistry calculations (using density functional theory, DFT) and experimental measurements (using XPS) on the series of nine non-ferrocene-containing [Mn(β-diketonato)3] complexes shown in Fig. 1 (with β-diketonato = dipivaloylmethanato (1), acetylacetonato (2), benzoylacetonato (3), dibenzoylmethanato (4), trifluoroacetylacetonato (5), trifluorothenoylacetonato (6), trifluorofuroylacetonato (7), trifluorobenzoylacetonato (8) and hexafluoroacetylacetonato (9)). Results are also compared with previously published results23 on [MnIII(ferrocenyl-β-diketonato)3] complexes.2 Results and discussion
The spectra obtained by X-ray photoelectron spectroscopy (XPS) of 3d transition metal complexes containing unpaired d electrons, frequently contain a variety of several features which arise from the electronic structure of the material. [Mn(β-diketonato)3] complexes are high-spin paramagnetic octahedral complexes, with a d-electron occupation of (t2g)3(eg)1.28 Since the electronic configurations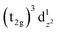 and
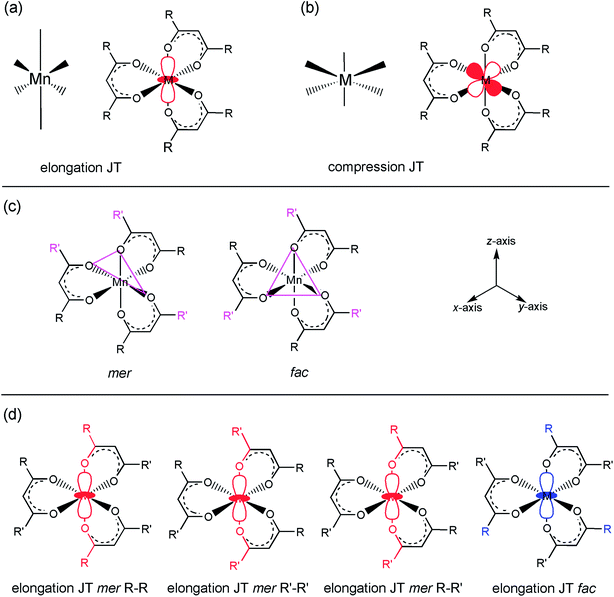
and 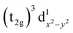 are degenerate, Jahn–Teller geometric distortion of [Mn(β-diketonato)3] complexes occurs in order to remove the degeneracy, with a consequent lowering in symmetry and energy.29 Elongation Jahn–Teller distortion in [Mn(β-diketonato)3] complexes is characterised by two long bonds along the z-direction, as well as four short bonds in the xy-plane, and a d-electron occupation of
are degenerate, Jahn–Teller geometric distortion of [Mn(β-diketonato)3] complexes occurs in order to remove the degeneracy, with a consequent lowering in symmetry and energy.29 Elongation Jahn–Teller distortion in [Mn(β-diketonato)3] complexes is characterised by two long bonds along the z-direction, as well as four short bonds in the xy-plane, and a d-electron occupation of 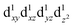 , with the highest occupied molecular orbital (HOMO) of mainly dz2 character. The elongation along the z-direction stabilises the energy of the dz2 HOMO. Compression Jahn–Teller distortion in [Mn(β-diketonato)3] complexes on the other hand, is characterised by two short bonds along the z-direction, as well as four long bonds in the xy-plane, and a d-electron occupation of
, with the highest occupied molecular orbital (HOMO) of mainly dz2 character. The elongation along the z-direction stabilises the energy of the dz2 HOMO. Compression Jahn–Teller distortion in [Mn(β-diketonato)3] complexes on the other hand, is characterised by two short bonds along the z-direction, as well as four long bonds in the xy-plane, and a d-electron occupation of 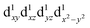 instead, with the HOMO of mainly dx2−y2 character. Elongation along the x and y-directions in this case, stabilises the energy of the dx2−y2 HOMO.25,27 The two electronic states
instead, with the HOMO of mainly dx2−y2 character. Elongation along the x and y-directions in this case, stabilises the energy of the dx2−y2 HOMO.25,27 The two electronic states 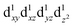 and
and 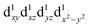 , respectively leading to elongation and compression Jahn–Teller distortion in [Mn(β-diketonato)3] complexes, are illustrated in Fig. 2(a) and (b), for [Mn(β-diketonato)3] complexes containing a symmetrically substituted β-diketonato ligand (RCOCHCOR′)−, with groups R = R′.
, respectively leading to elongation and compression Jahn–Teller distortion in [Mn(β-diketonato)3] complexes, are illustrated in Fig. 2(a) and (b), for [Mn(β-diketonato)3] complexes containing a symmetrically substituted β-diketonato ligand (RCOCHCOR′)−, with groups R = R′.
2.1 DFT study
The influence of the ligands upon the geometry, energy and electronic structure of [Mn(β-diketonato)3] complexes, can conveniently be studied by quantum chemical calculations. DFT calculations conducted on both the two electronic states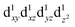 and
and 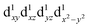 of complexes 1–9, show that for 1–9, the elongation Jahn–Teller distortion geometry is generally more stable than the compression Jahn–Teller distortion geometry, see Table 1.26,30 Both elongation31 as well as compression Jahn–Teller distortion32,33 has experimentally been observed in crystal structures obtained for [Mn(β-diketonato)3] complexes. However, compression Jahn–Teller distortion as observed for [Mn(acac)3],33 has actually been attributed to an unusual consequence of unknown crystal packing effects,34 stating that typically the tetragonal elongation is the “natural” Jahn–Teller effect of [Mn(acac)3], in agreement with the DFT calculations obtained in this study (Hacac = acetylacetone).
of complexes 1–9, show that for 1–9, the elongation Jahn–Teller distortion geometry is generally more stable than the compression Jahn–Teller distortion geometry, see Table 1.26,30 Both elongation31 as well as compression Jahn–Teller distortion32,33 has experimentally been observed in crystal structures obtained for [Mn(β-diketonato)3] complexes. However, compression Jahn–Teller distortion as observed for [Mn(acac)3],33 has actually been attributed to an unusual consequence of unknown crystal packing effects,34 stating that typically the tetragonal elongation is the “natural” Jahn–Teller effect of [Mn(acac)3], in agreement with the DFT calculations obtained in this study (Hacac = acetylacetone).
| No | Relative energy in eV | ||
|---|---|---|---|
| a From ref. 30.b From ref. 26.c Compression Jahn–Teller input geometry spontaneously converged to elongation Jahn–Teller geometry. | |||
| 1 | [Mn(dpm)3]a | 0.000 | 0.036 |
| 2 | [Mn(acac)3]a | 0.000 | 0.038 |
| 3 | [Mn(ba)3]a | 0.000 (mer Ph–Ph)a | 0.056 (fac)a |
| 0.015 (mer Ph–Me)a | |||
| 0.025 (mer Me–Me)a | |||
| 0.020 (fac)a | |||
| 4 | [Mn(dbm)3]a | 0.000 | 0.058 |
| 5 | [Mn(tfaa)3] | 0.000 (mer CF3–CF3) | c |
| 0.030 (mer Me–CF3) | |||
| 0.064 (mer Me–Me) | |||
| 0.044 (fac) | |||
| 6 | [Mn(tfth)3]b | 0.000 (mer Th–Th) | c |
| 0.016 (mer CF3–CF3) | |||
| 0.025 (mer CF3–Th) | |||
| 0.090 (fac) | |||
| 7 | [Mn(tffu)3] | 0.000 (mer CF3–CF3) | c |
| 0.019 (mer CF3–Fu) | |||
| 0.055 (mer Fu–Fu) | |||
| 0.085 (fac) | |||
| 8 | [Mn(tfba)3] | 0.000 (mer CF3–CF3) | c |
| 0.023 (mer CF3–Ph) | |||
| 0.041 (mer Ph–Ph) | |||
| 0.071 (fac) | |||
| 9 | [Mn(hfaa)3]a | 0.000 | 0.037 |
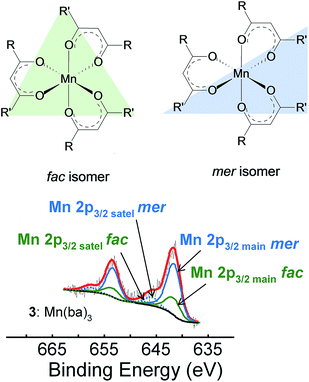
When the [Mn(β-diketonato)3] complex contains an unsymmetrically substituted β-diketonato ligand (with groups R ≠ R′, as for complexes 3, 5–8), then two geometrical isomers are possible, namely both the fac and mer isomers, depending on the arrangement of the unsymmetrical β-diketonato ligands around the metal; see Fig. 2(c). In the case of unsymmetrical complexes, the elongation Jahn–Teller distortion of the mer isomer can occur along any of the three different Oβ-diketonato–Mn–Oβ-diketonato′ bonds, leading to as much as three different bond stretch Jahn–Teller elongation mer isomers for the electronic state 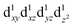 , as illustrated in Fig. 2(d).26,30 The OLYP/TZP energies for these four (including the one fac) different elongation [MnIII(β-diketonato)3] isomers of unsymmetrical complexes 3, 5–8, are given in Table 1. The DFT calculations show that for these unsymmetrical complexes 3, 5–8, (i) the most stable isomer is a mer isomer, (ii) the fac isomer is generally slightly less stable than the different mer isomers, and (iii) the energy differences between the different bond stretch isomers of [Mn(β-diketonato)3] complexes are small. This result indicates that an experimental sample of [Mn(β-diketonato)3] can contain a mixture of all four different bond stretch isomers (and possibly also different electronic states), in dynamic equilibrium with one other. This result agrees with the fact that experimental crystal structures of both the fac15,35 and mer isomers26 of [Mn(β-diketonato)3] complexes have been characterised.
, as illustrated in Fig. 2(d).26,30 The OLYP/TZP energies for these four (including the one fac) different elongation [MnIII(β-diketonato)3] isomers of unsymmetrical complexes 3, 5–8, are given in Table 1. The DFT calculations show that for these unsymmetrical complexes 3, 5–8, (i) the most stable isomer is a mer isomer, (ii) the fac isomer is generally slightly less stable than the different mer isomers, and (iii) the energy differences between the different bond stretch isomers of [Mn(β-diketonato)3] complexes are small. This result indicates that an experimental sample of [Mn(β-diketonato)3] can contain a mixture of all four different bond stretch isomers (and possibly also different electronic states), in dynamic equilibrium with one other. This result agrees with the fact that experimental crystal structures of both the fac15,35 and mer isomers26 of [Mn(β-diketonato)3] complexes have been characterised.
While the DFT study focused on the energy and character of the different [Mn(β-diketonato)3] isomers, which are mainly determined by the valence Mn-d electrons, an X-ray photoelectron spectroscopy (XPS) study of a series of these [Mn(β-diketonato)3] complexes, gave insight into the energies and properties of the core electrons of Mn and other elements present in [Mn(β-diketonato)3].
2.2 XPS study
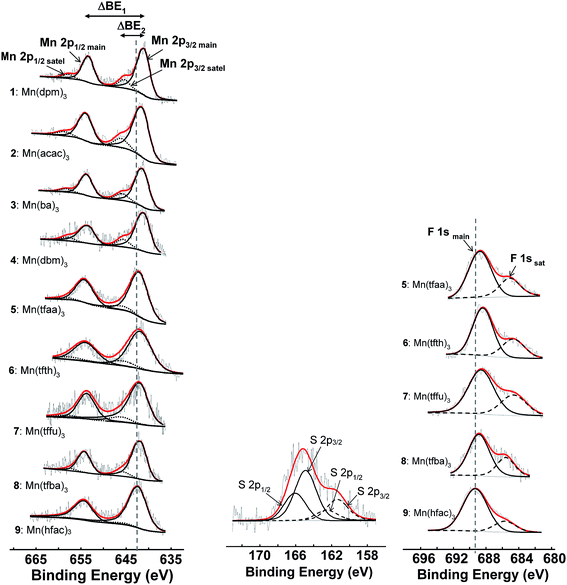
X-ray photoelectron spectroscopy (XPS) is a convenient characterisation technique to study the chemical and oxidation states of the elements present in a sample.36 The binding energy position, shape and intensity of a photoelectron line can additionally provide a wealth of information regarding the magnetic character,37 electronic properties38,39 and metal–ligand bond of the elements under investigation.40–42The XPS of complexes 1–9 in this study all displayed the Mn 2p (641–652 eV), C 1s (ca. 284.5 eV) and O 1s (ca. 528.8 eV) photoelectron lines, while the XPS of the five fluorine-containing complexes 5–9 also showed the F 1s photoelectron line (main line at ca. 688.5 eV; satellite line at ca. 684.9 eV) and complex 6 displayed the S 2p photoelectron lines (main line at ca. 164.7 eV; satellite at ca. 161.2 eV). The Mn 2p region of the XPS spectra of these non-ferrocene containing [MnIII(β-diketonato)3] complexes 1–9 of this study, is shown in Fig. 3, with selected data tabulated in Table 2. The Mn 2p3/2 and Mn 2p1/2 photoelectron lines are located respectively between 641.67–642.38 eV and 652.82–653.56 eV, with the presence of a satellite peak (due to the shake-up mechanism) at a binding energy of ca. 4 eV higher than the main Mn 2p photoelectron lines. The maximum binding energy of the main Mn 2p3/2 peak of all the [Mn(β-diketonato)3] complexes 1–9, is found at ca. 3 eV higher than the binding energy of the Mn 2p3/2 of the free manganese metal Mn0, which is located at ca. 639 eV.43 This 3 eV shift to a higher binding energy indicates that Mn in fact exists in oxidation state III in the complex, which is further confirmed by the ca. 641 eV binding energy reported for other MnIII species,44 as well as by the 641.2 eV previously reported in literature for [Mn(acac)3], complex 2, in ref. 45.
| Complex | ΣχR = 3(χR + χR′) a | νCO (cm−1) | BE Mn 2p3/2main (eV) | ΔBE1b (eV) | BE Mn 2p3/2sat (eV) | ΔBE2c (eV) | Iratiod | BE Mn 2p3/2main mer (eV) | BE F 1smain (eV) | BE F 1ssat (eV) | Iratiog |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Sum of the Gordy scale group electronegativities, see ref. 24.b ΔBE1 = BEMn 2p1/2main − BEMn 2p3/2main.c ΔBE2 = BEMn 2p3/2sat − BEMn 2p3/2main.d Iratio = ratio between the intensities of the satellite and main Mn 2p3/2 photoelectron lines = (IMn 2p3/2sat)/(IMn 2p3/2main).e The R-groups of the ligands of the complex are equivalent, hence the complex does not show mer and fac isomers.f The R-groups of the ligands of the complexes do not contain F.g Iratio = ratio between the intensities of the satellite and main F 1s photoelectron lines = (IF 1ssat)/(IF 1smain). | |||||||||||
| [Mn(dpm)3], 1 | 13.62 | 1591 | 641.67 | 11.88 | 645.87 | 4.20 | 0.16 | 641.49 | —f | —f | —f |
| [Mn(acac)3], 2 | 14.04 | 1590 | 641.73 | 11.91 | 646.09 | 4.36 | 0.17 | —e | —f | —f | —f |
| [Mn(ba)3], 3 | 13.65 | 1591 | 641.69 | 11.89 | 645.98 | 4.29 | 0.16 | 641.51 | —f | —f | —f |
| [Mn(dbm)3], 4 | 13.26 | 1592 | 641.67 | 11.80 | 645.99 | 4.32 | 0.17 | —e | —f | —f | —f |
| [Mn(tfaa)3], 5 | 16.05 | 1598 | 642.05 | 12.21 | 645.81 | 3.76 | 0.11 | 641.87 | 688.49 | 684.88 | 0.41 |
| [Mn(tfth)3], 6 | 15.33 | 1602 | 641.93 | 12.02 | 645.83 | 3.90 | 0.13 | 641.75 | 688.20 | 684.60 | 0.45 |
| [Mn(tffu)3], 7 | 15.39 | 1597 | 641.97 | 12.04 | 645.84 | 3.87 | 0.13 | 641.79 | 688.33 | 684.48 | 0.44 |
| [Mn(tfba)3], 8 | 15.66 | 1601 | 641.99 | 12.12 | 645.83 | 3.84 | 0.11 | 641.81 | 688.48 | 684.63 | 0.42 |
| [Mn(hfac)3], 9 | 18.06 | 1639 | 642.38 | 12.38 | 645.73 | 3.35 | 0.05 | —e | 689.32 | 685.70 | 0.23 |
Both the Mn 2p3/2 and Mn 2p1/2 photoelectron lines exhibit broad peaks, with a fairly high value of 4–6 eV measured for the full width at half maximum (FWHM). The FWHM of the obtained peaks is rather large in comparison with the FWHM of 2.28 eV, reported for the Mn 2p1/2 photoelectron line of the cubic alloy Al60Pd25Mn15, which was measured at a pass energy of 29.5 eV,46 and in comparison to the Mn 2p1/2 photoelectron line of elemental Mn0, which gave a FWHM of 2.52 eV (measured at a pass energy of 35.75 eV).47 The unexpectedly high FWHM value observed for these MnIII photoelectron lines might be due to various reasons:
(i) MnIII is known to exist in a high spin paramagnetic state,48,49 and the FWHM of high spin MnIII is generally higher than that of a low spin Mn complex. For example, the FWHM of high spin MnIII in Mn2O3 was measured as 1.75 eV, while the FWHM of low spin MnVI in MnO2 was found to be only 0.91 eV (both measured at 20 eV pass energy).50
(ii) A higher pass energy allows for a larger number of electrons to reach the detector, but the energy determination becomes less precise, causing the FWHM to increase. In this study a large pass energy had to be used in order to obtain well defined peaks (the same pass energy of 93.90 eV was used for all the complexes in the Mn 2p region), which contributed to the higher FWHM. For example, the FWHM of elemental Mn0 is 1.14 eV at 25 eV pass energy, 0.79 eV at 20 eV pass energy and 0.74 eV at 10 eV pass energy,50 therefore obtaining smaller FWHM values with decreasing pass energy.
(iii) The larger than expected FWHM value is also an indication of more than one species most probably being present in the sample, namely the fac as well as different bond stretch mer isomers, as discussed in the previous section.
(iv) The large FWHM value could also be attributed to final state effects, such as the coupling of the angular momenta of the unpaired electrons of the core level after photoionisation, with one of the four unpaired 3d electrons of high-spin MnIII of the outer orbitals (causing multiplet splitting), as well as crystal field- and electrostatic interactions.51,52 Multiplet splitting is discussed in a separate section below.
| BEMn 2p3/2 = 0.1532 ΣχR + 639.6; R2 = 0.993 (for complexes 1–9) |
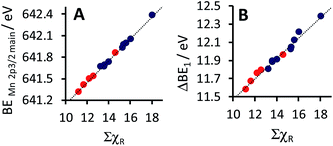 | ||
| Fig. 4 Relationship between ΣχR, the sum of the Gordy scale group electronegativities of the R-groups on the β-diketonato ligand of complexes 1–14, with (A) the maximum binding energy (BEMn 2p3/2main) of the main Mn 2p3/2 photoelectron line, as well as with (B) the spin orbit splitting of the maximum binding energy (ΔBE1) of the main Mn 2p3/2 and Mn 2p1/2 photoelectron lines (ΔBE1 = BEMn 2p1/2main − BEMn 2p3/2main). Data of complexes 1–9 from this study is shown in blue (from Table 2) and data of complexes 10–14 from a previous study in red (from ref. 23). | ||
For comparative reasons, data of previously published [MnIII(ferrocenyl-β-diketonato)3] complexes, containing an additional Fe metal,23 is also considered at this point. Considering published data23 from a previous study of the [MnIII(ferrocenyl-β-diketonato)3] complexes 10–14, containing at least one ferrocenyl group on the β-diketonato ligand (with ligands ferrocenyl-β-diketonato = (FcCOCHCOCF3)− (10), (FcCOCHCOCH3)− (11), (FcCOCHCOPh)− (12), (FcCOCHCOFc)− (13) as well as (ferrocenyl-β-diketonato)3 = ((FcCOCHCOFc)−)2(FcCOCHCOCH3)− (14), where Fc = ferrocenyl), and including this data in the linear fit of BEMn 2p3/2 with ΣχR, it was found that the above equation stays basically the same, within experimental accuracy (see Fig. 4A), namely:
| BEMn 2p3/2 = 0.1503 ΣχR + 639.6; R2 = 0.995 (for complexes 1–14) |
This result illustrates that the above equation can be used to a high degree of accuracy, to also determine the binding energy of related [Mn(β-diketonato)3] complexes, if the electronegativity χR of the groups attached to the β-diketonato ligands (RCOCHCOR′)−, is known.
Spin-orbit splitting is an initial state effect, and the magnitude of the separation is dependent on the degree of spin density delocalisation of the electrons in the valence orbitals.65–67 Thus it is expected that a more electron withdrawing group (with a higher degree of spin density delocalisation) would be associated with a larger spin–orbit splitting. The correlation between the sum of the Gordy group electronegativities (ΣχR) and the spin–orbit splitting (ΔBE1) between the Mn 2p3/2 and Mn 2p1/2 photoelectron lines (see Fig. 4B), is in agreement with the above.
It was found that the spin–orbit splitting ΔBE1 of the main Mn 2p3/2 and 2p1/2 peaks for complexes 1–9, increases linearly as ΣχR increases (see Fig. 4B):
| ΔBE1 = 0.1185 ΣχR + 10.25; R2 = 0.970 (for complexes 1–9) |
| ΔBE1 = 0.1110 ΣχR + 10.36; R2 = 0.972 (for complexes 1–14) |
Here again we observe that enlarging the ΣχR range, by including Fe-containing ligands from the previous study23 of [MnIII(ferrocenyl-β-diketonato)3] complexes 10–14 into the linear fit of ΔBE1 with ΣχR, does not have any significant influence on the accuracy of the fit or the linear relationship. The presence or absence of the second metal Fe in the ligand of these [MnIII(diketonato)3] complexes, does not influence the binding energies of the Mn 2p XPS peaks any more than merely the normal effect expected from the electronegativity of the ferrocenyl group.
As mentioned earlier and shown in Fig. 3, the Mn 2p3/2 shake-up satellite peak is located at ca. 4 eV higher binding energy than the main Mn 2p3/2 peak (see also columns 4 and 6 in Table 2). It previously has been shown for paramagnetic Cu(II) complexes, that the intensity of the satellite peak in comparison with the main metal M 2p3/2 peak, namely Iratio = (IM 2p3/2sat)/(IM 2p3/2main), increases (non-linearly) as the spin density of the ligands attached to the Cu metal increases.69 In this study it has been found however, that the ratio of the intensity of the satellite peak towards the main Mn 2p3/2 peak, Iratio = (IMn 2p3/2sat)/(IMn 2p3/2main), is inversely proportional to ΣχR, which is a measure of the electronegativity of the different ligands. This “opposite” trend will be explained below.
Satellite peaks are generally caused by one of two phenomena. In the first instance, shake-up peaks commonly observed for paramagnetic transition metals occur, when the kinetic energy of the emitted photoelectron is being reduced due to energy transfer to a non-photoelectron of the same element (causing the non-photoelectron to be in an exited state), which leads to a “shake-up” peak at a higher binding energy than the main line.70 On the other hand, shake-up peaks also occur due to a strong configuration interaction in the final state, involving significant ligand-to-metal charge transfer instead (for example in Ni(II) and Co(II)), which results in an “extra” 3d electron, compared with the initial state.71 The excited energy transfer satellite mechanism in the first instance dominates over the latter charge-transfer satellite mechanism, when firstly the difference between the maximum binding energies of the satellite and the main metal peaks is large (namely the charge transfer energy, ΔBE2), when secondly the core-hole-d-electron Coulomb attraction energy is small, and thirdly when there are many empty 3d orbitals.72 However, for the d4 complexes 1–9 in this study, with as many as 6 empty 3d orbitals each, the observed shake-up peaks can best be interpreted in terms of the latter instance, namely a ligand-to-metal charge transfer during photoionisation, since ΔBE2 is only a small value (less than 5 eV). Also, in the F-containing [MnIII(β-diketonato)3] complexes 5–9, the corresponding shake-down peaks are observed for the main F 1s photoelectron line, as well as for the main S 2p line of the S-containing complex 6 (see Section 2.2.4 below). Comparable shake-down satellites were reported in a previous study, for the photoelectron lines associated with the Fe 2p electrons of the Fe-containing ligand, for related [MnIII(ferrocenyl-β-diketonato)3] complexes.23 In addition, the latter charge transfer model also accounts quantitatively for the observed intensities of the satellites to the main peaks (Iratio),72 as was found in this study for complexes 1–9.
The electron configuration of Mn(III) in complexes 1–9 is 1s22s22p63s23p63d4. However, after ligand-to-metal charge transfer during photoionisation of a 2p electron, the final state is 1s22s22p53s23p63d4+n, where n ≈ 1 denotes the amount of charge transferred from the ligands to the Mn metal.45 Ligand-to-metal charge transfer during photoionisation, therefore leads to a change in spin density. As more charge is transferred from the ligand to the metal (leading to the “extra” 3d electron during photoionisation), the larger the satellite charge transfer peak (shake-up peak) of the metal becomes. The inversely proportional relationship observed between ΣχR and Iratio (Fig. 5B) affirms that β-diketonato ligands with higher ΣχR values (for example ΣχR = 18.06 for complex 9), indicating stronger electron withdrawing properties than others, would obviously transfer less charge to the Mn metal centre during photoionisation of a 2p electron, than the ligands of, for example complex 4, with a smaller value of ΣχR = 13.26, therefore withdrawing much less electrondensity. In literature, this result also agrees with the linear increase observed for the intensity ratio, Iratio, with the decrease of the Pauling electronegativity of the halides,73,74 for three series of metal-dihalides, namely CoX2, FeX2 and MnX2, where X = F, Cl or Br. The same increase in Iratio with decreasing ΣχR can be seen in the example of the Mn-dihalides series from literature: MnF2 (Iratio = 0.06, χF = 3.98), MnCl2 (Iratio = 0.28, χF = 3.16) and MnBr2 (Iratio = 0.38, χF = 2.96).72 However, metal-to-ligand charge transfer, as observed for Cu(II) in literature,69 results in the opposite trend between Iratio and ligand electronegativity (than ligand-to-metal charge transfer) during photoexcitation. The linear relationship obtained between the Iratio of the Mn 2p3/2 peaks and ΣχR, the sum of the Gordy scale group electronegativities of the R-groups on the ligands, fits the following linear equation (see Fig. 5B):
| Iratio = −0.0256 ΣχR + 0.5172; R2 = 0.970 (for complexes 1–9) |
| Iratio = −0.0248 ΣχR + 0.5040; R2 = 0.971 (for complexes 1–14) |
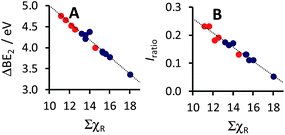 | ||
| Fig. 5 Relationship between ΣχR, the sum of the Gordy scale group electronegativities of the R-groups on the β-diketonato ligand, of complexes 1–14, with (A) the charge transfer energy (ΔBE2), namely the difference between the maximum binding energy of the main Mn 2p3/2 photoelectron line and the satellite Mn 2p3/2 photoelectron line (ΔBE2 = BEMn 2p3/2sat − BEMn 2p3/2main), as well as with (B) the intensity ratio (Iratio) of the satellite to the main Mn 2p3/2 photoelectron lines, Iratio = (IMn 2p3/2sat)/(IMn 2p3/2main). Data of complexes 1–9 from this study is shown in blue (from Table 2) and data of complexes 10–14 from literature in red (from ref. 23). | ||
| BEF 1s = 0.3834 ΣχR + 682.39; R2 = 0.979 (for the F-containing complexes 5–9) |
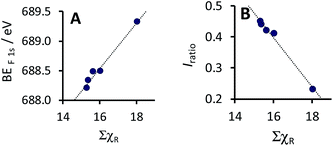 | ||
| Fig. 6 Relationship between ΣχR, the sum of the Gordy scale group electronegativities of the R-groups on the β-diketonato ligand, of the five CF3-containing complexes 5–9, with (A) the maximum binding energy (BEF 1smain) of the main F 1s photoelectron line, as well as with (B) the intensity ratio (Iratio) of the satellite and main F 1s photoelectron lines, Iratio = (IF 1ssatel)/(IF 1smain). Data from Table 2. | ||
The substructure of the F 1s photoelectron lines displays a strong shake-down satellite peak, ca. 3.7 eV lower than the main F 1s photoelectron line. This shake-down peak of the main F 1s photoelectron line is associated with the corresponding shake-up peak of the Mn 2p photoelectron, which depicts the ligand-to-metal charge transfer during photoionisation. The shake-down peak of the F 1s photoelectron line can be considered representative of some of the charge transfer from the F-containing ligand to the Mn metal.
The Iratio of the F 1s peaks (Iratio = (IF 1ssat)/(IF 1smain)) is a measure of the amount of charge transferred from the CF3-containing ligand to the Mn metal of these five complexes 5–9. The intensity ratio, and thus the amount of charge transferred from the fluorine-containing ligand to the Mn metal decreases, as the electronegativity of the CF3-containing ligand (ΣχR) increases (see Fig. 6B). This inversely proportional linear relationship obtained between the Iratio of the satellite to the main F 1s peaks with electronegativity ΣχR, fits the following equation (see Fig. 5B):
| Iratio = −0.0798 ΣχR + 1.674; R2 = 0.989 (for the F-containing complexes 5–9) |
The above result implies that the symmetrically substituted ligands in complex 9, containing as much as six strongly electron withdrawing CF3 groups, will subsequently transfer even less charge to the Mn metal in complex 9, than in complexes 5–8, which each contain only three electron withdrawing CF3 groups. This observed result agrees with the lowest amount of charge “received” by Mn in complex 9 (Fig. 5B), as given by Iratio = (IMn 2p3/2sat)/(IMn 2p3/2main) = 0.05 (see the point farthest to the right in Fig. 5B).
The observed ligand-to-metal charge transfer during photoionisation, occurs from the β-diketonato ligands to Mn. Therefore charge is also transferred from the other atoms on the β-diketonato ligand to the Mn metal. For example, in complex 6, containing sulphur in the groups substituted on the ligand, the main S 2 p3/2 peak at 164.8 eV also exhibits a shake-down charge transfer satellite peak at 161.5 eV, with a spin–orbit splitting (ΔBE1) of 3.3 eV, as seen in Fig. 3. It however was not possible to also identify the satellite peaks associated with the C and O lines in complexes 1–9, due to the presence of adventitious C and O.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the two Gaussian peaks were fitted in a ratio of 3
1, the two Gaussian peaks were fitted in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
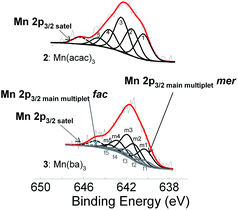
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 into the original Mn 2p peaks for the unsymmetrical complexes 3, 5–8. The Mn(III) β-diketonato complexes containing unsymmetrically substituted β-diketonato ligands (3, 5–8), were further simulated to have two Mn main 2p3/2 peaks, as well as two satellite peaks; one satellite structure is associated with the three more stable mer bond stretch isomers (at lower energy) and the other satellite peak with the less stable fac isomer (at higher energy). The difference in binding energy between the mer and the fac isomers is ca. 0.01 eV. The mer and fac simulation of complex 3 is shown in Fig. 7, as an example for the other unsymmetrical complexes.
1 into the original Mn 2p peaks for the unsymmetrical complexes 3, 5–8. The Mn(III) β-diketonato complexes containing unsymmetrically substituted β-diketonato ligands (3, 5–8), were further simulated to have two Mn main 2p3/2 peaks, as well as two satellite peaks; one satellite structure is associated with the three more stable mer bond stretch isomers (at lower energy) and the other satellite peak with the less stable fac isomer (at higher energy). The difference in binding energy between the mer and the fac isomers is ca. 0.01 eV. The mer and fac simulation of complex 3 is shown in Fig. 7, as an example for the other unsymmetrical complexes.
However, the FWHM obtained above is still larger than expected. A more accurate fitting of the peaks due to additional multiplet splitting, commonly observed in high spin paramagnetic complexes, is therefore needed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.35
1.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 for the five splitted 2p peaks. Fig. 8 shows a simulation of the theoretical multiplet splitting ratio as calculated by Gupta and Sen,51,52 simulated for both complex 2 (representative of complexes 1, 2, 4 and 9 with symmetric ligands), as well as for complex 3 (representative of complexes 3, 5–8 with unsymmetric ligands, in this case fitting both the fac and mer isomers). These multiplet splitting peaks consist of five components (labelled peaks 1–5 for symmetrical complex 2, as well as f1–f5 (fac) and m1–m5 (mer) for the unsymmetrical complex 3). For complex 2, the CHI squared value decreased from 1.8 to 1.3 when multiplet peaks instead of one single peak were fitted for the Mn 2p3/2 photoelectron line, indicating a good fit. The CHI squared value for unsymmetrical complex 3, also decreased further from 1.5 (where merely two main peaks for the fac and mer isomers were fitted for complex 3) to a CHI squared value of 1.3, when also applying the multiplet splitting peak fitting. The overall FWHM of the Mn 2p3/2 photoelectron line when using multiplet splitting, decreased (from the original range of 3.9–6.2 eV, before applying any simulation, see Section 2.2.5) to a range of 1.4–1.8 eV, which is in the same order as was found for related free Mn ions: For example, the calculated Gupta and Sen multiplet splitting distribution for Mn2+ and Mn3+ free ions, when applied to Mn2+ in MnO, and to Mn3+ in manganite, resulted in FWHM values of the multiplet peaks of 1.7 for Mn2+ and 1.25 eV for Mn3+,77 which are similar to the above improved FWHM values for the Mn 2p3/2 peaks (range of 1.4–1.8 eV), after fitting the multiplet splitting.
0.3 for the five splitted 2p peaks. Fig. 8 shows a simulation of the theoretical multiplet splitting ratio as calculated by Gupta and Sen,51,52 simulated for both complex 2 (representative of complexes 1, 2, 4 and 9 with symmetric ligands), as well as for complex 3 (representative of complexes 3, 5–8 with unsymmetric ligands, in this case fitting both the fac and mer isomers). These multiplet splitting peaks consist of five components (labelled peaks 1–5 for symmetrical complex 2, as well as f1–f5 (fac) and m1–m5 (mer) for the unsymmetrical complex 3). For complex 2, the CHI squared value decreased from 1.8 to 1.3 when multiplet peaks instead of one single peak were fitted for the Mn 2p3/2 photoelectron line, indicating a good fit. The CHI squared value for unsymmetrical complex 3, also decreased further from 1.5 (where merely two main peaks for the fac and mer isomers were fitted for complex 3) to a CHI squared value of 1.3, when also applying the multiplet splitting peak fitting. The overall FWHM of the Mn 2p3/2 photoelectron line when using multiplet splitting, decreased (from the original range of 3.9–6.2 eV, before applying any simulation, see Section 2.2.5) to a range of 1.4–1.8 eV, which is in the same order as was found for related free Mn ions: For example, the calculated Gupta and Sen multiplet splitting distribution for Mn2+ and Mn3+ free ions, when applied to Mn2+ in MnO, and to Mn3+ in manganite, resulted in FWHM values of the multiplet peaks of 1.7 for Mn2+ and 1.25 eV for Mn3+,77 which are similar to the above improved FWHM values for the Mn 2p3/2 peaks (range of 1.4–1.8 eV), after fitting the multiplet splitting.
3 Experimental section
3.1 Synthesis
A series of nine β-diketonato manganese(III) complexes of formula [Mn(RCOCHCOR)3], with symmetrically substituted groups R = C(CH3)3 [1]; (CH3) [2]; (C6H5) [4] and (CF3) [9], or of formula [Mn(RCOCHCOR′)3], with unsymmetrically substituted groups R = (CH3) and R′ = (C6H5) [3]; and also R = (CF3) and R′ = (CH3) [5], (OC4H3S) [6], (OC4H3S) [7] or (C6H5) [8], were prepared and characterised according to published methods.783.2 XPS measurements
The X-ray photoelectron spectroscopic (XPS) analysis was carried out with a PHI 5000 Versaprobe, using monochromatic Al Kα X-ray radiation, with hν = 1486.6 eV. The hemispherical analyser pass energy, used to record the high resolution spectra, was maintained at 29.35 eV for C 1s and O 1s, and at 93.90 eV for Mn 2p, S 2p, and F 1s, in order to obtain peaks with good resolution, using 1 eV per step. In order to acquire a neutral charge on the surface of the sample, a low energy electron beam was utilised. All the binding energies of the photoelectron spectra were referenced against the lowest binding energy of the C–C simulated adventitious C 1s photoelectron line, which was set at 284.8 eV. The XPS data was analysed, utilising the Multipak version 8.2c computer software.793.3 DFT calculations
Density functional theory (DFT) calculations were conducted with the ADF (Amsterdam Density Functional) 2013 programme.80 The GGA (Generalised Gradient Approximation) functional OLYP (Handy-Cohen and Lee-Yang-Parr),81–84 a all-electron TZP (Triple ζ polarised) basis set, and spin-unrestricted open shell calculations were used for geometry optimisations. The OLYP functional proved to be a good choice for correctly calculating the ground state of high spin 3d metal-complexes.26,85–89 To further support the reliability of the computational method used in this work, the S = 1 (two unpaired electrons) and S = 2 (four unpaired electrons) spin states of [Mn(acac)3] as representative example of [Mn(β-diketonato)3] complexes, were optimized using a selection of functionals, see Table 3. These results indicate that the OLYP, B3LYP, CAM-B3LYP (B3LYP with long-range correction), M06-D3 (M06 with the Grimme empirical dispersion correction) and M065X functionals correctly predicted the experimental high state of S = 2 of [Mn(acac)3], in agreement with experimental EPR studies.284 Conclusions
A DFT study of the energy and character of the Frontier orbitals of [Mn(β-diketonato)3] complexes shows that a highest occupied molecular orbital (HOMO) of mainly dz2 character is responsible for the elongation of two Mn-ligand bonds along the z-direction. [Mn(β-diketonato)3] complexes containing an unsymmetrically substituted β-diketonato ligand, can have one fac and three mer bond stretch isomers. An XPS study of the Mn 2p core electron binding energies, provides insight into the influence of the electronic environment caused by the electron donating/withdrawing properties of the β-diketonato ligands, on the binding energy strength. The main and satellite peaks of the Mn 2p XPS photoelectron lines were fitted with multiplet splitting peaks, as calculated for the free Mn(III) ion by Gupta and Sen, as well as with satellite shake-up peaks, due to charge transfer. Two sets of multiplet splitting peaks, representing the mer and fac isomers, were simulated for the Mn 2p3/2 photoelectron lines of those [Mn(β-diketonato)3] complexes containing unsymmetrically substituted β-diketonato ligands. The relative intensity of the satellite peak of both the Mn 2p signal of the metal and the F 1s signal in the five fluorine-containing ligands, is related to the amount of charge transferred between the β-diketonato ligands and the MnIII metal centre.Acknowledgements
The authors acknowledge financial support from the South African National Research Foundation and the Central Research Fund of the University of the Free State, Bloemfontein, South Africa. The High Performance Computing facility of the UFS and the Norwegian Supercomputing Program (NOTUR, Grant No. NN4654K) is acknowledged for computer time.References
- (a) M. Viciano-Chumillas, S. Tanase, I. Mutikainen, U. Turpeinen, L. J. de Jongh and J. Reedijk, Inorg. Chem., 2008, 47, 5919 CrossRef CAS PubMed; (b) P. Artus, C. Boskovic, J. Yoo, W. E. Streib, L.-C. Brunel, D. N. Hendrickson and G. Christou, Inorg. Chem., 2001, 40, 4199 CrossRef CAS PubMed.
- J. Yoo, A. Yamaguchi, M. Nakano, J. Krzystek, W. E. Streib, L.-C. Brunel, H. Ishimoto, G. Christou and D. N. Hendrickson, Inorg. Chem., 2001, 40, 4604 CrossRef CAS PubMed.
- G. E. Granroth, M. W. Meisel, M. Chaparala, T. Jolicœur, B. H. Ward and D. R. Talham, Phys. Rev. Lett., 1996, 77, 1616 CrossRef CAS PubMed.
- J. S. Miller, C. Vazquez, N. L. Jones, R. S. McLean and A. J. Epstein, J. Mater. Chem., 1995, 5, 707 RSC.
- A. L. Barra, A. Caneschi, D. Gatteschi and R. Sessoli, J. Am. Chem. Soc., 1995, 117, 8855 CrossRef CAS.
- J. S. Miller, C. Vazquez, J. C. Calabrese, R. S. McLean and A. J. Epstein, Adv. Mater., 1994, 6, 217 CrossRef CAS.
- J. S. Miller, J. C. Calabrese, R. S. McLean and A. J. Epstein, Adv. Mater., 1992, 4, 498 CrossRef CAS.
- A. Caneschi, D. Gatteschi, R. Sessoli, A. L. Barra, L. C. Brunel and M. Guillot, J. Am. Chem. Soc., 1991, 113, 5873 CrossRef CAS.
- (a) R. K. Sodhi and S. Paul, Catal. Lett., 2011, 141, 608 CrossRef CAS; (b) Y.-F. Wang and S. Chiba, J. Am. Chem. Soc., 2009, 131, 12570 CrossRef CAS PubMed.
- R. van Gorkum, E. Bouwman and J. Reedijk, Inorg. Chem., 2004, 43, 2456 CrossRef CAS PubMed.
- R. I. Khusnutdinov, N. A. Shchadneva, A. R. Baiguzina, Y. Y. Lavrentieva and U. M. Dzhemilev, Russ. Chem. Bull., 2002, 51, 2074 CrossRef CAS.
- (a) J. R. Bryant, J. E. Taves and J. M. Mayer, Inorg. Chem., 2002, 41, 2769 CrossRef CAS PubMed; (b) K. A. Campbell, M. R. Lashley, J. K. Wyatt, M. H. Nantz and R. D. Britt, J. Am. Chem. Soc., 2001, 123, 5710 CrossRef CAS PubMed.
- P. Magnus, A. H. Payne, M. J. Waring, D. A. Scott and V. M. Lynch, Tetrahedron Lett., 2000, 41, 9725 CrossRef CAS.
- M. J. S. Dewar and T. Nakaya, J. Am. Chem. Soc., 1968, 90, 7134 CrossRef CAS.
- S. Carli, E. Benazzi, L. Casarin, T. Bernardi, V. Bertolasi, R. Argazzi, S. Caramori and C. A. Bignozzi, Phys. Chem. Chem. Phys., 2016, 18, 5949 RSC.
- I. R. Perera, A. Gupta, W. Xiang, T. Daeneke, U. Bach, R. A. Evans, C. A. Ohlin and L. Spiccia, Phys. Chem. Chem. Phys., 2014, 16, 12021 RSC.
- K. A. Campbell, D. A. Force, P. J. Nixon, F. Dole, B. A. Diner and R. D. Britt, J. Am. Chem. Soc., 2000, 122, 3754 CrossRef CAS.
- R. D. Britt, J. M. Peloquin and K. A. Campbell, Annu. Rev. Biophys. Biomol. Struct., 2000, 29, 463 CrossRef CAS PubMed.
- K. A. Campbell, E. Yikilmaz, C. V. Grant, W. Gregor, A.-F. Miller and R. D. Britt, J. Am. Chem. Soc., 1999, 121, 4714 CrossRef CAS.
- I. Fridovich, Annu. Rev. Biochem., 1995, 64, 97 CrossRef CAS PubMed.
- K. M. Faulkner, S. I. Liochev and I. Fridovich, J. Biol. Chem., 1994, 269, 23471 CAS.
- E. Paparazzo, J. Electron Spectrosc. Relat. Phenom., 2006, 154, 38 CrossRef CAS.
- B. E. Buitendach, E. Erasmus, J. W. Niemantsverdriet and J. C. Swarts, Molecules, 2016, 21, 1427 CrossRef PubMed.
- For a comprehensive list of Gordy scale group electronegativities related to β-diketonato R-groups, see J. Conradie, J. Chem. Soc., Dalton Trans., 2015, 44, 1503 RSC.
- R. Freitag, T. J. Muller and J. Conradie, J. Chem. Crystallogr., 2014, 44, 352 CrossRef CAS.
- R. Gostynski, P. H. van Rooyen and J. Conradie, J. Mol. Struct., 2016, 1119, 48 CrossRef CAS.
- R. Freitag and J. Conradie, J. Chem. Educ., 2013, 90, 1692 CrossRef CAS.
- S. L. Dexheimer, J. W. Gohdes, M. K. Chan, K. S. Hagen, W. H. Armstrong and M. P. Klein, J. Am. Chem. Soc., 1989, 111, 8923 CrossRef CAS.
- S. Geremia and N. Demitri, J. Chem. Educ., 2005, 82, 460 CrossRef CAS.
- J. Conradie, Comput. Theor. Chem., 2016, 1087, 1 CrossRef CAS.
- Cambridge Structural Database (CSD), Version 5.36, May 2015 update. CSD, reference codes: ACACMN21, ACACMN22, ACACMN22, ACACMN23, ACACMN24, JINPIF, JINPIF01, NOSHUA, QAYYEU, XESPIW.
- P. Magnus, A. H. Payne, M. J. Waring, D. A. Scott and V. Lynch, Tetrahedron Lett., 2000, 41, 9725 CrossRef CAS.
- J. P. Fackler Jr and A. Avdeef, Inorg. Chem., 1974, 13, 1864 CrossRef.
- J. Krzystek, G. J. Yeagle, J.-H. Park, M. W. Meisel, R. D. Britt, L.-C. Brunel and J. Telser, Inorg. Chem., 2003, 42, 4610 CrossRef CAS PubMed.
- B. E. Buitendach, E. Erasmus, M. Landman, J. W. Niemantsverdriet and J. C. Swarts, Inorg. Chem., 2016, 55, 1992 CrossRef CAS PubMed.
- J. W. Niemantsverdriet, Microscopy and Imaging, in Spectroscopy in Catalysis: An Introduction, Wiley-VCH Verlag GmbH & Co. KGaA, 3rd edn, Weinheim, Germany, 2007 Search PubMed.
- I. L. Eremenko, S. E. Nefedov, A. A. Sidorov, M. A. Golubnichaya, P. V. Danilov, V. I. Ikorskii, Y. G. Shvedenkov, V. M. Novotortsev and I. I. Mioseev, Inorg. Chem., 1999, 38, 3764 CrossRef CAS.
- P. Pfluger and G. B. Street, J. Chem. Phys., 1984, 80, 544 CrossRef CAS.
- C. Battistoni, J. L. Dormann, D. Fiorani, E. Paparazzo and S. Viticoli, Solid State Commun., 1981, 39, 581 CrossRef CAS.
- A. E. Bocquet, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 3771 CrossRef CAS.
- A. V. Naumkin, T. M. Ivanova, A. V. Shchukarev, A. A. Sidorov, M. A. Kiskin, V. M. Novotortsev and I. L. Eremenko, Russ. J. Inorg. Chem., 2008, 53, 1614 CrossRef.
- A. E. Bocquet, J. Electron Spectrosc. Relat. Phenom., 1996, 82, 87 CrossRef CAS.
- V. DiCastro and G. Polzonetti, J. Electron Spectrosc. Relat. Phenom., 1989, 48, 117 CrossRef CAS.
- L.-T. Chang, C.-Y. Wang, J. Tang, T. Nie, W. Jiang, C.-P. Chu, S. Aranfin, L. He, M. Afsal, L.-J. Chen and K. L. Wang, Nano Lett., 2014, 14, 1823 CrossRef CAS PubMed.
- A. J. Nelson, J. G. Reynolds and J. W. Roos, J. Vac. Sci. Technol., A, 2000, 18, 1072 CAS.
- C. J. Jenks, S.-L. Chang, J. W. Anderegg, P. A. Thiel and D. W. Lynch, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 6301 CrossRef CAS.
- A. R. Chourasia and D. R. Chopra, Surf. Sci. Spectra, 1995, 3, 74 CrossRef CAS.
- S. Wang, W.-R. He, M. Ferbinteanu, Y.-H. Li and W. Huang, Polyhedron, 2013, 52, 1199 CrossRef CAS.
- B. J. Kennedy and K. S. Murry, Inorg. Chem., 1985, 24, 1552 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717 CrossRef CAS.
- R. P. Gupta and S. K. Sen, Phys. Rev. B: Solid State, 1974, 10, 71 CrossRef CAS.
- R. P. Gupta and S. K. Sen, Phys. Rev. B: Solid State, 1975, 12, 15 CrossRef CAS.
- J. W. Niemantsverdriet, Spectroscopy in Catalysis, John Wiley & Sons, 3rd edn, Weinheim, 2007 Search PubMed.
- E. Erasmus, J. Conradie, A. Muller and J. C. Swarts, Inorg. Chim. Acta, 2007, 360, 2277 CrossRef CAS.
- E. Erasmus and J. C. Swarts, New J. Chem., 2013, 37, 2862 RSC.
- R. Liu, J. Conradie and E. Erasmus, J. Electron Spectrosc. Relat. Phenom., 2016, 206, 46 CrossRef CAS.
- J. Conradie and E. Erasmus, Polyhedron, 2016, 119, 142 CrossRef CAS.
- J. J. C. Erasmus and J. Conradie, Dalton Trans., 2013, 42, 8655 RSC.
- M. M. Conradie, J. J. C. Erasmus and J. Conradie, Polyhedron, 2011, 30, 2345 CrossRef CAS.
- M. M. Conradie, J. Conradie and E. Erasmus, Polyhedron, 2014, 79, 52 CrossRef CAS.
- A. Kuhn and J. Conradie, Electrochim. Acta, 2010, 56, 257 CrossRef CAS.
- H. Cao, X. Wu, G. Wang, J. Yin, G. Yin, F. Zhang and J. Liu, J. Phys. Chem. C, 2012, 116, 21109 CAS.
- M. Salavati-Niasari, F. Mohandes, F. Davar and K. Saberyan, Appl. Surf. Sci., 2009, 256, 1476 CrossRef CAS.
- M. Liu, G.-J. Zhang, Z.-R. Shen, P.-C. Sun, D.-T. Ding and T.-H. Chen, Solid State Sci., 2009, 11, 118 CrossRef CAS.
- J. C. Carver, G. K. Schweitzer and T. A. Carlson, J. Chem. Phys., 1972, 57, 973 CrossRef CAS.
- T. M. Ivanova, A. V. Shchukarev, A. V. Naumkin, A. A. Sidorov, M. A. Kiskin, V. M. Novotortsev and I. L. Eremenko, Russ. J. Inorg. Chem., 2008, 53, 1929 CrossRef.
- E. Agostinelli, C. Battistoni, D. Fiorani, G. Mattogno and M. Nogues, J. Phys. Chem. Solids, 1989, 50, 269 CrossRef CAS.
- Y. G. Borod'ko, S. I. Vetchinkin, S. L. Zimont, I. N. Ivleva and Y. M. Shul'ga, Chem. Phys. Lett., 1976, 42, 264 CrossRef.
- M. S. Ioffe and Y. G. Borod'ko, J. Electron Spectrosc. Relat. Phenom., 1977, 11, 235 CrossRef CAS.
- A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564 CrossRef CAS.
- J. S. H. Q. Perera, D. C. Frost and C. A. McDowell, J. Chem. Phys., 1980, 72, 5151 CrossRef CAS.
- J. Park, S. Ryu, M.-S. Han and S.-J. Oh, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10867 CrossRef CAS.
- L. Pauling, J. Am. Chem. Soc., 1932, 54, 3570 CrossRef CAS.
- L. Pauling, Nature of the Chemical Bond, Cornell University Press, 1960 Search PubMed.
- E. Ünveren, E. Kemnitz, S. Hutton, A. Lippitz and W. E. S. Unger, Surf. Interface Anal., 2004, 36, 92 CrossRef.
- A. P. Grosvenor, M. C. Biesinger, R. S. C. Smart and N. S. McIntyre, Surf. Sci., 2006, 600, 1771 CrossRef CAS.
- H. W. Nesbitt and D. Banerjee, Am. Mineral., 1998, 83, 305 CrossRef CAS.
- R. Freitag and J. Conradie, Electrochim. Acta, 2015, 158, 418 CrossRef CAS.
- F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, ULVAC-PHI, Inc., Enzo, Chigasaki, Japan, 1995 Search PubMed.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. F. Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS.
- N. C. Handy and A. J. Cohen, Mol. Phys., 2001, 99, 403 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 37, 785 CrossRef.
- B. G. Johnson, P. M. W. Gill and J. A. Pople, J. Chem. Phys., 1993, 98, 5612 CrossRef CAS.
- T. V. Russo, R. L. Martin and P. J. Hay, J. Chem. Phys., 1994, 101, 7729 CrossRef CAS.
- M. Swart, A. R. Groenhof, A. W. Ehlers and K. Lammertsma, J. Phys. Chem. A, 2004, 108, 5479 CrossRef CAS.
- J. Conradie and A. Ghosh, J. Phys. Chem. B, 2007, 111, 12621 CrossRef CAS PubMed.
- J. Conradie and A. Ghosh, J. Chem. Theory Comput., 2007, 3, 689 CrossRef CAS PubMed.
- M. M. Conradie and J. Conradie, Electrochim. Acta, 2015, 152, 512 CrossRef CAS.
- J. Conradie, T. Wondimagegn and A. Ghosh, J. Phys. Chem. B, 2008, 112, 1053 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2010 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |