Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Feasibility of USPIOs for T1-weighted MR molecular imaging of tumor receptors†
Zhetao Liuad,
Jiali Caib,
Huilan Suc,
Jingxing Yanga,
Wenshe Suna,
Yongjie Maa,
Shiyuan Liu*b and
Chunfu Zhang *ad
*ad
aState Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai 200030, China. E-mail: cfzhang@sjtu.edu.cn
bChangzheng Hospital, Secondary Military Medical University, Shanghai 200003, China. E-mail: cjr.liushiyuan@vip.163.com
cState Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China
dDepartment of Nuclear Medicine, Rui Jin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China
First published on 20th June 2017
Abstract
Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles have been extensively explored for T2- and T1-weighted magnetic resonance imaging (MRI). However, whether USPIOs could be simultaneously used for T2- and T1-weighted MR tumor receptor imaging is seldom reported. Therefore, in the current study, SPECT/MRI dual-functional probes targeting αvβ3 integrin receptors was developed based on USPIOs to examine the feasibility of T2- and T1-weighted dual MRI of tumor receptors. The probes were around 4.5 nm, had superior T1 and T2 MRI contrast effects in water suspensions and high specificity for αvβ3 integrin. After being incubated with αvβ3 positive tumor cells, MR imaging of cell suspensions indicated that the T2 contrast effect of the probe was pronounced and enhanced compared to that in water suspensions at the same concentration, while the T1 contrast effect vanished. After being intravenously administered into tumor bearing mice, the probes could specifically accumulate in tumors as revealed by SPECT/CT imaging. T2-Weighted MRI showed a hypo-intense signal in the tumor region and the signal intensity enhanced with prolongation of time, while for T1-weighted MRI, no hyper-intense signal was observed in the same tumor area. Transmission electron microscopy of tumor tissues revealed that the probes aggregated in cell organelles after targeting αvβ3 integrin. Our study suggested that USPIOs with both superior T1 and T2 contrast effects could only be used for T2-weighted, but not for T1-weighted MR tumor receptor imaging due to aggregation of the particles in cell organelles.
1. Introduction
Superparamagnetic iron oxide (SPIO) nanoparticles are conventionally used as MRI T2 contrast agents, producing negative contrast in T2-weighted images due to the magnetic inhomogeneity induced by their strong magnetic moment.1,2 Because of their high sensitivity and biocompatibility, dextran-coated SPIOs, i.e. Feridex, have been approved by the FDA for diagnosis of liver focal lesions using MRI.3,4 In recent years, SPIOs have also been extensively explored for theranostics of cancers.1,5,6 For example, monoclonal antibodies, peptides and aptamers specific for different antigens have been attached to SPIOs for tumor detection and therapy. Their efficacies have been established in ex vivo and animal studies.7–9 However, the intrinsic dark signal in a T2-weighted MR image may mislead diagnosis because lesions or tumors labelled with T2 agents could be confused with other hypo-intense areas such as bleeding, calcification or metal deposition.10,11 Moreover, the susceptibility artefacts distort the background image. For these reasons, T1 contrast agents are more desirable than T2 agents for accurate high-resolution imaging.Paramagnetic compounds with a large number of unpaired electrons, including Gd3+, Mn2+ and Fe3+, are usually used for T1 contrast agents. T1 contrast effect is induced by the interactions between protons of water molecules and electron spins of the metal ions.12 Currently, the majority of T1 contrast agents are gadolinium complex such as Gd-DTPA. This kind of contrast agent is limited by their nonspecificity to target, quick removal by renal excretion and relatively low sensitivity.13 Early attempts to create targeted T1-weighted molecular imaging agents with this complex to characterize tissues based on the presence of pathognomonic biosignatures initially failed because the payload of metal per homing unit (e.g., antibody) reaching the target site was inadequate to produce detectable signal amplification. As a result, nano formulations with high gadolinium surface payloads were frequently prepared and intensively explored for T1-weighted MR molecular imaging.12,14,15 However, it has been found that gadolinium contrast agents have long-term toxicity and have the risk of inducing nephrogenic system fibrosis (NSF) in patients with impaired kidney function, especially in older patients.16 Therefore, T1 MRI contrast agents with high sensitivity and biocompatibility are more desirable.
Iron oxides are more biocompatible than Gd-based materials because the iron species are rich in human blood,17 which are mostly stored as ferritin in the body. SPIOs with different formulations have been proved by FDA for diagnosis and therapy of diseases.3 For example, ferumoxytol, a carboxylized dextran-coated SPIOs, has been approved for treatment of iron-deficiency anaemia in adults with chronic kidney disease in 2009 as Feraheme. The application dose is 510 mg.18 However, the commonly used SPIOs are not appropriate for T1 MRI contrast agents due to their low r1 value and large r2/r1 ratio, a defining parameter indicating whether the contrast agent can be employed as a positive or negative agent.19 However, the magnetic property of SPIOs is strongly dependent on their size.5,20 When the size of SPIOs decreases, the magnetic moment of the particles declines rapidly due to the reduction in the volume magnetic anisotropy and spin disorders on the surface of the nanoparticles,21 whereas iron ions with 5 (Fe3+) or 6 (Fe2+) unpaired electrons exposed on the particle surface are increased, which is very beneficial to suppress the T2 effect and maximize the T1 contrast effect.22 It has been suggested that a core size of approximately 5 nm is optimal to form a T1 contrast agent based on iron oxide nanoparticles.23 Ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs) have been demonstrated a good T1 MRI contrast agent.24–26 Kim et al. have synthesized extremely small-sized iron oxide nanoparticles (ESIONs) of less than 4 nm and demonstrated the ESIONs had a great potential as T1 MRI contrast agent in clinical settings.27 Recently, we have developed a novel approach to produce polyacrylic acid (PAA) coated USPIOs (PAA@USPIOs) in large scale.28 The PAA@USPIOs (around 4.5 nm) have a superior T1 contrast effect and are highly effective for MRI angiography. However, whether USPIOs with good T1 contrast effect could be used for receptor-targeted, T1-weighted MR molecular imaging is still unknown and few works have been performed in this regard.
Therefore, in this study, we prepared a αvβ3 integrin-targeted SPECT/MRI dual functional probe (RGD-99mTc-PAA@USPIOs) based on PAA@USPIOs developed previously and evaluated its performance for T1- and T2-weighted dual MR tumor receptor imaging. We found that the probes have good T1 and T2 contrast effect in water suspensions; however, after targeting αvβ3 integrin, the T1 contrast effect vanished and the probes only demonstrated T2 contrast effect.
2. Experimental section
2.1 Preparation of RGD peptide-conjugated, 99mTc-labeled USPIOs probe (RGD-99mTc-PAA@USPIOs)
USPIOs coated with polyacrylic acid (PAA@USPIOs) were synthesized via a polyol method according to our previous reports.28 For preparation of RGD-99mTc-PAA@USPIOs, PAA@USPIOs were first modified with ethylene diamine. Specifically, PAA@USPIOs (4.5 mg) and ethylene diamine (13.5 mg) were mixed into 2-(N-morpholino)ethanesulfonic acid (MES) buffer (3 mL, pH = 4.5). After adjusting the pH to 5.5, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloridecrystalline (EDC, 20 mg) was then added and the mixture was stirred for 3 h at room temperature. Ethylene diamine modified PAA@USPIOs was retrieved by ultrafiltration (Millipore, MWCO 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and washed with phosphate buffer saline (PBS, pH = 8.5) three times. To couple RGD peptide and label 99mTc, ethylene diamine derivatized USPIOs were further modified with maleimide-PEG-succinimidyl valerate (MAL-PEG-SVA) and diethylenetriaminepentaacetic acid (DTPA) dianhydride simultaneously. In detail, the aminated USPIOs (5 mg) were dispersed into 0.5 mL of PBS (pH = 8.5), into which MAL-PEG-SVA (MW = 3400, 6.0 mg) and DTPA dianhydride (C14H19N3O8, MW = 357.32, 0.6 mg) were added and the mixture was stirred for about 30 min at room temperature. Subsequently, the USPIOs were collected by ultrafiltration (Millipore, MWCO 100
000) and washed with phosphate buffer saline (PBS, pH = 8.5) three times. To couple RGD peptide and label 99mTc, ethylene diamine derivatized USPIOs were further modified with maleimide-PEG-succinimidyl valerate (MAL-PEG-SVA) and diethylenetriaminepentaacetic acid (DTPA) dianhydride simultaneously. In detail, the aminated USPIOs (5 mg) were dispersed into 0.5 mL of PBS (pH = 8.5), into which MAL-PEG-SVA (MW = 3400, 6.0 mg) and DTPA dianhydride (C14H19N3O8, MW = 357.32, 0.6 mg) were added and the mixture was stirred for about 30 min at room temperature. Subsequently, the USPIOs were collected by ultrafiltration (Millipore, MWCO 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), washed with PBS (pH = 7.4) three times, and finally suspended into 0.5 mL of PBS (pH = 7.4). For RGD peptide conjugation, cyclic RGD peptide c(RGDyC) (abbreviated RGD, 0.3 mg) was added into the above suspensions and gently stirred overnight at room temperature. The RGD-conjugated USPIOs were ultrafiltrated (Millipore, MWCO 100
000), washed with PBS (pH = 7.4) three times, and finally suspended into 0.5 mL of PBS (pH = 7.4). For RGD peptide conjugation, cyclic RGD peptide c(RGDyC) (abbreviated RGD, 0.3 mg) was added into the above suspensions and gently stirred overnight at room temperature. The RGD-conjugated USPIOs were ultrafiltrated (Millipore, MWCO 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), washed with PBS (pH = 7.4), and eventually dispersed into 0.5 mL of PBS (pH = 7.4). The peptide conjugation efficiency was determined with the Ellman method by measuring the free sulfhydryl groups in the peptide in the reaction media before and after conjugation spectrophotometrically.29,30
000), washed with PBS (pH = 7.4), and eventually dispersed into 0.5 mL of PBS (pH = 7.4). The peptide conjugation efficiency was determined with the Ellman method by measuring the free sulfhydryl groups in the peptide in the reaction media before and after conjugation spectrophotometrically.29,30
For 99mTc labeling, 2 mg of RGD peptide-conjugated USPIOs were dispersed into a mixture of ammonium acetate (90 μL, 0.25 M) and tartrate buffer (30 μL, 50 mM), then 10 μL of freshly prepared stannous chloride dihydrate solution (4 mg mL−1 in tartrate buffer) was added, followed by 200 μL 99mTc-pertechnetate generator eluate (2 mCi). The mixture was vortexed for about 30 min at room temperature. The labeled USPIOs were retrieved by ultrafiltration and washed with PBS (pH = 7.4) three times.31,32
99mTc radiolabeling efficiency and its stability on the probe were evaluated by radio-thin layer chromatography (AR2000, Bioscan, Washington, USA) using acetone as the mobile phase. In this system, 99mTc-labeled USPIOs remain at the origin, while 99mTc-pertechnetate migrates to retardation factor (Rf) = 0.7–0.9. The labeling efficiency was calculated by dividing the radioactivity retained at the origin to the total radioactivity added. To assess the radiochemical stability of 99mTc in the physiological condition, the probe RGD-99mTc-PAA@USPIOs was co-incubated with 200 μL of fresh mouse plasma at 37 °C for different periods of time. Stability of 99mTc was expressed as a percentage of radioactivities retained on the particles to the radioactivity of the probes.33
In addition, RGD peptide-conjugated, technetium (Tc)-labeled USPIOs (RGD-Tc-PAA@USPIOs) were also prepared as a “cold” probe using NaTcO4 as a precursor under the same conditions as those for 99mTc labeling. At the same time, scramble peptide c(RADyC) (abbreviated RAD) conjugated-, 99mTc- or Tc-labeled PAA@USPIOs were also prepared as control probes (RAD-99mTc-PAA@USPIOs, RAD-Tc-PAA@USPIOs).
2.2 Characterizations of the probes
The morphology and core size of the probes were investigated by transmission electron microscopy (TEM, JEOL2010) at an accelerating voltage of 200 kV. The average core size was determined by measuring the diameters of more than 100 particles in the TEM images using ImageJ analysis software (NIH). The hydrodynamic sizes and zeta potentials were analysed by using a dynamic light scattering (DLS) instrument (NanoZS, Malvern, UK). The T1 and T2 relaxation times were determined using a 1.41 T (60 MHz) Bruker mq60 nuclear magnetic resonance analyzer (Bruker, Karlsruhe, Germany) at 37 °C. For this purpose, the probes were diluted in a series of concentration, which were measured using an atomic absorption spectrophotometer (AAS, Z-2000, Hitachi, Japan). Inversion recovery and multi-echo CPMG sequences were used to determine the T1 and T2 relaxation times of the probe samples, and thus calculate the R1 (1/T1) and R2 (1/T2) relaxation rates of each sample. The R1 and R2 were plotted against probe concentration (mM, in iron) to respectively determine the longitudinal (r1) and transverse (r2) relaxivities from the slope of the linear fit. The T1 and T2 relaxation times and thus the r1 and r2 relaxivities were also determined using a 3 T clinical MRI scanner (TrioTim, Siemens, Germany) at the room temperature. The measurement setup and imaging parameters were detailed in ESI.† To study the effect of surface modifications on MRI property of the USPIOs, relaxivities of the particles at each step of probe preparation were evaluated.2.3 MRI of RGD-Tc-PAA@USPIOs suspensions
To evaluate the MRI performance of the probe, RGD-Tc-PAA@USPIOs was diluted in deionized water in plastic vials. To avoid susceptibility artefacts from the surrounding air in the scans, all the samples were placed in a water-containing plastic container at room temperature. MRI was performed with a 3 T MRI scanner (TrioTim, Siemens, Germany) using a clinical head coil with T1- (TR = 500 ms, TE = 15 ms, average 3, FOV = 100 mm, matrix = 192 × 192, slice thickness = 2 mm) and T2-weighted spin-echo sequence (TR = 2000 ms, TE = 37 ms, average 3, FOV = 100 mm, matrix = 192 × 192, slice thickness = 2 mm).2.4 Cytotoxicity assay
H1299 cells, a non-small lung cancer cell line, were provided by Shanghai Institutes for Biological Sciences, CAS (Shanghai, China), grown in DMEM medium supplemented with 10% FBS, and maintained at 37 °C under a humidified atmosphere containing 5% CO2. Cytotoxicity of the probes was evaluated by the typical 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assays using the cold probe (RGD-Tc-PAA@Fe3O4).33,34 For this purpose, the cells were seeded in a 96-well plate with 1 × 104 cells per well and cultured with the media containing various concentrations of probes (0.15, 0.5 and 1.5 mM in iron) for different period of time. After incubation, the culture media were removed and the cells were washed with PBS (pH 7.4) three times. Subsequently, 100 μL aliquots of MTT solution were added. After incubation for another 4 h, the media were replaced with 100 μL of dimethyl sulfoxide per well, and the absorbance was monitored by a microplate reader at a wavelength of 490 nm. The cell viability was expressed as the percentage of absorbance of the cells incubated with the probes to that of the cells maintained in a normal culture medium.2.5 In vitro cell binding and specificity
H1299 cells, a non small-lung cancer cell line, overexpresses αvβ3 integrin.35 Specificity of the probes for αvβ3 integrin was examined by Prussian blue staining and AAS quantifications of intracellular iron contents of the cells treated with the probes. For Prussian blue staining, H1299 cells were seeded on glass coverslips and cultured in six-well plates with media containing RGD-Tc-PAA@USPIOs, RAD-Tc-PAA@USPIOs or RGD-Tc-PAA@USPIOs plus free RGD peptide (10 μM) at the concentration of 0.5 mM (in iron) for 1 h. After incubation, the cells were washed with PBS (0.1 M, pH 7.4) three times and then fixed with paraformaldehyde (4%). The fixed cells were stained with 10% Prussian blue for 5 min, a mixture of 10% Prussian blue and 20% HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 30 min, and nuclear fast red for 5 min successively. Slides were examined by optical microscopy using a Leica DMLB microscope (Leica Microsystems Inc, Buffalo Grove, Illinois). For quantifications of intracellular iron content, the cells (3 × 106) were collected and digested with aqua regia at 60 °C for 1 h, and then the intracellular iron content was determined by AAS.
1) for 30 min, and nuclear fast red for 5 min successively. Slides were examined by optical microscopy using a Leica DMLB microscope (Leica Microsystems Inc, Buffalo Grove, Illinois). For quantifications of intracellular iron content, the cells (3 × 106) were collected and digested with aqua regia at 60 °C for 1 h, and then the intracellular iron content was determined by AAS.
2.6 MR cell imaging
After treated with the probes, the cells (1 × 106) were homogenously suspended in gelatin (2%, 500 μL) in plastic vials and placed in a water tank. T1- and T2-weighted MRI was performed with a 3 T MRI scanner (TrioTim, Siemens, Germany) using the same parameters as those for probe imaging aforementioned. In addition, MR imaging of RGD-Tc-PAA@USPIOs-treated cells suspended in gelatin at different concentrations (in iron) was also performed.2.7 SPECT/CT and MR imaging
All experiments were performed in compliance with the National Regulations for the Administration of Affairs Concerning Experimental Animals and approved by the animal protection and care committee of Shanghai Jiao Tong University. H1299 tumor xenograft was conducted by implanting tumor cells (1 × 106) under the left limb of BALB/c mice (Slaccas, Shanghai, China). Tumors were allowed to grow over the next 3–4 weeks. For SPECT/CT imaging, tumor-bearing mice (five mice per group) were intravenously injected with RGD-99mTc-PAA@USPIOs, RAD-99mTc-PAA@USPIOs, or RGD-99mTc-PAA@USPIOs plus free RGD peptide (0.15 mM, 100 μL) at the radioactive dose of 3.7 MBq. SPECT/CT scans were performed at 0.5, 1, 3, 6 h post probe injection using a small-animal imaging system (Bioscan, Washington, USA) and the images were obtained at 32 projections over 360 °C (radius of rotation = 7.6 cm, 30 s per projection). The CT images were used to provide anatomical references to the tumor location. Reconstructed data from SPECT and CT were visualized and co-registered using InVivoScope provided by the manufacturer.After SPECT/CT imaging, the mice were euthanized and dissected. The major organs (tumor, heart, liver, spleen, lung, kidney, stomach, intestine, brain, bone, pancreas, bladder, muscle) were harvested and weighed. The radioactivity associated with each organ was determined by a γ-counter along with 3 × 0.5 mL aliquots of the diluted standard representing 100% of the injected dose. The mean activities were used to obtain the percentage of injected dose per gram of tissue (% ID per g).
MR imaging of tumors was performed using the cold probe. H1299 tumor bearing mice (five mice per group) were treated with RGD-Tc-PAA@USPIOs, RAD-Tc-PAA@USPIOs, or RGD-Tc-PAA@USPIOs plus free RGD (0.15 mM, 100 μL) at the dose of 100 μmol Fe per kg bodyweight.34 MR imaging was conducted using a 3 T MRI scanner (Signa Excite HDx, GE, Milwaukee, Wisconsin) equipped with a customized coil. For image acquisition and determination of T1 and T2 relaxation times of tumors before and after probe injection, T1 mapping sequence (TR = 3000 ms, TE = 15 ms, and inversion delays of 500, 1000, 1500, 2000, 2500, 3000, and 3500 ms) and T2 mapping sequence (TR = 3000 ms, TE = 20–160 ms, 8 echo, matrix = 128 × 128, FOV = 150 mm, slice thickness = 2 mm) were utilized.
2.8 Histological studies
After MR imaging, the mice were euthanized. The tumors were removed, imbedded in OCT glue (Sakura Finetek Inc, Torrance, California) and then frozen with nitrogen. 10 μm sections were made using a cryotome (CM1850; Leica Microsystems GmbH). The sections were first fixed in acetone for 10 min at 4 °C and then air-dried for 30 min.To verify RGD-Tc-PAA@USPIOs targeting tumor angiogenic vessels, Prussian staining was performed. The procedure for the tissue staining was same as that for cell staining. The slides were examined by optical microscopy using a Leica DMLB microscope (Leica Microsystems Inc, Buffalo Grove, Illinois).
To identify the conditions of the probes in tumors, TEM examinations of tumor tissues were conducted. The procedure for the sample preparation was same as that for cells described previously. Micrographs were taken with TEM operating at an acceleration voltage of 80 kV (Philip CM-120, Eindhoven, The Netherlands).
To identify the expression of αvβ3 integrin, immunohistological staining of tumor tissues against αvβ3 integrin was performed. To this end, the sections were treated with a primary rat-anti-mouse CD61 monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dilution; BD Biosciences) and a biotinylated goat-anti-rat IgG (BD Bioscience) in combination with streptavidin-horseradish peroxidase (HRP) and the DAB detection system. The tumor sections were counterstained with hematoxylin and returned to blue by using an ammonia solution. In addition, tumor vessels were also stained against CD31.
50 dilution; BD Biosciences) and a biotinylated goat-anti-rat IgG (BD Bioscience) in combination with streptavidin-horseradish peroxidase (HRP) and the DAB detection system. The tumor sections were counterstained with hematoxylin and returned to blue by using an ammonia solution. In addition, tumor vessels were also stained against CD31.
2.9 Statistical evaluation
All data were presented as means ± standard deviations (SD). Statistical analysis of intracellular iron content (AAS data) and biodistributions of the probes were conducted by using a Wilcoxon rank sum test. A p value of <0.05 was considered to indicate significant differences between groups.3. Results and discussion
3.1 Synthesis and characterizations of RGD-99mTc-PAA@USPIOs
PAA@USPIOs was prepared by polyol method in the presence of PAA. The TEM size and zeta potential of PAA@USPIOs were 4.5 ± 0.5 nm and −55 mV, respectively. The longitudinal (r1) and transversal (r2) relaxivities were 8.67 and 25.36 mM−1 s−1, with r2/r1 ratio of 2.93. PAA@USPIOs was highly effective for MRI angiography. To conjugate RGD peptides and label 99mTc, PAA@USPIOs was first modified with ethylene diamine. After the modification, zeta potential of the USPIOs raised to −28 mV. Then, the aminated PAA@USPIOs was further derivatized by MAL-PEG-SVA and diethylene triamine pentaacetic acid (DTPA) dianhydride simultaneously. Both of the substances could form covalent bonds with the primary amine present on the aminated PAA@USPIOs through amide bonds. The RGD peptides (c(RGDyC)) were then covalently conjugated to the USPIOs through thiol–maleimide linkages between the peptide and PEG. The RGD conjugation efficiency was about 98% as measured by Ellman method. 99mTc was labeled onto the USPIOs by complexing 99mTc with DTPA.33,36 The labeling efficiency, as verified by RTLC, was 95%. The procedure for preparation of the probe was shown in Scheme 1. Once fully labeled, the RGD-99mTc-PAA@USPIOs were purified using size exclusion filters and size exclusion chromatography with disposable columns containing Sephadex G-25 medium, using saline as the eluent. The purified RGD-99mTc-PAA@USPIOs were highly stable in mouse plasma, retaining around 95% of the initial 99mTc content after 24 h incubation at 37 °C (Fig. 1A).We also prepared USPIOs without 99mTc for use as cold probes, labeling instead with technetium using NaTcO4 as a precursor (RGD-Tc-PAA@USPIOs). The TEM size of RGD-Tc-PAA@USPIOs was 4.5 ± 1.2 nm, similar to PAA@USPIOs and the probes were well separated from each other without observable aggregation in deionized water (Fig. 1B). Zeta potential of the probes was found to be around −32 mV. In order to test the stability of the probe, we measured the hydrodynamic size of the probes in mouse serum and PBS using DLS for different periods of time. In both cases, the hydrodynamic sizes were 102 ± 2 nm and 95 ± 3 nm, respectively, and did not change significantly during 24 h (Fig. 1C). Fig. 1D showed the 1/T1 and 1/T2 relaxation rates of the probes at 1.41 T as a function of the iron concentrations. It was found that the relaxation rates varied linearly with the iron concentrations. The longitudinal (r1) and transversal (r2) relaxivities were 9.34 mM−1 s−1 and 24.64 mM−1 s−1, respectively, with r2/r1 ratio of 2.64, better than other clinically approved USPIOs-based T1 contrast agent.1 Moreover, after each step of surface modification, both the longitudinal and transverse relaxivities of the particles were similar to those of PAA@USPIOs, indicating that the surface modifications did not significantly affect the relaxation properties of the particles (Table S1†). However, consistent with previous reports, the r2 relaxivity increased, while the r1 decreased at a higher magnetic field (3 T, Table S1†).37
To investigate the MR signal enhancement effects, the aqueous solutions of RGD-Tc-PAA@USPIOs at different concentrations (in iron) were measured on a clinical 3 T MRI scanner. As shown in Fig. 1E, RGD-Tc-PAA@USPIOs induced a dark signal on the T2-weighted images and a bright signal on the T1-weighted images, in line with other USPIOs contrast agents.38 The T2 signal intensity decreased in a concentration-dependent manner.39 However, T1-weighted images showed an increasing enhancement with a marked brightening until a given iron concentration, but for higher concentrations the signal decreased and darkening was observed. Our results were consistent with previous observations, and the phenomenon might arise from overdose effects.40 The overdose effect is attributed to increasing T2 shortening at higher doses, which reduces the signal intensity and cancel out the signal-enhancing effect of T1 shortening even at the short echo times used.41
3.2 Cytotoxicity of the probes
Cytotoxicity of the probes was evaluated in vitro with MTT reduction assay. H1299 cells were incubated with RGD-Tc-PAA@USPIOs at different concentrations (0.15, 0.5, 1.5 mM in iron) for different period of time (3, 6, 12, 24 h) (Fig. 1F). The results indicated that the viability of the cells was not affected by the presence of the probes even up to 1.5 mM for 24 h (∼95%), suggesting that similar to PAA@USPIOs demonstrated previously;28 our probes were noncytotoxic and safe for further in vivo use.42 This result also indicated that the surface modifications did not alter the safety profile of the USPIOs.3.3 Specificity of the probes
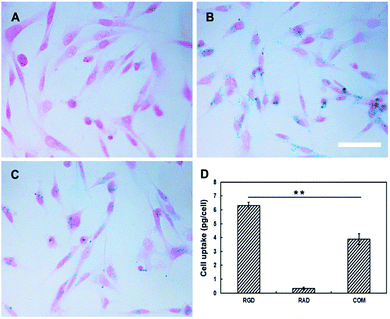
To evaluate the specificity of the probes for αvβ3, H1299 cells were cultured with media containing RGD-Tc-PAA@USPIOs, RAD-Tc-PAA@USPIOs, or RGD-Tc-PAA@USPIOs plus free RGD peptide (10 μM) at the concentration of 0.5 mM (in iron) for 1 h. Prussian blue staining revealed that cell uptake of RGD-Tc-PAA@USPIOs was greater than that of RAD-Tc-PAA@USPIOs, and the uptake was suppressed by free RGD peptide (Fig. 2A–C). Consistent with Prussian blue staining, AAS quantification indicated that cell uptake of RGD-Tc-PAA@USPIOs and RAD-Tc-PAA@USPIOs was 6.5 ± 0.2 and 0.6 ± 0.1 pg per cell, respectively, and the uptake was reduced to 3.8 ± 0.4 pg per cell after inhibition by free peptide (p < 0.01) (Fig. 2D). These observations suggested that RGD-Tc-PAA@USPIOs could specifically target αvβ3 integrin and the cellular uptake of the probes was mediated by the receptor.8,363.4 MRI of cell suspensions
As demonstrated previously, RGD-Tc-PAA@USPIOs was a good T1 MRI contrast agent in relatively lower concentrations. To determine whether the probes could still act as T1 MRI contrast agent after targeting tumor cells, MR imaging of H1299 cells (1 × 106) treated with the probes was performed. In contrast to the dispersed isolated probes in water suspensions, the signal enhancement in T1-weighted MRI images of the probe-treated cells was negative and the signal loss was similar to that in the T2-weighted MRI images. The dark signal intensity lessened after competition with free RGD peptide. Even for the RAD-Tc-PAA@USPIOs-treated cells, the signal intensity also decreased marginally (Fig. 3A). To exclude the possible overdose effect observed in probe water suspensions, we suspended the probe-treated cells at concentrations (in iron) same as those of probe suspensions for MRI. As shown in Fig. 3B, even at the lowest cell concentration (0.05 mM), T1-weighted MR signal also decreased compared to that of the plain cells. Different from the dispersed isolated probes, the positive enhancement was no longer present, whereas the darkening effect increased over the whole concentration range. As observed in Prussian blue staining, αvβ3 integrin targeting induced cell uptake of the probe. Previous studies indicated that the receptor-mediated uptake would induce the probes to be accumulated and clustered in cell lysosomes.34 Clustering would dramatically enhance the T2 effect, while diminish the T1 effect of USPIOs.43,44To evaluate the physical status of the probes in cells, TEM examinations of the cells were performed. In line with Prussian blue staining and AAS quantification, TEM microscopies revealed that cell ingestion of RGD-Tc-PAA@USPIOs (Fig. 3D) was more than that of RAD-Tc-PAA@USPIOs (Fig. 3C) and free RGD peptide competition reduced the ingestion (Fig. 3E). Both RGD-Tc-PAA@USPIOs and RAD-Tc-PAA@USPIOs were internalized by cells and densely packed in cell lysosomes (arrowheads).7
Change of physical status from the dispersed isolated particles in a water solution to aggregations in cytoplasm may be the reason for the probe loosing MRI T1 performance. The precondition for USPIOs used as T1 contrast agent is that the USPIOs should have a high r1 and a relatively lower r2, thus lower r2/r1 ratio.22,45 Clustering of USPIOs would dramatically enhance the T2 contrast effect (r2), while weakening the T1-shortening effect (r1), resulting in high r2/r1 ratio and limiting USPIOs to be a T1 contrast agent.46 Our results were actually in accordance with previous observations that intracellular confinement of magnetite nanoparticles within micrometric endosomes led to a significant decrease of the r1 relaxivity compared to that of the dispersed isolated nanoparticles. Consequently, for T1-weighted sequences, the signal intensity fell essentially, so the positive enhancement no longer existed.37,47 The possible explanation was that the intracellular compartmentalization would restrict water diffusion and/or particle diffusion and thereby limited the T1 effect of the USPIOs in cells.37,47
3.5 MRI and SPECT/CT imaging of tumors
To investigate the potential of the probe for T1-weighted MR tumor receptor imaging, next, the specificity of the probe for αvβ3 integrin in vivo was first assessed by SPECT/CT imaging. H1299 tumor bearing mice were intravenously injected with RGD-99mTc-PAA@USPIOs, RAD-99mTc-PAA@USPIOs or RGD99mTc-PAA@USPIOs plus free RGD (3.7 MBq). SPECT/CT imaging revealed that tumor accumulation of RGD-99mTc-PAA@USPIOs was obvious 30 min post injection and augmented with the prolongation of time. Six hours post injection, strong radioactive signal was observed in tumor region. Moreover, the accumulation was greater than that of RAD-99mTc-PAA@Fe3O4 at each time point examined, and reduced in the presence of free RGD peptide competition (Fig. 4A). Consistent with SPECT/CT imaging, biodistribution studies indicated that tumor accumulations of RGD-99mTc-PAA@USPIOs, RAD-99mTc-PAA@USPIOs and RGD-99mTc-PAA@USPIOs plus free RGD were 4.98 ± 0.70, 0.90 ± 0.23, and 2.25 ± 0.03 ID% per g (**p < 0.01), respectively. These observations indicated that the RGD-99mTc-PAA@USPIOs specifically target αvβ3 integrin in vivo.33,34T2- and T1-weighted MRI were performed using the cold probe and a 3 T MRI scanner by an alternate scanning manner. For T2-weighted MRI, inhomogeneous dark signals were observed in tumor regions for mice receiving RGD-Tc-PAA@Fe3O4 probe 1 h post injection and the hypo-intense signals further decreased with the prolongation of time (Fig. 5A). Accordingly, the T2 relaxation time changes of the tumors before and after probe injection were 28 ± 6 ms (1 h), 36 ± 5 ms (3 h) and 44 ± 8 ms (6 h), respectively. For mice treated with RGD-Tc-PAA@Fe3O4 plus free RGD peptide, the inhomogeneous dark signals in tumor regions could still be observed, but were less pronounced. For the control mice treated with RAD-Tc@Fe3O4 probes, the decrease in the MR signal intensity in the tumor regions was only marginal. These results were consistent with previous reports that iron oxide nanoparticles concentrated at the target site generated dark or negative contrast in T2-weighted images.48 However, for T1-weighted MRI, no bright signals in tumor regions were observed for mice receiving RGD-Tc-PAA@Fe3O4 (Fig. 5B), even if the probes were present in the tumor regions as clearly observed in T2-weighted MRI. The T1 relaxation time changes of the tumors before and after probe injection were 6 ± 4 ms (1 h), 7 ± 5 ms (3 h) and 8 ± 2 ms (6 h), respectively. Due to the superior T1 contrast effect of the probes, bright signals in tumor region were expected, similar as those from gadolinium-based molecular imaging probes.10,33 Our results were actually consistent with previous report on liver MR imaging using USPIOs-based T1 contrast agent,49 in which T1-weighted MRI signal intensity in liver region was first increased by 26% shortly after injection of the USPIOs and then gradually decreased, in a manner similar to that in T2-weighted MRI. The possible reason was that shortly after injection, the USPIOs were still in blood pool of liver and can perform well as T1 contrast agent. Once internalized by Kuffer cells in sinus hepaticus, the T1 effect diminished due to aggregation of the particles in cytoplasm. Considering the results of MR cell imaging, we speculated that RGD-Tc-PAA@Fe3O4 probe may also clustered in tumor after targeting tumor cells and made them not suitable for T1-weighted MR imaging.
 | ||
| Fig. 5 (A and B) T2- and T1-weighted MRI of mice intravenously injected with RGD-Tc-PAA@USPIOs (RGD), control probe (RAD-Tc-PAA@USPIOs) (RAD) and RGD-Tc-PAA@USPIOs plus free RGD peptide (COM). | ||
USPIOs have been derivatized with varieties of biomolecules, such as peptides, aptamers and antibodies, targeting different biomarkers for MR molecular imaging of cancers.50,51 RGD-containing peptides have high affinity to αvβ3 integrin receptor, which is overexpressed on endothelial cells during angiogenesis, but barely detectable in most normal organs.52 Therefore, it is widely used for diagnostic imaging. In addition to targeting tumor angiogenic vessels, our probe RGD-99mTc-PAA@USPIOs could also address H1299 tumor cells after extravasation from tumor vessels. Therefore, compared to probes binding to receptors that only express on tumor cells, the targeting efficiency and thus the detection sensitivity of our probes for H1299 tumor might be higher.
3.6 Histological studies
After MRI, the mice were sacrificed, the tumors were removed, and histological studies of the tumor tissues were performed. Microscopic examination of tumor sections stained with Prussian blue revealed that RGD-Tc-PAA@USPIOs registered the tumor angiogenic vessels (Fig. 6A) and its targeting efficiency was reduced after competition with free RGD peptide. RAD-Tc-PAA@USPIOs was also found in the tumors, though to a lesser extent, which indicated nonspecific uptake due to the enhanced permeation and retention (EPR) effect.53 To identify the physical conditions of the probes in tumors, TEM examinations of tumor tissues were also performed (Fig. 6A). TEM microscopies showed that the probes accumulated and packed in cytoplasmic vesicles, similar as that observed in cell suspensions. Aggregation of the probes within cells may be the reason for poor T1-weighted effect in tumors. Immunostaining of the tumor tissues against CD31 (Fig. 6B left) and CD61 (Fig. 6B right) indicated that H1299 tumors were highly vascular and some of the tumor vessels were αvβ3 positive.4. Conclusions
In summary, we have developed a αvβ3 integrin targeted, SPECT/MRI dual functional molecular imaging probe based on USPIOs. The probes had superior T1 and T2 MRI contrast effects in water suspensions and high specificity for αvβ3 integrin. After targeting αvβ3 integrin, however, its performance as T1 positive contrast agent was significantly suppressed and only T2 contrast effect was manifested both in vitro and in vivo due to clustering of the probe in cell vesicles. Our study suggested that for MR tumor receptor imaging, USPIOs even with good T1 contrast effect could only be used for T2-weighted imaging.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 81571729, 81230030) and grants from the State Key Laboratory of Oncogenes and Related Genes (90-15-03).Notes and references
- N. Lee, D. Yoo, D. Ling, M. H. Cho, T. Hyeon and J. Cheon, Chem. Rev., 2015, 115, 10637–10689 CrossRef CAS PubMed.
- B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901–986 CrossRef CAS PubMed.
- A. Tanimoto and S. Kuribayashi, Eur. J. Radiol., 2006, 58, 200–216 CrossRef PubMed.
- D. D. Stark, R. Weissleder, G. Elizondo, P. F. Hahn, S. Saini, L. E. Todd, J. Wittenberg and J. T. Ferrucci, Radiology, 1988, 168, 297–301 CrossRef CAS PubMed.
- L. Wu, A. Mendoza-Garcia, Q. Li and S. Sun, Chem. Rev., 2016, 116, 10473–10512 CrossRef CAS PubMed.
- J. E. Rosen, L. Chan, D.-B. Shieh and F. X. Gu, Nanomedicine: Nanotechnology, Biology and Medicine, 2012, 8, 275–290 CrossRef CAS PubMed.
- H. Zhu, L. Zhang, Y. Liu, Y. Zhou, K. Wang, X. Xie, L. Song, D. Wang, C. Han and Q. Chen, Sci. Rep., 2016, 6, 39245 CrossRef CAS PubMed.
- R. Misri, D. Meier, A. C. Yung, P. Kozlowski and U. O. Häfeli, Nanomedicine: Nanotechnology, Biology and Medicine, 2012, 8, 1007–1016 CrossRef CAS PubMed.
- F. Ai, C. A. Ferreira, F. Chen and W. Cai, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 619–630 CrossRef CAS PubMed.
- H. B. Na, J. H. Lee, K. An, Y. I. Park, M. Park, I. S. Lee, D.-H. Nam, S. T. Kim, S.-H. Kim, S.-W. Kim, K.-H. Lim, K.-S. Kim, S.-O. Kim and T. Hyeon, Angew. Chem., Int. Ed., 2007, 46, 5397–5401 CrossRef CAS PubMed.
- Z. Li, B. Tan, M. Allix, A. I. Cooper and M. J. Rosseinsky, Small, 2008, 4, 231–239 CrossRef CAS PubMed.
- Y.-K. Peng, S. C. E. Tsang and P.-T. Chou, Mater. Today, 2016, 19, 336–348 CrossRef CAS.
- Z. Gao, T. Ma, E. Zhao, D. Docter, W. Yang, R. H. Stauber and M. Gao, Small, 2016, 12, 556–576 CrossRef CAS PubMed.
- S. Ghiani, M. Capozza, C. Cabella, A. Coppo, L. Miragoli, C. Brioschi, R. Bonafè and A. Maiocchi, Nanomedicine: Nanotechnology, Biology and Medicine, 2017, 13, 693–700 CrossRef CAS PubMed.
- A. H. Schmieder, P. M. Winter, T. A. Williams, J. S. Allen, G. Hu, H. Zhang, S. D. Caruthers, S. A. Wickline and G. M. Lanza, Radiology, 2013, 268, 470–480 CrossRef PubMed.
- P. H. Kuo, E. Kanal, A. K. Abu-Alfa and S. E. Cowper, Radiology, 2007, 242, 647–649 CrossRef PubMed.
- R. Chen, D. Ling, L. Zhao, S. Wang, Y. Liu, R. Bai, S. Baik, Y. Zhao, C. Chen and T. Hyeon, ACS Nano, 2015, 9, 12425–12435 CrossRef CAS PubMed.
- B. Schiller, P. Bhat and A. Sharma, Clin. Ther., 2014, 36, 70–83 CrossRef CAS PubMed.
- P. A. Rink and R. N. Muller, Eur. J. Radiol., 1999, 9, 998–1004 CrossRef PubMed.
- E. D. Smolensky, H.-Y. E. Park, Y. Zhou, G. A. Rolla, M. Marjanska, M. Botta and V. C. Pierre, J. Mater. Chem. B, 2013, 1, 2818–2828 RSC.
- R. H. Kodama, J. Magn. Magn. Mater., 2009, 200, 359–372 CrossRef.
- G. Wang, X. Zhang, A. Skallberg, Y. Liu, Z. Hu, X. Mei and K. Uvdal, Nanoscale, 2014, 6, 2953–2963 RSC.
- U. I. Tromsdorf, O. T. Bruns, S. C. Salmen, U. Beisiegel and H. Weller, Nano Lett., 2009, 9, 4434–4440 CrossRef CAS PubMed.
- J. P. Finn, K.-L. Nguyen, F. Hana, Z. Zhou, I. Salusky, I. Ayada and P. Hu, Clin. Radiol., 2016, 71, 796–806 CrossRef CAS PubMed.
- J. Huang, L. Wang, X. Zhong, Y. Li, L. Yang and H. Mao, J. Mater. Chem. B, 2014, 2, 5344–5351 RSC.
- D. Ling and T. Hyeon, Small, 2013, 9, 1450–1466 CrossRef CAS PubMed.
- B. H. Kim, N. Lee, H. Kim, K. An, Y. I. Park, Y. Choi, K. Shin, Y. Lee, S. G. Kwon, H. B. Na, J.-G. Park, E.-Y. Ahn, Y.-W. Kim, W. K. Moon, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2011, 133, 12624–12631 CrossRef CAS PubMed.
- Y.-P. Rui, B. Liang, F. Hu, J. Xu, Y.-F. Peng, P.-H. Yin, Y. Duan, C. Zhang and H. Gu, RSC Adv., 2016, 6, 22575–22585 RSC.
- G. L. Ellman, K. D. Courtney, V. Andres Jr and R. M. Featherstone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- M. L. Hu, Methods Enzymol., 1994, 233, 380–385 CAS.
- S. Sudipta Chakraborty, K. S. Sharma, A. Rajeswari, K. V. Vimalnath, H. D. Sarma, U. Pandey, Jagannath, R. S. Ningthoujam, R. K. Vatsa and A. Dash, J. Mater. Chem. B, 2015, 3, 5455–5466 RSC.
- D. Cheng, D. Li, C. Zhang, H. Tan, C. Wang, L. Pang and H. Shi, ACS Appl. Mater. Interfaces, 2015, 7, 2847 CAS.
- Y. Yang, L. Zhang, J. Cai, X. Li, D. Cheng, H. Su, J. Zhang, S. Liu, H. Shi and Y. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 1525–1531 Search PubMed.
- C. Zhang, X. Xie, S. Liang, M. Li, Y. Liu and H. Gu, Nanomedicine: Nanotechnology, Biology and Medicine, 2011, 8, 996–1006 CrossRef PubMed.
- M. Irigoyen, M. J. Pajares, J. Agorreta, M. P. Ponz-Sarvisé, E. Salvo and M. D. Lozano, Mol. Cancer, 2010, 9, 130–142 CrossRef PubMed.
- S. Xue, C. Zhang, Y. Yang, L. Zhang, D. Cheng, J. Zhang, H. Shi and Y. Zhang, J. Biomed. Nanotechnol., 2015, 11, 1027 CrossRef CAS PubMed.
- G. H. Simon, J. Bauer, O. Saborovski, Y. Fu, C. Corot, M. F. Wendland and H. E. Daldrup-Link, Eur. J. Radiol., 2006, 16, 738–745 CrossRef PubMed.
- E. Peng, F. Wang and J. M. Xue, J. Mater. Chem. B, 2015, 3, 2241–2276 RSC.
- M. Li, H. Gu and C. Zhang, Nanoscale Res. Lett., 2012, 7, 204 CrossRef PubMed.
- L. Xiao, J. Li, D. F. Brougham, E. K. Fox, N. Feliu, A. Bushmelev, A. Schmidt, N. Mertens, F. Kiessling, M. Valldor, B. Fadeel and S. Mathur, ACS Nano, 2011, 5, 6315–6324 CrossRef CAS PubMed.
- M. Taupitz, J. Schnorr, C. Abramjuk, S. Wagner, H. Pilgrimm, H. Hunigen and B. Hamm, J. Magn. Reson. Imag., 2000, 12, 905–911 CrossRef CAS PubMed.
- L. Wang, J. Du, Y. Zhou and Y. Wang, Nanomedicine: Nanotechnology, Biology and Medicine, 2017, 13, 455–469 CrossRef CAS PubMed.
- A. Roch, Y. Gossuin, R. N. Muller and P. Gillis, J. Magn. Magn. Mater., 2005, 293, 532–539 CrossRef CAS.
- F. Xu, C. Cheng, D.-X. Chen and H. Gu, ChemPhysChem, 2012, 13, 336–341 CrossRef CAS PubMed.
- F. Hu and Y. S. Zhao, Nanoscale, 2012, 4, 6235–6243 RSC.
- L. Calucci, A. Grillone, E. R. Riva, V. Mattoli, G. Ciofani and C. Forte, J. Phys. Chem. C, 2017, 121, 823–829 CAS.
- C. Billotey, C. Wilhelm, M. Devaud, J. C. Bacri, J. Bittoun and F. Gazeau, Magn. Reson. Med., 2003, 49, 646–654 CrossRef CAS PubMed.
- M. A. Abakumov, N. V. Nukolova, M. Sokolsky-Papkov, S. A. Shein, T. O. Sandalova, H. M. Vishwasrao, N. F. Grinenko, I. L. Gubsky, A. M. Abakumov, A. V. Kabanov and V. P. Chekhonin, Nanomedicine: Nanotechnology, Biology and Medicine, 2015, 11, 825–833 CrossRef CAS PubMed.
- Z. Li, P. W. Yi, Q. Sun, H. Lei, H. L. Zhao, Z. H. Hua Zhu, S. C. Smith, M. B. Lan and G. Q. Lu, Adv. Funct. Mater., 2012, 12, 2387–2393 CrossRef.
- S. Y. Lee, S. I. Jeon, S. Jung, I. J. Chung and C.-H. Ahn, Adv. Drug Delivery Rev., 2014, 76, 60–78 CrossRef CAS PubMed.
- K. Zarschler, L. Rocks, N. Licciardello, L. Boselli, E. Polo, K. P. Garcia, L. D. Colac, H. Stephan and K. A. Dawson, Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12, 1663–1701 CrossRef CAS PubMed.
- E. Ruoslahti, Annu. Rev. Cell Dev. Biol., 1996, 12, 697–715 CrossRef CAS PubMed.
- X. Tong, Z. Wang, X. Sun, J. Song, O. Jacobson, G. Niu, D. O. Kiesewetter and X. Chen, Theranostics, 2016, 6, 2039–2051 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04903j |
| This journal is © The Royal Society of Chemistry 2017 |