Open Access Article
Open Access ArticleInternally plasticized PVC materials via covalent attachment of aminated tung oil methyl ester
Puyou Jiaa,
Lihong Huab,
Xiaohui Yangab,
Meng Zhang*ab,
Qianqian Shanga and
Yonghong Zhou *a
*a
aInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry (CAF), National Engineering Lab for Biomass Chemical Utilization, Key Lab on Forest Chemical Engineering, State Forestry Administration, Key Lab of Biomass Energy and Materials, 16 Suojin North Road, Nanjing 210042, Jiangsu Province, P. R. China. E-mail: yhzhou777@sina.com; zhangmeng82@163.com
bResearch Institute of Forestry New Technology, Chinese Academy of Forestry, Dongxiaofu-1 Xiangshan Road, Beijing 100091, P. R. China
First published on 9th June 2017
Abstract
We developed an internal plasticizer of aminated tung oil methyl ester for the production of non-migration, phthalate-free flexible and internally plasticized poly(vinyl chloride) (PVC) materials. The chemical structure of the synthesized aminated tung oil methyl ester and the internally plasticized PVC materials were characterized. The obtained internally plasticized PVC materials presented a lower glass transition temperature (Tg) and were more flexible than pure PVC. The thermal stability of internally plasticized PVC materials was less thermally stable compared to those of pure PVC due to the active secondary amine groups of aminated tung oil methyl ester. However, the modified PVC materials presented no migration for self-plasticizing PVC films but 15.7% weight loss for 50 wt% of the PVC/Dioctyl phthalate (DOP) system. It is expected that the PVC materials can be widely used in areas with high migration resistance requirements.
1. Introduction
Polyvinyl chloride (PVC) is one of the most important produced thermoplastic materials, which has been widely used in food packing, toys, wire cables and medical devices.1–3 A third of PVC in the world has been used to produce flexible PVC products. Soft PVC products contain a large amount of plasticizer. Phthalate esters accounted for 70% of the global plasticizer demand in 2014.4 However, the phthalate esters easily migrate from the polymer matrix into the environment during processing and use with increasing time, which decreased the properties of the polymer products, and the potential toxicity of phthalate esters to the human body was reported.5–8The effective strategy to avoid the migration of plasticizers is developing alternative plasticizers such as epoxidized vegetable oil,9–11 polymer plasticizer,12–14 polyol ester15 and phosphate plasticizer.16,17 These alternative plasticizers can suppress the migration from PVC products in a certain degree, but these small molecule plasticizers will migrate from PVC products with increasing time. Polymer plasticizers with low plasticizing effect cannot be used as main plasticizer. Therefore, covalent attachment of plasticizer onto the PVC chains to prevent plasticizer from migrating has been paid more attention. Internal plasticizing effect makes the distance between internally plasticized PVC chains larger than pure PVC, and decreases interaction force, which further promotes the PVC chain mobility. Navarro et al.18 reported the internal plasticized method by displacement of chlorine with phthalate-based thiol additives and obtained good plasticized efficiency. Though the modified PVC materials exhibited zero migration, flexibility of the materials was reduced compared with PVC/phthalate systems. Earla et al.19,20 reported the covalent attachment of phthalate derivatives onto the PVC chains via click chemistry. The obtained internally plasticized PVC materials gave lower Tg than PVC/diethylhexyl phthalate (DEHP) system, which indicated that the plasticizing strategy was successful. The click chemistry opens new gates for internally plasticized PVC materials. Besides, a series of novel internal plasticizers such as propargyl ether cardanol,21 propargyl ether triethyl citrate,22 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) containing castor oil based derivative23 and hyperbranched polyglycerol24 were reported. Yang et al. synthesized a bio-based cardanol-based plasticizer, which was covalently linked to PVC chain using click reaction. The obtained internally plasticized PVC materials presented lower Tg than pure PVC, excellent thermal stability and near-zero migration comparing with PVC/DOP blends. Lee et al. described that hyperbranched polyglycerol was click grafted on the PVC chains in the same way.24 Recently, we developed the propargyl ether triethyl citrate and DOPO containing castor oil based derivative for preparing internally plasticized PVC materials, which were covalently attached on the PVC chain, respectively. Excellent plasticizing efficiency was obtained. However, the click chemistry strategy was usually carried out with copper ion catalyst such as cuprous bromide, cuprous iodide and blue copperas. These copper ion catalysts were hard to remove and limited the PVC materials further application. The catalysts-free method was paid more attention. Navarro et al.25,26 reported the substitution of chlorine with trichlorotriazine-based sodium thiolates with different aliphatic chains via one-pot procedure. Inexpensive trichlorotriazine and copper ion catalyst-free was used, which extended the application of the method in internally plasticizing PVC materials. However, these PVCs were obtained via one-pot procedure and experienced complicated reaction process, which caused the productivity of each step hard to calculate, or more impurities existed in these PVCs than single reaction.
In order to obtain soft and pure PVC materials without migration, the displacement of chlorine with aminated derivatives via single reaction should be give more attention, because the strategy is similar to click reaction and one-pot procedure but without catalyst and impurities. In recent years, the studies of displacement of chlorine with aminated derivatives have been investigated and used in removing heavy metal ions of industrial wastewater, Knoevenagel condensation as catalyzer and preparing ultrafiltration membranes.27–29 The modified PVC polymers with aminated castor oil was used to remove heavy metal ions of industrial wastewater, reported by Ahamed et al.27 The displacement of chlorine with tetraethylenepentamine and 2-aminoethanol was used to prepare catalysts for the Knoevenagel condensation and Heck reactions, which were reported by Dong et al. and Huang et al.,28,29 respectively. The aminated-PVC (PVC–NH2) coated quartz crystal microbalance (QCM) immunosensor was also used to detect bacillus anthracis spores.30 In addition, the displacement of chlorine with amidation derivatives was usually to modify PVC surfaces for preparing functional ultrafiltration membranes with improved antifouling properties.31–33 Covalent immobilization of trypsin and chymotrypsin onto aminated-PVC microspheres was also descried by Li et al.34 However, the displacement of chlorine with aminated derivatives via single reaction for plasticizing PVC materials has never reported.
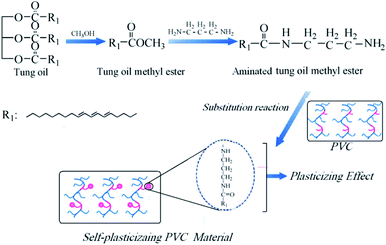
Herein, we present a kind of aminated tung oil methyl ester as a novel renewable and non-migration plasticizer for self-plasticizing PVC materials. Aminated tung oil methyl ester was synthesized via alcoholysis with methanol and ammoxidation with propylenediamine. The self-plasticizing PVC materials were obtained from substitution reaction of PVC with aminated tung oil methyl ester, which was used as an internal plasticizer. The self-plasticizing PVC materials exhibited better flexibility than pure PVC, and presented zero migration comparing with that of PVC/Dioctyl phthalate (DOP) system. The internal mechanism was also discussed. Thus, we anticipate that aminated tung oil methyl ester can be used as a bio-based alternative plasticizer for producing food packaging, toys and medical derives.
2. Experimental section
2.1. Materials
Methanol, potassium hydroxide, sulfuric acid, propylenediamine, N,N-dimethylformamide (DMF), DOP were kindly provided by Nanjing Chemical Reagent Co., Ltd. All of these raw materials and reagents are analytical grade and used without further purification. Polyvinyl chloride (PVC) was supplied by Hanwha (KM-31, South Korea). Tung oil was obtained from the Nanjing Daziran Fine chemicals Co. Ltd (≥95% iodine value is 167).2.2. Synthesis of tung oil methyl ester
Tung oil (100 g, 0.1 mol), anhydrous methanol (20 g, 0.625 mol) and KOH (0.9 g, 0.016 mol) was mixed into a 500 mL flask with mechanical agitator and condenser tube. The mixture was stirring at 45 °C for 6 h. Then the mixture was settled in the liquid separating funnel after neutralizing with sulfuric acid solution. The upper compounds were washed with acid solution and distilled water. Tung oil methyl ester was gotten after removing water using a rotary evaporator.2.3. Synthesis of aminated tung oil methyl ester
Tung oil methyl ester and propylenediamine with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 were dissolved methanol. The mixture was charged into a 250 mL flask and stirring at 60 °C for 12 h to finish the reaction. The aminated tung oil methyl ester was obtained after removing methanol and unreacted propylenediamine with reduced pressure distillation. The synthetic route of aminated tung oil methyl ester was presented in Fig. 1.
1.5 were dissolved methanol. The mixture was charged into a 250 mL flask and stirring at 60 °C for 12 h to finish the reaction. The aminated tung oil methyl ester was obtained after removing methanol and unreacted propylenediamine with reduced pressure distillation. The synthetic route of aminated tung oil methyl ester was presented in Fig. 1.
2.4. Synthesis of self-plasticizing PVC materials
Five grams of PVC (5 g) and aminated tung oil methyl ester was dissolved in 80 mL of DMF. The mixture was stirred at 80 °C for 2 h. After that, the self-plasticizing PVC material was obtained after washing with 10 wt% aqueous methanol solution and drying in a DHG-91401A electrothermal blowing dry box (Shanghai Jinghong Experimental Equipment Co., Ltd.) at 60 °C for 24 h. The composition of reactants was showed in Table 1.| Self-plasticizing PVC materials | PVC (g) | Aminated tung oil methyl ester (g) | Solvent | Temperature (°C) | Reaction time (h) |
|---|---|---|---|---|---|
| T1 | 5 | 1 | DMF | 80 | 2 |
| T2 | 5 | 2 | DMF | 80 | 2 |
| T3 | 5 | 3 | DMF | 80 | 2 |
| T4 | 5 | 4 | DMF | 80 | 2 |
2.5. Preparation of self-plasticizing PVC films
Self-plasticizing PVC material (5 g) was dissolved in 60 mL of THF and stirred at 40 °C for 20 min until the solution presented transparent. After that, the solution was poured into a glass Petri dish (12 cm diameter) and the dried in a drying box at 60 °C for 24 h to completely remove residual THF. Then the PVC film with a thickness of approximately 0.2 mm was obtained. Pure PVC film and 50 wt% DOP/PVC system were obtained using the same method.2.6. Characterization
1H Nuclear magnetic resonance (NMR) and 13C NMR spectra of the products synthesized were performed on an AV-300 NMR spectrometer (Bruker Instrument Crop., Germany) at a frequency of 400 MHz. The process was carried out using CDCl3 as solvent and tetramethylsilane (TMS) as an internal standard. The NMR data were processed using MestReNova software (Santiago de Compostela, Spain).
Weight-average molecular weight and dispersity of PVC and self-plasticizing PVC materials were investigated on an gel permeation chromatograph (GPC) measurement made by Waters, USA at 30 °C (flow rate: 1 mL min−1, column: mixed PL gel 300 × 718 mm, 25 μm) using HPLC-grade THF as solvent. PVC and self-plasticizing PVC material was brought into THF solutions with a known concentration of 1–5mg mL−1.
The thermogravimetric analysis (TGA) tests were carried out using a TG209F1 TGA thermal analysis instruments (Netzsch Instrument Corp., Germany) in N2 atmosphere (50 mL min−1) at a heating rate of 10 °C min−1. The data were collected while the oven temperature was ranging from 40 °C to 600 °C.
Differential scanning calorimeter (DSC) measurement were carried out under N2 atmosphere using a NETZSCH DSC 200 PC analyzer. The temperature was over a range of −40–120 °C at a heating of 20 °C min−1. Approximately 5–10 mg of PVC and self-plasticizing PVC materials was weight and sealed in a 40 μL aluminum crucible, and immediately detected using DSC measurement. The DSC data were collected from first cycle of heating.
| Degree of migration = [(W1 − W2)/W1)] × 100, | (1) |
3. Results and discussion
3.1. Structure of self-plasticizing PVC materials
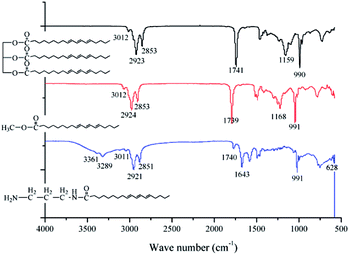
In order to ensure a high purity of the aminated tung oil methyl ester at mild reaction condition, the synthesis of target product was experienced two stages of alcoholysis and aminolysis reaction. The IR spectra of the tung oil, tung oil methyl ester and aminated tung oil methyl ester synthesized for self-plasticizing PVC materials were monitored and shown in Fig. 2. The strong characteristic absorption peaks were labeled in Fig. 2. As seen from the IR spectrum of tung oil, the strong absorption peaks appeared at 3012, 2923, 2853, 1741, 1159 and 991 cm−1, which are attributed to CH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ,
, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, –C–H, C
C–H, –C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–C bonds, respectively35–38. Absorption peaks appeared in the IR spectrum of tung oil methyl ester were presented similar absorption patterns comparing with that of tung oil. This is because the same type of chemical bond such as C
O and C–C bonds, respectively35–38. Absorption peaks appeared in the IR spectrum of tung oil methyl ester were presented similar absorption patterns comparing with that of tung oil. This is because the same type of chemical bond such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,
C, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, –C–H, C
C–H, –C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–C bonds can be found in the chemical structure of tung oil methyl ester.35–38 In the FT-IR spectrum of aminated tung oil methyl ester, the shrinking of the C
O and C–C bonds can be found in the chemical structure of tung oil methyl ester.35–38 In the FT-IR spectrum of aminated tung oil methyl ester, the shrinking of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristic absorption peak at 1740 cm−1 with concomitant appearance of the –NH at 3361 cm−1 and 3289 cm−1, which is assigned to amine N–H stretching vibration, the absorption peak at 1643 cm−1 which represents the rocking vibration of N–H, the absorption peak at 1030 cm−1 which is corresponded to the vibration of C–N, and the weak and broad peaks at 628 cm−1 are attributed to N–H twisting vibrations,39 is indicative of the formation of the aminated tung oil methyl ester.
O characteristic absorption peak at 1740 cm−1 with concomitant appearance of the –NH at 3361 cm−1 and 3289 cm−1, which is assigned to amine N–H stretching vibration, the absorption peak at 1643 cm−1 which represents the rocking vibration of N–H, the absorption peak at 1030 cm−1 which is corresponded to the vibration of C–N, and the weak and broad peaks at 628 cm−1 are attributed to N–H twisting vibrations,39 is indicative of the formation of the aminated tung oil methyl ester.
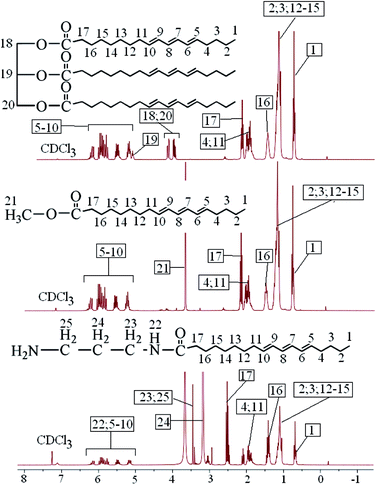
The chemical structures of tung oil, tung oil methyl ester and aminated tung oil methyl ester were also analyzed by 1H NMR, all the peaks in the 1H NMR spectrum were easily assigned, and no side products were observed, as shown in the 1H NMR spectrum of tung oil (Fig. 3), the peak at 0.88 ppm was attributed the protons of methyl groups on the unsaturated long fatty acid chains. The strong peak at 1.25 ppm was assigned to the protons of methylene groups. The protons of methylene groups connected the carbonyl groups appeared at 2.25 ppm, and the signals appeared at 4.13, 4.26 and 5.24 ppm were attributed to the methylene groups and methylene groups derived from glyceride groups. The protons appeared at 1.59, 2.02, and 2.14 ppm were corresponded to the other methylene groups. The protons of olefin groups appeared at the range of 5.33–6.17 ppm.35–38 Comparing with the 1H NMR spectrum of tung oil, a new and strong signal appeared at 3.65 ppm in the 1H NMR spectrum of tung oil methyl ester, which was assigned to the protons of methyl groups connected to ester bonds, and the signals attributed to the methylene groups and methylene groups derived from glyceride groups cannot be observed at 4.13, 4.26 and 5.24 ppm, indicating that the alcoholysis reaction was completed.35–38 As seen from the 1H NMR spectrum of aminated tung oil methyl ester, the appearance of new peaks at 3.18, 3.45 and 3.67 ppm were assigned to the methylene groups derived from propane diamine, and the new peaks appeared at around 6.16 ppm was attributed to the protons of amine groups,39 which indicated that the ammonolysis reaction was completed.
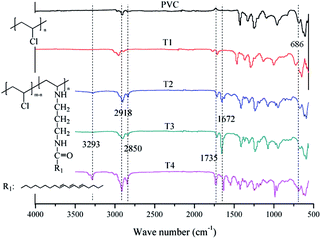
In order to analyze the chemical structure of the self-plasticizing PVC materials, IR and 1H NMR measurements were carried out, and the IR and 1H NMR spectra of the self-plasticizing PVC materials were shown in Fig. 4 and 5, respectively. As seen from the Fig. 4, it is clearly that these peaks appeared at 3293, 2918, 2850, 1735, and 1672 cm−1, which are assigned to the –NH stretching vibrations, C–H (sp3), C–H (sp2), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H rocking vibration, respectively,35–40 were increased gradually with more displacement of chlorines. In addition, the peaks at around 686 cm attributed to the C–Cl,19–23 which appeared weak gradually with more aminated tung oil methyl ester connected to the chemical structure of self-plasticizing PVC mistrals, indicating the self-plasticizing PVC materials were gotten.
O and N–H rocking vibration, respectively,35–40 were increased gradually with more displacement of chlorines. In addition, the peaks at around 686 cm attributed to the C–Cl,19–23 which appeared weak gradually with more aminated tung oil methyl ester connected to the chemical structure of self-plasticizing PVC mistrals, indicating the self-plasticizing PVC materials were gotten.
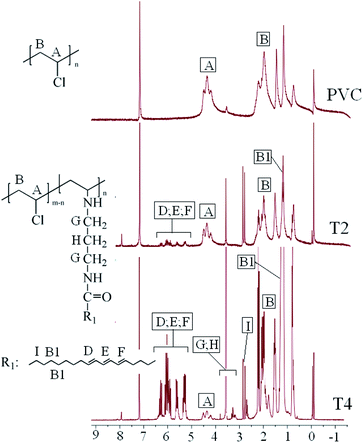
In order to further study the chemical structure of self-plasticizing PVC materials, 1H NMR measurements were carried out and the 1H NMR spectra of PVC and self-plasticizing PVC materials were showed in Fig. 5. As seen from Fig. 5, the peak at 4.45 ppm was attributed to protons of C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –Cl (peak A of 1H NMR spectra of PVC), and the peak at 2.06 ppm was assigned to protons of C
–Cl (peak A of 1H NMR spectra of PVC), and the peak at 2.06 ppm was assigned to protons of C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2 (peak B of 1H NMR spectra of PVC). New peak appeared in the 1H NMR spectra of self-plasticizing PVC materials with more displacement of chlorines comparing with 1H NMR spectra of PVC. As seen from the 1H NMR spectra of self-plasticizing PVC materials (T2 and T4), it is clearly to observe that the signals at 1.23, 2.80, 3.58 and 5.29–6.29 ppm, which were attributed to the protons of –C
2 (peak B of 1H NMR spectra of PVC). New peak appeared in the 1H NMR spectra of self-plasticizing PVC materials with more displacement of chlorines comparing with 1H NMR spectra of PVC. As seen from the 1H NMR spectra of self-plasticizing PVC materials (T2 and T4), it is clearly to observe that the signals at 1.23, 2.80, 3.58 and 5.29–6.29 ppm, which were attributed to the protons of –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–, O
2–, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–, –NH–C
2–, –NH–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C
2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C
2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–NH– and –C
2–NH– and –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) – respectively,35–40 appearing stronger with more displacement of chlorines, but the signal at 4.5 ppm corresponding to the protons of C
– respectively,35–40 appearing stronger with more displacement of chlorines, but the signal at 4.5 ppm corresponding to the protons of C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –Cl presented weaker gradually,19–23,40 indicating the reaction between PVC and aminated tung oil methyl ester was completed.
–Cl presented weaker gradually,19–23,40 indicating the reaction between PVC and aminated tung oil methyl ester was completed.
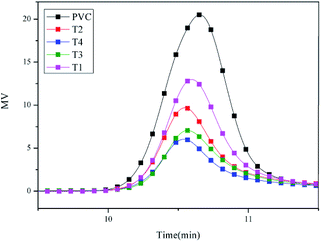
The GPC analysis was used to detect the molecular weight and dispersity of PVC and self-plasticizing PVC materials. It can be used as a method to evaluate the reaction level by examining the molecular the molecular weight change.41,42 GPC spectra of PVC and self-plasticizing PVC material were shown in Fig. 6. The data of number average molecular weight (Mn), weight-average molecular weight (MW), Z-average molecular weight (MZ) and dispersity were presented in Table 2. As seen from Table 2, Mn, MW and MZ of PVC materials increased gradually from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 18
100, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 23
900 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 to 23
000 g mol−1 to 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, 30
800, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 39
200 and 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 with increasing displacement of chlorines. Besides, GPC peak of self-plasticizing PVC materials demonstrated a clear shift to higher molecular weight region comparing with that of PVC, indicating that the chlorine atoms in PVC were substituted with aminated tung oil methyl ester. All of self-plasticizing PVC materials showed a single GPC peak with a clear shift to a higher molecular weight region illustrated that no homopolymer contamination of coupling reactions. Fig. 6 presented an interesting trend that peak area of self-plasticizing PVC materials decreased gradually with increasing substitution of chlorine atoms in PVC with aminated tung oil methyl ester. The decrease in peak area was attributed to filtration of highly branched self-plasticizing PVC materials by organic membrane during dissolution process with chromatographic purity as solvent.
200 g mol−1 with increasing displacement of chlorines. Besides, GPC peak of self-plasticizing PVC materials demonstrated a clear shift to higher molecular weight region comparing with that of PVC, indicating that the chlorine atoms in PVC were substituted with aminated tung oil methyl ester. All of self-plasticizing PVC materials showed a single GPC peak with a clear shift to a higher molecular weight region illustrated that no homopolymer contamination of coupling reactions. Fig. 6 presented an interesting trend that peak area of self-plasticizing PVC materials decreased gradually with increasing substitution of chlorine atoms in PVC with aminated tung oil methyl ester. The decrease in peak area was attributed to filtration of highly branched self-plasticizing PVC materials by organic membrane during dissolution process with chromatographic purity as solvent.
| PVC materials | Number average molecular weight (g mol−1) | Weight-average molecular weight (g mol−1) | Z-average molecular weight (g mol−1) | Dispersity (g mol−1) |
|---|---|---|---|---|
| PVC | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| Y1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.2 |
| Y2 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.2 |
| Y3 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.3 |
| Y4 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.3 |
3.2. Property of self-plasticizing PVC materials
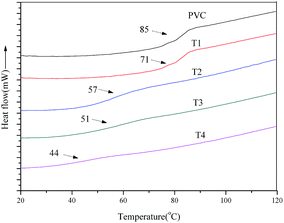
Glass transition temperature (Tg) was used to evaluate internal plasticized effect of aminated tung oil methyl ester on PVC, which was detected using DSC measurements, and the glass transition temperature (Tg) was summarized in Table 3. Pure PVC is with stiff backbone and high glass transition temperature (Tg) at around 90 °C. The addition of plasticizer into the PVC polymer will increase distance of PVC chains and make macromolecule structure polymer easy to move, increasing free volume of polymer and reducing Tg.43–45 The substituting of chlorine atoms with aminated tung oil methyl ester will increase distance between PVC chains and reduce intermolecular force. An internal plasticization will be expected to reduce Tg of PVC materials. Fig. 7 presented the DSC curves of PVC materials. As seen from Table 3 and Fig. 7, it can be found that a single change in heat capacity of Tg could be observed in the DSC curves of all self-plasticizing PVC materials. Tg of self-plasticizing PVC materials decreased from 85 °C to 44 °C with increasing substitution of chlorine atoms, which indicated that aminated tung oil methyl ester can be used as a internal plasticized plasticizer for PVC.| PVC materials | Td (°C) | Tp1 (°C) | Tp2 (°C) | Tg (°C) |
|---|---|---|---|---|
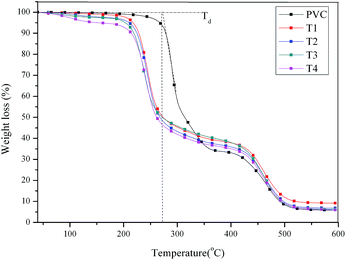
| PVC | 278.4 | 291.5 | 467.4 | 85 |
| T1 | 224.7 | 244.8 | 460.1 | 71 |
| T2 | 223.7 | 244.2 | 458.4 | 57 |
| T3 | 219.6 | 241.8 | 458.2 | 51 |
| T4 | 217.1 | 237.5 | 468.1 | 44 |
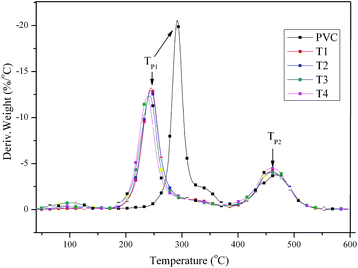
Plasticized PVC materials generally require higher thermal stability than that of pure PVC, because ester bonds or benzene groups with high thermal stability in the chemical structure of plasticizers such as dioctyl tetrahydrophthalate (DOTP) and epoxidized soybean oil, limiting the thermal degradation of those segments with lower thermal stability chain segments, then improving the thermal degradation stability of plasticized PVC materials.46 In the study, thermal stability of PVC and self-plasticizing PVC materials were investigated, the TGA and DTG curves obtained for PVC and self-plasticizing PVC materials were presented in Fig. 8 and 9, respectively, and the thermal data including thermal degradation temperature (Td) and peak temperature of thermal degradation (Tp1 and Tp2) were summarized in Table 3. As observed from Fig. 8 and 9, all PVC materials showed two thermal degradation stages, dehydrochlorination of PVC occurred at the first stage. The second stage was attributed to cyclization of conjugated polyene sequences.47,48 An interesting phenomenon can be found from Table 3, the Td, Tp1 and Tp2 for pure PVC was 278.4 °C, 291.5 °C and 467.4, but these characteristic temperatures decreased gradually with increasing substitution of chlorine atoms with aminated tung oil methyl ester, indicating the self-plasticizing PVC materials were less thermal stable than pure PVC due to the active secondary amine groups.
The substitution of chlorine atom with aminated tung oil methyl ester was able to change distance of PVC chains to separation of PVC chain segments, and then increase the lubricating property of PVC chains. It made the PVC materials more flexible. The tensile data were presented in Table 4. Tensile strength decreased from 30.63 MPa to 18.52 MPa with increasing substitution of chlorine atom, and the elongation at break increased from 163.03% to 360.30%, indicating the PVC was plasticized effectively.
| PVC materials | Tensile strength (MPa) | Elongation at break (%) | Modulus of elasticity (MPa) |
|---|---|---|---|
| PVC | 30.63 ± 0.87 | 163.03 ± 5.41 | 203.79 ± 3.25 |
| T1 | 25.74 ± 0.78 | 210.63 ± 1.28 | 161.01 ± 4.00 |
| T2 | 25.41 ± 0.17 | 240.52 ± 7.12 | 157.10 ± 3.28 |
| T3 | 21.22 ± 0.86 | 290.38 ± 6.52 | 130.01 ± 7.45 |
| T4 | 18.52 ± 0.48 | 360.32 ± 7.10 | 90.14 ± 4.10 |
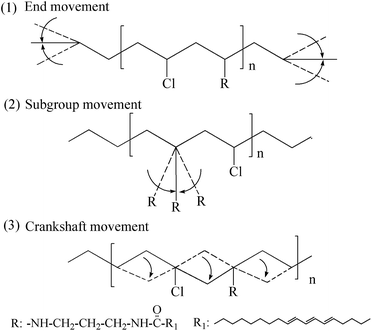
Fig. 10 showed internal plasticizing mechanism of self-plasticizing PVC materials. The internal plasticizing mechanism can be illustrated with free volume theory.
The free volume of internally plasticized PVC was controlled by the three factors: end movement, subgroup movement and crankshaft movement. The displacement of chlorine with aminated tung oil methyl ester increases distance of PVC chains and makes them easier to move, further increases free volume of PVC chains. The increase of free volume promote the end movement, subgroup movement and crankshaft movement, which makes PVC present flexible and easy to be processed. However, the substituting of chlorine atoms with aminated tung oil methyl ester must be controlled in a certain degree, or the cross-linking of polymer with many aminated tung oil methyl ester chains will cause anti plasticizing effect.
PVC products without migration not only keep the properties long time stable, but also decrease threat to human body from the phthalate plasticizers. In this study, leaching tests were used to evaluate durability in n-hexane at 50 °C for 2 h. The property of n-hexane is similar to the many oils such as gasoline, diesel oil and so on. It is usually used to estimate leaching stability of plasticizers in organic solvents. The degree of migration for self-plasticizing was compared with 50 wt% PVC/DOP system, the results illustrated no migration for self-plasticizing PVC films but 15.7% of weight loss for 50 wt% PVC/DOP system.
4. Conclusions
In this study, an internal plasticizer of aminated tung oil methyl ester was synthesized via alcoholysis and aminolysis reaction, which was covalently linked to PVC for preparing self-plasticizing PVC materials. The GPC results showed that Mn, MW and MZ of self-plasticizing PVC materials increased gradually from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 18
100, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 23
900 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 to 23
000 g mol−1 to 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, 30
800, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 39
200 and 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 with more substituting of chlorine atoms, and Tg decreased from 85 °C to 44 °C. The active secondary amine groups of aminated tung oil methyl ester decreased thermal stability of self-plasticizing PVC materials. However, the modified PVC materials presented no migration for self-plasticizing-PVC films but 15.7% of weight loss for 50 wt% of PVC/DOP system. Therefore, it was expected that aminated tung oil methyl ester can be used as a bio-based alternative plasticizer for producing these PVC products with high migration resistance retirements such as food packing, toys and medical derives.
200 g mol−1 with more substituting of chlorine atoms, and Tg decreased from 85 °C to 44 °C. The active secondary amine groups of aminated tung oil methyl ester decreased thermal stability of self-plasticizing PVC materials. However, the modified PVC materials presented no migration for self-plasticizing-PVC films but 15.7% of weight loss for 50 wt% of PVC/DOP system. Therefore, it was expected that aminated tung oil methyl ester can be used as a bio-based alternative plasticizer for producing these PVC products with high migration resistance retirements such as food packing, toys and medical derives.
Acknowledgements
This work was supported by the Fundamental Research Funds of Research Institute of Forestry New Technology (CAFYBB2016SY027), Fundamental Research Funds from Jiangsu Province Biomass and Materials Laboratory (JSBEM-S-2017010); Natural Science Foundation of Jiangsu Province of China (Grants BK20150072); and the President of the Chinese Academy of Forestry Foundation (Grant No. CAFYBB2014QB021).References
- L. M. D. Espinosa, A. Gevers, B. Woldt, M. Graß and M. A. R. Meier, Green Chem., 2014, 16, 1883–1896 RSC.
- M. D. Silva, M. G. A. Vieira and A. C. G. Maçumoto, Polym. Test., 2011, 30, 478–784 CrossRef.
- Y. Saeki and T. Emura, Prog. Polym. Sci., 2002, 27, 2055–2131 CrossRef CAS.
- A. H. Tullo, Chem. Eng. News, 2015, 96, 16–18 Search PubMed.
- U. Heudorf, V. Mersch-Sundermann and J. Angerer, Int. J. Hyg. Environ. Health, 2007, 210, 623–634 CrossRef CAS PubMed.
- N. R. Janjua, G. K. Mortensen, A. M. Andersson, B. Kongshoj, N. E. Skakkebak and H. C. Wulf, Environ. Sci. Technol., 2007, 41, 5564–5570 CrossRef CAS PubMed.
- J. A. Tickner, T. Schettler, T. Guidotti, M. McCally and M. Rossi, Am. J. Ind. Med., 2001, 39, 100–111 CrossRef CAS PubMed.
- J. Zhou, B. Chen and Z. Cai, Environ. Sci. Pollut. Res., 2015, 22, 5092–5099 CrossRef CAS PubMed.
- J. Chen, X. Li, Y. Wang, J. Huang, K. Li, X. Nie and J. Jiang, Eur. J. Lipid Sci. Technol., 2016 DOI:10.1002/ejlt.201600216.
- J. M. Ferri, M. D. Samper, D. García-Sanoguera, M. J. Reig, O. Fenollar and R. Balart, J. Mater. Sci., 2016, 51, 5356–5366 CrossRef CAS.
- B. W. Chieng, N. A. Ibrahim, Y. Y. Then and Y. Y. Loo, Molecules, 2014, 19, 16024–16038 CrossRef PubMed.
- A. Jarray, V. Gerbaud and M. Hemati, Prog. Org. Coat., 2016, 101, 195–206 CrossRef CAS.
- E. M. Zahran, A. New, V. Gavalas and L. G. Bachas, Analyst, 2014, 139, 757–763 RSC.
- M. Guzmán and E. A. Murillo, Polym. Eng. Sci., 2015, 55, 2526–2533 Search PubMed.
- P. Y. Jia, M. Zhang, L. Hu, G. Feng, C. Bo and Y. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 2187–2193 CrossRef CAS.
- J. Chen, Z. Liu, X. Li, P. Liu, J. Jiang and X. Nie, Polym. Degrad. Stab., 2016, 126, 58–64 CrossRef CAS.
- P. Jia, L. Hu, G. Feng, C. Bo, J. Zhou, M. Zhang and Y. Zhou, RSC Adv., 2017, 7, 897–903 RSC.
- R. Navarro, M. P. Perrino, M. G. Tardajos and H. Reinecke, Macromolecules, 2010, 43, 2377–2381 CrossRef CAS.
- A. Earla, L. Li, P. Costanzo and R. Braslau, Polymer, 2016, 109, 1–12 CrossRef.
- A. Earla and R. Braslau, Macromol. Rapid Commun., 2014, 35, 666–671 CrossRef CAS PubMed.
- P. Yang, J. Yan, H. Sun, H. Fan, Y. Chen, F. Wang and B. Shi, RSC Adv., 2015, 5, 16980–16985 RSC.
- P. Jia, L. Hu, G. Feng, C. Bo, M. Zhang and Y. Zhou, Mater. Chem. Phys., 2017, 190, 25–30 CrossRef CAS.
- P. Jia, L. Hu, M. Zhang, G. Feng and Y. Zhou, Eur. Polym. J., 2017, 87, 209–220 CrossRef CAS.
- K. W. Lee, J. W. Chung and S. Kwak, Macromol. Rapid Commun., 2016, 37 DOI:10.1002/marc.201600533.
- R. Navarro, M. P. Perrino, C. García, C. Elvira, A. Gallardo and H. Reinecke, Macromolecules, 2016, 49, 2224–2227 CrossRef CAS.
- R. Navarro, M. P. Perrino, C. García, C. Elvira, A. Gallardo and H. Reinecke, Polymers, 2016 DOI:10.3390/polym8040152.
- I. S. Ahamed, A. K. Ghonaim, A. A. AbdelHakim, M. M. Moustafa and A. H. K. El-Din, J. Appl. Sci. Res., 2008, 4, 1946–1958 CAS.
- F. Dong, Y. Q. Li and R. F. Dai, Chin. Chem. Lett., 2007, 18, 266–268 CrossRef CAS.
- X. J. Huang, F. Dong, L. Chen and Y. Q. Li, Monatsh. Chem., 2008, 139, 1447–1451 CrossRef CAS.
- A. Oztuna, H. Nazir and M. Baysallar, J. Coat., 2014, 1–8 CrossRef.
- J. Zhu, Y. Su, X. Zhao, Y. Li, J. Zhao, X. Fan and Z. Jiang, Ind. Eng. Chem. Res., 2014, 53, 14046–14055 CrossRef CAS.
- S. Bigot, G. Louarn, N. Kébir and F. Burel, Appl. Surf. Sci., 2013, 283, 411–416 CrossRef CAS.
- C. Wu, S. Liu, Z. Wang, J. Zhang, X. Wang, X. Lu, Y. Jia and W. Hung, J. Membr. Sci., 2016, 517, 64–72 CrossRef CAS.
- D. F. Li, H. C. Ding and T. Zhou, J. Agric. Food Chem., 2013, 61, 10447–10453 CrossRef CAS PubMed.
- T. Lacerda, A. F. Carvalho and A. Gandini, RSC Adv., 2014, 4, 26829 RSC.
- K. Huang, Z. Liu, J. Zhang, S. Li, M. Li, J. Xia and Y. Zhou, Biomacromolecules, 2014, 15, 837–843 CrossRef CAS PubMed.
- X. Yang, S. Li, J. Xia, J. Song, K. Huang and M. Li, Ind. Crops Prod., 2015, 63, 17–25 CrossRef CAS.
- C. Meiorin, M. I. Aranguren and M. A. Mosiewicki, Eur. Polym. J., 2015, 67, 551–560 CrossRef CAS.
- K. Huang, Y. Zhang, M. Li, J. Lian, X. Yang and J. Xia, Prog. Org. Coat., 2012, 74, 240–247 CrossRef CAS.
- Z. Huang, C. Feng, H. Guo and X. Huang, Polym. Chem., 2016, 7, 3034–3045 RSC.
- B. F. Bowers, B. Huang, X. Shu and B. C. Miller, Constr. Build. Mater., 2014, 50, 517–523 CrossRef.
- J. Geng, H. Li and Y. Sheng, Int. J. Pavement Res.Technol., 2014, 7, 77–82 Search PubMed.
- P. H. Daniels, J. Vinyl Addit. Technol., 2009, 15, 219–223 CrossRef CAS.
- G. Wypych, Handbook of plasticizers, Elsevier, Toronto, Canada, 2012, ch. 10 Search PubMed.
- W. Shi, Z. Shi and P. Jiang, Plasticizers and their applications, Beijing, China, 2002 Search PubMed.
- P. Jia, M. Zhang, L. Hu, J. Zhou, G. Feng and Y. Zhou, Polym. Degrad. Stab., 2015, 121, 292–302 CrossRef CAS.
- Y. T. Pan and D. Wang, RSC Adv., 2015, 5, 27837–27843 RSC.
- L. L. Pan, G. Y. Li, Y. C. Su and J. S. Lian, Polym. Degrad. Stab., 2012, 97, 1801–1806 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |