Open Access Article
Open Access ArticleEmission characteristics and relationships among PCDD/Fs, chlorobenzenes, chlorophenols and PAHs in the stack gas from two municipal solid waste incinerators in China†
Tianjiao Wang ,
Tong Chen*,
Binbin Lin,
Xiaoqing Lin,
Mingxiu Zhan and
Xiaodong Li
,
Tong Chen*,
Binbin Lin,
Xiaoqing Lin,
Mingxiu Zhan and
Xiaodong Li
State Key Laboratory of Clean Energy Utilization, Institute for Thermal Power Engineering, Zhejiang University, Hangzhou 310027, People's Republic of China. E-mail: chentong@zju.edu.cn
First published on 18th September 2017
Abstract
An extensive investigation was conducted to understand polychlorinated dibenzo-p-dioxin and furan (PCDD/F) formation mechanisms and their relationships with chlorobenzenes (CBzs), chlorophenols (CPhs) and polycyclic aromatic hydrocarbons (PAHs) in the stack gas from two fluidized bed municipal solid waste incinerators in China. The toxic equivalent quantity (TEQ) value and the concentration of target compounds changed with the incinerator operating conditions. CPhs and PAHs were much more sensitive to operation conditions and were affected more easily by change. Only 2-monochlorophenol revealed a negative linear correlation (R2 ≥ 0.7). More than half of the PAHs revealed an adequate correlation model with PCDD/F concentration (R2 > 0.6), while CBzs showed almost perfect correlations with PCDD/Fs (R2 ≥ 0.8, significance level α ≤ 0.05). 123-Trichlorobenzene, 1234-tetrachlorobenzene and pentachlorobenzene revealed the best positive linear correlation (R2 > 0.9). PCDFs were revealed to be the best target compounds for indication due to the similar formation variation trend to that of other precursors. Unary and multiple linear regression equations with high coefficients of determination between several CBz, PAHs and PCDD/Fs, TEQ and PCDFs were established. The detailed relationships among PCDD/F homologues, isomers and other compounds and their formation mechanism were also discussed.
1. Introduction
Large amounts of organic pollutants are produced and emitted from municipal solid waste incineration. Most of them are toxic, hazardous, and harmful to human beings. Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans are considered the most toxic and most worrying. Besides PCDD/Fs, other common organic pollutants such as chlorobenzenes (CBzs), chlorophenols (CPhs) and polycyclic aromatic hydrocarbons (PAHs) are also studied, typical precursors in the formation of PCDD/Fs.Numerous research studies have been conducted to understand how PCDD/Fs are formed and have suggested many kinds of mechanisms. The widely known and accepted conclusions are the formation from precursors and de novo synthesis from carbon.1–3 Investigations into formation mechanisms have found the relationship between PCDD/Fs and other compounds, such as CBzs, CPhs and PAHs. They maybe co-formed by similar mechanisms or may serve as reactants to form PCDD/Fs.
Generally there are two ways to form PCDD/Fs from precursors in different temperature ranges. One is the rearrangement reactions of chlorinated precursors, CPhs and CBzs in the gas named high-temperature homogeneous reactions, in the temperature range of 500–800 °C. Other is the low-temperature heterogeneous reactions, in the temperature range of 200–400 °C.4,5 Among the most abundant aromatic compounds found in incinerator emissions, CPhs have the most similar structure with PCDD/Fs, thought to be the easiest to form PCDD/Fs.6,7 High-temperature, gas-phase reactions of 2,4,6-trichlorophenol were found to form rather high yields of polychlorinated dibenzo-p-dioxins (PCDDs).8 The oxidation of dichlorophenols at 600 °C could also produce large yields of PCDFs. Different structure had different results. 3,4-DCP produced the largest yields of PCDFs with two or more chlorine substituents, while 2,6-DCP did not produce tri- or tetra-chlorinated PCDF congeners.9 Apart from the homogeneous reactions of CPhs, the heterogeneous reactions are very important for PCDD/Fs formation. Many studies found several CPhs could produce PCDD/Fs on the surface of supported metal oxides with significant yields over the temperature range 200–500 °C.10–13 There is also a thought that the PCDDs are mainly formed by chlorophenol condensation, while the PCDFs are formed from a non- or a low-chlorinated precursor followed by further chlorination reactions.14 Several catalytic and condensation reaction models from CP to PCDD/Fs have already been reported to understand the mechanism.3 Apart from CPhs, CBzs are also investigated to generate PCDD/Fs at proper temperature with catalysts. However the formation rate and yield from CBzs are poorer than that from CPhs.15–17
Besides chlorination as a dominant route for PCDF formation, PAHs were also observed to form PCDFs and the incorporation of oxygen from the outside of PAH molecules resulted in PCDFs.18 PCDF can be formed directly from fluorenone, biphenyl and fluorene.19 Anthracene and chlorinated anthracenes can generate PCDD/Fs.20 Under the same conditions, several PAHs generate PCDFs and its derivatives even more than activated carbon.18,21 Furthermore, CPhs, polychlorinated naphthalene, and CBzs were found to come from PAHs.22
These PCDD/F mechanistic studies have also made efforts for the possibility of establishing indicator compounds for faster and less costly predictive monitoring of PCDD/F concentrations and TEQs. As concerned it's difficult to directly monitor PCDD/Fs with such a low level of concentrations, on-line monitoring indicators to obtain PCDD/F emissions through a mechanistic relationship between the indicators and PCDD/Fs has become a prospective technology to detect PCDD/Fs. Vacuum ultra-violet single-photon ionization ion trap time-of-flight mass spectrometer (TOFMS), Resonance-Enhanced Multiphoton Ionization (REMPI) TOFMS and Laser Induced Breakdown Spectroscopy have already been applied to monitoring air toxic emissions from diesel generators, aircraft ground equipment and municipal solid waste incinerators (MSWIs).23–30 Many studies on potential indicator compounds were performed. Chlorinated hydrocarbons (e.g., CPhs, CBzs), polychlorinated biphenyls (PCBs), PAHs, low-volatile organohalogen compounds (LVOH), CO and some specific PCDD/Fs were all investigated as toxic equivalent quantity (TEQ) indicator compounds, and revealed good correlations with PCDD/Fs especially CBzs and PAHs.31–47
Since emissions and formations of PCDD/Fs from waste incineration are really complicated, and varieties of obvious and potential factors (e.g. incinerator types, air pollution control devices, capacities of incinerators) may also affect results, there is no agreement on use of a single, “universal” TEQ indicator and TEQ model. Among existing research a single indicator compound is usually used to establish a good relationship. And the correlation studies are always confined to the total concentration of TEQ of PCDD/Fs. Advanced research on relationship with potential indicator and detailed analysis are still needed, especially in China with current lack of successful and useful correlation model for online monitoring dioxins, not only in the case of standard emissions, but also in diagnostic cases with old MSWI improvement.
This paper investigated multiple potential indicator compounds, such as CBzs, CPhs, PAHs, and PCDD/Fs emission characteristics from two fluidized bed municipal solid waste incinerators at the same time.
With these results, the implications for determining formation mechanisms were addressed. The detailed investigation of existed relationships among PCDD/Fs, different precursors and indicators was performed. Multiple data statistical analysis methods were used to reveal the detailed relationships between PCDD/Fs and other pollutants. Some good correlation models of indicators were also established, including single and multiple linear relationships.
2. Materials and methods
2.1 Sampling and treatment
Thirteen samples were collected from the stack gas of two typical municipal waste solid incinerators (MSWIs), just after the fabric filter. The two incinerators are both the circulating fluidized bed (CFB) furnaces of the same plant in the eastern city of China. They have the same set furnace temperature, 700–900 °C, and the same air pollution control device (APCD). The APCDs both include selective non-catalytic reduction (SNCR) for NOx control, semi-dry scrubber for acid gas control, and fabric filter for particulate matter (PM) control. The obvious difference affecting pollution emissions between MSWI 1 and 2 may be is the addition of coal to waste. The mass ratio of waste and added coal of MSWI 1 is 4 while that of MSWI 2 is 9. Coal added is a common condition for better waste incineration in CFB furnaces and also makes a significant effect on formation and emission of varieties of pollutants.48–50 A detailed comparison of two incinerators is shown in ESI S1.†All samples were taken for 2–3 h for approximately 2.0–4.0 m3 in volume using U.S. Method 23a.51 The filter part was analyzed for particle-phase targets and the XAD-2 resin for gas-phase targets. The collected water in the sampling trains was also analyzed for CBzs, CPhs and PAHs. Collected samples were extracted stepwise with methylene chloride and toluene and the extracts were concentrated separately to avoid losses of semivolatile compounds.52,53 After extraction, aliquots of the samples were taken for the target compounds analysis. The PCDD/Fs cleanup procedure and analysis were performed according to US EPA Method 23a, by HRGC/HRMS on a 6890 Series gas chromatograph (Agilent, USA) and coupled to a JMS-800D mass spectrometer (JEOL, Japan). The mean recoveries of standards for PCDD/Fs range from 55 to 125%, which are all within the acceptable 25 to 150% range. GC-ECD (GC 6890N, Agilent, USA) is used to analyze CBzs. Agilent 6460 triple quadrupole liquid chromatography/mass spectrometer (LC/MS) were used to detected CPhs. Agilent 6890N GC/5975B MSD is used for PAHs analysis. Detailed information on this MSWI was described in a previous study.54,55
There were several boiler shutdowns during the sampling due to feed clogging problems. The operating conditions and continuous emission monitor (CEM) data from the plant during the sampling are shown in the Table 2.
2.2 Statistical analysis
The relationships between CBzs, CPhs, PAHs, PCDD/F isomers and the TEQ values were analyzed. Parsons coefficients and coefficients of determination (R2) were calculated. And effective correlation models were established including unary linear regression and multiple linear regression analysis. In order to evaluate homologue and isomer distribution patterns according to sample types and to determine the relationship among these compounds, principal component analysis (PCA) and cluster analysis were used. PCA and cluster analysis are multivariate statistical analyses that allow evaluation of the absolute and relative importance of variables, as well as graphical representation of the same. All the statistical analysis was performed with SPSS 22.0 software.3. Results and discussions
3.1 PCDD/F, CBz, CPh and PAH emission levels with operating conditions
The operating conditions during the sampling periods and the concentrations of target compounds are presented in Table 1. The concentrations of CBzs, CPhs and PAHs were much higher than those of PCDD/Fs, consistent with previous studies.53,56–59 And all the compounds fluctuated with changes in operating conditions. The average concentration of PCDD/Fs from MSWI 2 was higher than that from MSWI 1, maybe due to the higher waste capacity and less added coal.48–50,58 The organic pollutant emission levels in the former 4 samples of MSWI 1 (sample 1-1, 1-2, 1-3, 1-4) were obviously different from the last 2 samples (sample 1-5, 1-6). Since the last 2 samples were collected after an overhaul of incinerator, incineration conditions affecting chlorinated pollutants perhaps became better. For instance ashes with metals attached to the flue and furnace inner surfaces were removed and the high temperature was more stable, which could destruct the formation of chlorinated organic compounds.1,60–63 However common pollutants such as SO2, NOx, were affected more by APCD. So the two parts revealed different levels especially for PCDD/Fs, while SO2 and NOx changed little. Sample 2-2, which had a shut-down and a start-up process, had the highest PCDD/F TEQ value. Sample 2-6, which was collected after a 30 minute shutdown and then startup, also revealed rather high concentrations of PCDD/Fs and TEQ values, and other compounds such as CBzs, CPhs and PAHs also presented an obvious increase in concentrations compared to the other data. Several previous studies52,64–68 also reported increased concentration of PCDDs/Fs and other related compounds during shut-down and startup processes. The advanced and detailed relationships on these compounds were described below.| Position | Sample number | Operation condition | Concentration | CEM data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCDD, ng N−1 m−3 (11% O2) | PCDF, ng N−1 m−3 (11% O2) | PCDD/Fs, ng N−1 m−3 (11% O2) | TEQ, ng N−1 m−3 (11% O2) | CBzs μg N−1 m−3 | CPhs μg N−1 m−3 | PAHs μg N−1 m−3 | SO2 mg N−1 m−3 | NOx mg N−1 m−3 | O2% | Boiler temperature °C | |||
| MSWI 1 | 1-1 | Normal | 4.79 | 83.4 | 88.2 | 24.6 | 50.4 | 141.0 | 2129.9 | 38.3 | 187.0 | 14.7 | 898.9 |
| 1-2 | Normal | 3.51 | 57.7 | 61.2 | 17.3 | 39.8 | 54.8 | 2648.8 | 15.4 | 144.6 | 14.1 | 932.7 | |
| 1-3 | Normal | 4.62 | 56.0 | 60.6 | 12.2 | 32.1 | 89.3 | 4986.5 | 12.0 | 201.9 | 15.3 | 851.2 | |
| 1-4 | Normal | 7.09 | 92.6 | 99.7 | 17.8 | 67.4 | 23.1 | 1903.0 | 2.8 | 210.2 | 17.5 | 729.2 | |
| 1-5 | After overhaul | 9.39 | 8.34 | 17.7 | 1.21 | 13.8 | 0.604 | 148.6 | 32.2 | 256.3 | 15.8 | 808.9 | |
| 1-6 | Normal | 3.08 | 5.93 | 9.01 | 1.05 | 10.9 | 21.7 | 520.5 | 15.4 | 233.7 | 14.9 | 838.8 | |
| MSWI 2 | 2-1 | Normal | 211.8 | 1384.8 | 1596.6 | 231.4 | 577.1 | 338.8 | 4989.6 | 57.7 | 80.6 | 11.5 | 827.7 |
| 2-2 | Shut down (40 min) and start up | 276.8 | 1276.1 | 1552.9 | 266.6 | 376.9 | 47.3 | 1937.8 | 30.0 | 150.1 | 14.3 | 776.9 | |
| 2-3 | Normal | 106.3 | 551.1 | 657.4 | 101.1 | 229.6 | 68.2 | 2102.8 | 12.7 | 161.1 | 15.1 | 783.5 | |
| 2-4 | Normal | 10.8 | 64.3 | 75.1 | 12.3 | 29.5 | 9.28 | 162.9 | 5.9 | 156.7 | 14.9 | 822.1 | |
| 2-5 | Normal | 20.7 | 127.9 | 148.6 | 27.7 | 18.7 | 165.1 | 801.2 | 9.6 | 125.5 | 13.4 | 867.0 | |
| 2-6 | Start up (shut down before sampling) | 57.3 | 398.7 | 456.0 | 118.4 | 186.8 | 716.8 | 3069.3 | 7.1 | 185.2 | 14.6 | 800.3 | |
| 2-7 | Normal | 21.6 | 114.8 | 136.5 | 25.6 | 98.5 | 45.4 | 101.2 | 3.4 | 235.8 | 15.6 | 753.3 | |
3.2 Relationships between CBzs, CPhs, PAHs, PCDD/Fs, and TEQ
Linear relationships are the most common and convenient indication models. And most of our data scatter diagrams also presented the trends. To compare and find the optimal situations, the coefficients of determination (R2) among CBzs, CPhs, PAHs, toxic chlorinated PCDD/Fs, and TEQ values were obtained from MSWI 1 and MSWI 2 samples and from a combined dataset of all MSWI 1 and MSWI 2 data, shown in Table 2. On the whole MSWI 2 showed a better correlation with PCDD/Fs for all of the target isomers. The most probable cause was the overhaul of MSWI 1. This operation possibly changed the incinerator greater and decreased the target compounds (sample 1-5, 1-6) so drastically, which could be found in Table 2. As to the target indicators, most of CBzs revealed a really good relationship with PCDD/Fs, as shown in previous studies.3 Nearly all CBz isomers except 14-dichlorobenzenes (14-DCBzs) and hexachlorobenzenes (HCBzs), had a good correlation with PCDD/F concentrations (R2 ≥ 0.8, significance level α ≤ 0.05). 123-Trichlorobenzene (123-TrCBz), 1234-tetrachlorobenzene (1234-TeCBz), and pentachlorobenzene (PCBz) revealed the best positive linear correlation, R2 > 0.9, from MSWI 2 data. High chlorinated chlorobenzenes had a better correlation than low chlorinated ones, such as 1234-tetrachlorobenzene (1234-TeCBz), PCBzs. Even in the conditions of MSWI 1, in which most compounds showed a rather poor correlation, TeCBzs also revealed high correlation with PCDD/F TEQ (R2 > 0.9). Due to the limitation of ionizing power in online monitoring dioxin systems, trichlorobenzenes (TrCBzs) were a better choice for indication. In previous studies 135-TrCBz was found to be the best69 and 124-TrCBz was used to indicate PCDD/Fs in the experiments of near-online REMPI TOFMS.30 While in this study 123-TrCBz had the highest concentration among TrCBz isomers and revealed the best correlation with PCDD/Fs, the highest R2 = 0.94 in the combined dataset.| Coefficients with PCDD/F concentrations | Coefficients with TEQ | ||||||
|---|---|---|---|---|---|---|---|
| CBz isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 | CBz isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 |
| a Means p value ≤ 0.05.b Means 0.05 < p value ≤ 0.1; no marks means p value > 0.1.c 0.00 means the value less than 0.005; — means the value couldn't be calculated due to undetected isomer concentrations in samples. | |||||||
| (a) CBz | |||||||
| 13 | 0.06 | 0.79a | 0.82a | 13 | 0.15 | 0.63a | 0.70a |
| 14 | 0.61b | 0.52b | 0.62a | 14 | 0.41 | 0.36 | 0.49a |
| 12 | 0.72a | 0.85a | 0.81a | 12 | 0.48 | 0.77a | 0.75a |
| DCBzs | 0.72a | 0.84a | 0.84a | DCBzs | 0.51b | 0.73a | 0.75a |
| 135 | 0.40 | 0.79a | 0.83a | 135 | 0.16 | 0.71a | 0.77a |
| 124 | 0.46 | 0.84a | 0.89a | 124 | 0.30 | 0.76a | 0.83a |
| 123 | 0.52b | 0.92a | 0.94a | 123 | 0.35 | 0.86a | 0.90a |
| TrCBzs | 0.52b | 0.89a | 0.92a | TrCBzs | 0.34 | 0.83a | 0.88a |
| 1235 + 1245 | 0.74a | 0.89a | 0.89a | 1235 + 1245 | 0.90a | 0.81a | 0.84a |
| 1234 | 0.75a | 0.93a | 0.94a | 1234 | 0.93a | 0.84a | 0.90a |
| TeCBzs | 0.74a | 0.92a | 0.94a | TeCBzs | 0.92a | 0.83a | 0.90a |
| 5 | 0.52b | 0.93a | 0.94a | 5 | 0.21 | 0.88a | 0.92a |
| 6 | 0.77a | 0.22 | 0.01 | 6 | 0.65a | 0.24 | 0.00 |
| Total CBzs | 0.96a | 0.91a | 0.93a | Total CBzs | 0.75a | 0.81a | 0.88a |
| CPh isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 | CPh isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 |
|---|---|---|---|---|---|---|---|
| (b) CPh | |||||||
| 2 | 0.70b | 0.07 | 0.33b | 2 | 0.85a | 0.06 | 0.33b |
| 3/4 | 0.22 | 0.07 | 0.02 | 3/4 | 0.41 | 0.14 | 0.06 |
| MCP | 0.15 | 0.19 | 0.23b | MCP | 0.31 | 0.23 | 0.27b |
| 26 | — | 0.00 | — | 26 | — | 0.01 | — |
| 23 | — | 0.56 | — | 23 | — | 0.44 | — |
| 25 | — | 0.25 | — | 25 | — | 0.13 | — |
| 24 | — | 0.00 | — | 24 | — | 0.01 | — |
| 34 | — | 0.00 | — | 34 | — | 0.01 | — |
| DCP | 0.18 | 0.00 | 0.04 | DCP | 0.42 | 0.02 | 0.09 |
| 234/246 | 0.50 | 0.01 | 0.01 | 234/246 | 0.40 | 0.00 | 0.04 |
| 245 | 0.56 | 0.01 | 0.00 | 245 | 0.42 | 0.00 | 0.00 |
| TrCP | 0.54 | 0.01 | 0.00 | TrCP | 0.41 | 0.00 | 0.02 |
| 2356 | 0.41 | 0.07 | — | 2356 | — | 0.01 | — |
| 2346 | 0.07 | — | — | 2346 | 0.27 | 0.14 | 0.03 |
| TeCP | 0.11b | 0.07 | 0.07 | TeCP | 0.25 | 0.01 | 0.03 |
| PCP | 0.10 | 0.06 | 0.07 | PCP | 0.00 | 0.02 | 0.09 |
| Total Cphs | 0.46 | 0.08 | 0.16 | Total Cphs | 0.55b | 0.05 | 0.15 |
| PAH isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 | PAH isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 |
|---|---|---|---|---|---|---|---|
| (c) PAH | |||||||
| NAP | 0.45 | 0.07 | 0.02 | NAP | 0.23 | 0.12 | 0.03 |
| ANY | 0.17 | 0.73a | 0.29b | ANY | 0.18 | 0.66a | 0.24b |
| ANA | 0.08 | 0.69a | 0.26b | ANA | 0.16 | 0.61a | 0.23b |
| FLU | 0.07 | 0.57a | 0.11 | FLU | 0.17 | 0.52b | 0.10 |
| PHE | 0.12 | 0.67a | 0.24b | PHE | 0.13 | 0.64a | 0.23b |
| ANT | 0.04 | 0.67a | 0.17 | ANT | 0.09 | 0.55b | 0.14 |
| FLT | 0.09 | 0.67a | 0.12 | FLT | 0.18 | 0.61a | 0.11 |
| PYR | 0.08 | 0.65a | 0.12 | PYR | 0.16 | 0.57a | 0.10 |
| BaA | 0.03 | 0.46b | 0.40a | BaA | 0.04 | 0.32 | 0.29a |
| CHR | 0.03 | 0.63a | 0.57a | CHR | 0.04 | 0.47b | 0.45a |
| BbF | 0.01 | 0.44b | 0.50a | BbF | 0.02 | 0.29 | 0.37a |
| BkF | 0.06 | 0.64b | 0.69a | BkF | 0.03 | 0.46b | 0.55a |
| BaP | 0.42 | 0.62b | 0.68a | BaP | 0.20 | 0.45b | 0.55a |
| IPY | 0.00 | 0.18 | 0.09 | IPY | 0.07 | 0.12 | 0.08 |
| DBA | 0.00 | 0.19 | 0.09 | DBA | 0.08 | 0.12 | 0.08 |
| BghiP | 0.00 | 0.18 | 0.08 | BghiP | 0.07 | 0.12 | 0.08 |
| PAHs | 0.25 | 0.57a | 0.20 | PAHs | 0.25 | 0.56b | 0.19 |
PAHs were the next significant indicators according to the results. In MSWI 2 more than half of PAHs had a relative obvious correlation with PCDD/Fs. Acenaphthylene (ANY) showed a positive linear correlation with PCDD/F concentrations, the highest R2 = 0.73, significance level ≤0.05, which was different from previous studies on naphthalene, phenanthrene and fluoranthene.32 It was also found the reason inducing poorer correlations of PAHs than that of CBzs both in MSWI 2 was the shut-down and start-up process during sample 2-2 collection. This operating condition varied caused obviously different changes in concentrations of different compounds. The PCDD/Fs concentration increased by 3% and the concentration of CBzs increased by 35%. However the concentrations of CPhs and PAHs increased by 86% and 61%, respectively. The drastic changes of CPhs and PAHs caused large deviation from the other data, and made an obvious effect on correlation analysis with PCDD/Fs. It could also be found CPhs and PAHs were much more sensitive to the condition changes and higher volatility also affected the data collection and statistics analysis.
Compared with CBzs and PAHs, CPhs had a really poor correlation with PCDD/Fs. Most of the coefficients were lower than 0.5. Maybe the samples collection process from stack gas and the pretreatment process caused relative large losses of some CPh isomers. However, 2-monochlorophenol (2-MCP) still revealed a negative linear correlation, of which the R2 value was 0.70 with PCDD/F concentrations and R2 value was up to 0.85 with TEQ.
In previous studies researchers focused much more on the total PCDD/Fs concentrations of TEQ. In order to find a more proper way to establish correlation models, we also investigated the correlations with PCDDs and PCDFs, respectively, shown in Table 3. For all these target compounds, the correlation with PCDFs was the best, compared with the results of PCDDs, PCDD/Fs and TEQ. Maybe these indicators and PCDFs have the similar variation trend of amounts, and these precursors were more closely correlated with PCDFs formation.33,61
| Coefficients with PCDD concentrations | Coefficients with PCDF concentrations | ||||||
|---|---|---|---|---|---|---|---|
| Isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 | Isomers | MSWI 1 | MSWI 2 | MSWI 1 + 2 |
| a Means p value ≤ 0.05.b Means 0.05 < p value ≤ 0.1; no marks means p value > 0.1.c 0.00 means the value less than 0.005; — means the value couldn't be calculated due to undetected isomer concentrations in samples. | |||||||
| (a) CBz | |||||||
| 13 | 0.22 | 0.66a | 0.73a | 13 | 0.08 | 0.81a | 0.84a |
| 14 | 0.18 | 0.41 | 0.52a | 14 | 0.56b | 0.54b | 0.63a |
| 12 | 0.00 | 0.68a | 0.68a | 12 | 0.72a | 0.87a | 0.83a |
| DCBzs | 0.00 | 0.68a | 0.71a | DCBzs | 0.72a | 0.87a | 0.86a |
| 135 | 0.09 | 0.60a | 0.70a | 135 | 0.37 | 0.81a | 0.86a |
| 124 | 0.08 | 0.68a | 0.76a | 124 | 0.44 | 0.87a | 0.90a |
| 123 | 0.08 | 0.79a | 0.85a | 123 | 0.49b | 0.93b | 0.95a |
| TrCBzs | 0.09 | 0.75a | 0.82a | TrCBzs | 0.49b | 0.91b | 0.94a |
| 1235 + 1245 | 0.10 | 0.73a | 0.78a | 1235 + 1245 | 0.77a | 0.91a | 0.90a |
| 1234 | 0.11 | 0.79a | 0.85a | 1234 | 0.78a | 0.95a | 0.95a |
| TeCBzs | 0.10 | 0.77a | 0.85a | TeCBzs | 0.78a | 0.94a | 0.96a |
| 5 | 0.08 | 0.86a | 0.90a | 5 | 0.49b | 0.94a | 0.94a |
| 6 | 0.01 | 0.09 | 0.00 | 6 | 0.75a | 0.25 | 0.01 |
| Total CBzs | 0.00 | 0.75a | 0.83a | Total CBzs | 0.95a | 0.92a | 0.95a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| (b) CPh | |||||||
| 2 | 0.22 | 0.00 | 0.20 | 2 | 0.71 | 0.10 | 0.36a |
| 3/4 | 0.24 | 0.03 | 0.01 | 3/4 | 0.25 | 0.07 | 0.03 |
| MCP | 0.38 | 0.08 | 0.13 | MCP | 0.19 | 0.21 | 0.25b |
| 26 | — | 0.02 | — | 26 | — | 0.00 | — |
| 23 | — | 0.37 | — | 23 | — | 0.60b | — |
| 25 | — | 0.34 | — | 25 | — | 0.22 | — |
| 24 | — | 0.01 | — | 24 | — | 0.00 | — |
| 34 | — | 0.02 | — | 34 | — | 0.00 | — |
| DCP | 0.02 | 0.01 | 0.01 | DCP | 0.19 | 0.00 | 0.04 |
| 234/246 | 0.18 | 0.03 | 0.00 | 234/246 | 0.52b | 0.01 | 0.01 |
| 245 | 0.22 | 0.03 | 0.01 | 245 | 0.58b | 0.01 | 0.00 |
| TrCP | 0.20 | 0.03 | 0.00 | TrCP | 0.56b | 0.01 | 0.00 |
| 2356 | — | 0.11 | — | 2356 | — | 0.06 | — |
| 2346 | 0.01 | 0.10 | 0.10 | 2346 | 0.12 | 0.06 | 0.06 |
| TeCP | 0.01 | 0.10 | 0.10 | TeCP | 0.11 | 0.06 | 0.06 |
| PCP | 0.70 | 0.12 | 0.19 | PCP | 0.43 | 0.08 | 0.15 |
| Total Cphs | 0.19 | 0.00 | 0.04 | Total Cphs | 0.33 | 0.01 | 0.09 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| (c) PAH | |||||||
| NAP | 0.04 | 0.04 | 0.00 | NAP | 0.46b | 0.08 | 0.02 |
| ANY | 0.07 | 0.54b | 0.18 | ANY | 0.19 | 0.76a | 0.28 |
| ANA | 0.22 | 0.49b | 0.17 | ANA | 0.10 | 0.73a | 0.28 |
| FLU | 0.24 | 0.57 | 0.06 | FLU | 0.09 | 0.61a | 0.12 |
| PHE | 0.06 | 0.47b | 0.17 | PHE | 0.13 | 0.70a | 0.26 |
| ANT | 0.21 | 0.48b | 0.11 | ANT | 0.05 | 0.71a | 0.18 |
| FLT | 0.22 | 0.47b | 0.07 | FLT | 0.11 | 0.71a | 0.13 |
| PYR | 0.21 | 0.44b | 0.07 | PYR | 0.10 | 0.68a | 0.13 |
| BaA | 0.15 | 0.27 | 0.26 | BaA | 0.03 | 0.49a | 0.43a |
| CHR | 0.16 | 0.44b | 0.43a | CHR | 0.03 | 0.66a | 0.60a |
| BbF | 0.03 | 0.25 | 0.34a | BbF | 0.01 | 0.48 | 0.53a |
| BkF | 0.04 | 0.45 | 0.55a | BkF | 0.07 | 0.67a | 0.71a |
| BaP | 0.00 | 0.42 | 0.53a | BaP | 0.41 | 0.65a | 0.71a |
| IPY | 0.15 | 0.20 | 0.09 | IPY | 0.00 | 0.18 | 0.08 |
| DBA | 0.15 | 0.21 | 0.09 | DBA | 0.00 | 0.18 | 0.08 |
| BghiP | 0.15 | 0.20 | 0.09 | BghiP | 0.00 | 0.17 | 0.08 |
| PAHs | 0.14 | 0.39 | 0.12 | PAHs | 0.27 | 0.60a | 0.22b |
Linear regression was the most widely used and convenient method. According to the analysis results, CBzs and PAHs were found to be the useful indicators to establish linear regression models for monitoring PCDD/Fs. The whole data set of MSWI 1 and MSWI 2 were found better to be analyzed for obtaining the significant linear regression models. Parson coefficients between PCDD/Fs concentrations, TEQ, PCDDs concentrations, PCDFs concentrations and each CBz, PAHs were supplemented in Table 4. This result correspond with the former analysis in Tables 2 and 3. Nearly all CBzs and several PAH compounds, such as benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), chrysene (CHR) and benzo(b)fluoranthene (BbF), showed a rather good significant level at 0.01 (two-tailed).
| PCDD/Fs concentration | TEQ | PCDDs concentration | PCDFs concentration | |
|---|---|---|---|---|
| a **Significant at 0.01 level (two-tailed); *significant at 0.05 level (two-tailed). | ||||
| (a) CBz | ||||
| 13-DCBz | 0.908** | 0.836** | 0.852** | 0.915** |
| 14-DCBz | 0.787** | 0.702** | 0.723** | 0.796** |
| 12-DCBz | 0.901** | 0.865** | 0.823** | 0.912** |
| 135-TrCBz | 0.913** | 0.879** | 0.834** | 0.925** |
| 124-TrCBz | 0.941** | 0.909** | 0.874** | 0.951** |
| 123-TrCBz | 0.970** | 0.950** | 0.924** | 0.975** |
| 1235/1245-TeCBz | 0.941** | 0.919** | 0.885** | 0.948** |
| 1234-TeCBz | 0.970** | 0.947** | 0.922** | 0.976** |
| PCBz | 0.962** | 0.951** | 0.939** | 0.963** |
| HCBz | −0.073 | −0.057 | −0.039 | −0.079 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (b) PAH | ||||
| NAP | 0.140 | 0.184 | 0.067 | 0.153 |
| ANY | 0.515 | 0.490 | 0.428 | 0.530 |
| ANA | 0.509 | 0.478 | 0.417 | 0.525 |
| FLU | 0.332 | 0.315 | 0.240 | 0.348 |
| PHE | 0.494 | 0.483 | 0.409 | 0.508 |
| ANT | 0.409 | 0.368 | 0.329 | 0.423 |
| FLT | 0.345 | 0.328 | 0.264 | 0.359 |
| PYR | 0.342 | 0.317 | 0.257 | 0.356 |
| BaA | 0.631* | 0.541 | 0.506 | 0.652* |
| CHR | 0.757** | 0.671* | 0.656* | 0.774** |
| BbF | 0.699** | 0.603* | 0.581* | 0.719** |
| BkF | 0.824** | 0.737** | 0.738** | 0.838** |
| BaP | 0.820** | 0.738** | 0.722** | 0.835** |
| IPY | −0.292 | −0.288 | −0.295 | −0.290 |
| DBA | −0.291 | −0.287 | −0.295 | −0.290 |
| BghiP | −0.288 | −0.284 | −0.292 | −0.286 |
Both unary and multiple linear regression models were established. For unary linear regression analysis, 123-TrCBz, 1234-TrCBz and PCBz were chosen to establish easy indicating models for use with the highest R2, 0.94 with PCDD/Fs concentrations and 0.9 with TEQ. Several PAH isomers could also be used for indication. Detailed correlation figures were shown in the ESI S2.†
Then the multiple linear regression analysis was also performed. The whole data of all CBz and PAHs were introduced as variables. The target variables were PCDD/Fs concentration, TEQ and PCDFs concentration, respectively. Several final multiple linear regression equations were obtained as follow. All the equations had good fitting degree of sample data and R2 was obviously advanced. Coefficients of regression equations were significant at 0.05 level. The collinearity of variables was weak and residual errors were independent. It was better to take advantage of diversified indicators in establishing correlation models. For instance, the coefficient became higher, 0.97 when 123-TrCBz and 135-TrCBz both introduced in correlation models, compared with the situations in which only one kind of chlorobenzenes used. Indication capacity made much progress. The same conclusion could also be found for PAHs. Compared with single indicator, varieties of indicators used at the same time benefits to the achievement of the aim of real online monitoring PCDD/Fs.
3.3 Relationship among PCDD/Fs homologues and other compounds and their formation mechanism
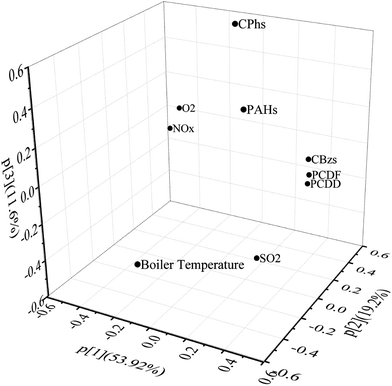
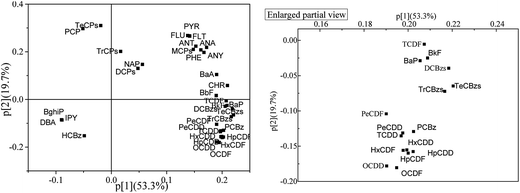
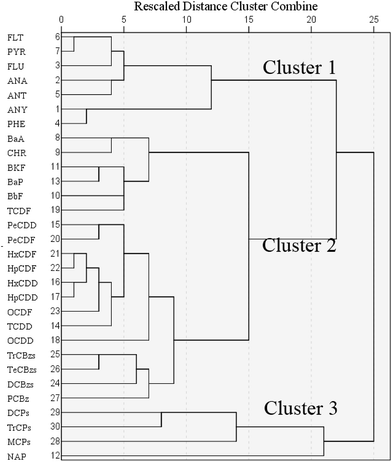
The relationship among these CEM data and target compounds was investigated with PCA (Fig. 1). Boiler temperature, NOx, O2 and SO2 were common parameters. Boiler temperature and O2 affect waste combustion conditions and have complicated influences on PCDD/Fs formation.1,3,60,62 Low temperature and poor oxygen content usually induce amounts of uncompleted combustion pollutants. SO2 is known as a common suppressor of PCDD/F formation, and in previous studies3,70,71 a negative relationship was revealed between SO2 concentrations with PCDD/Fs. In this PCA result boiler temperature and O2 stated more far away from PCDD/Fs. Maybe in this experimental data all the combustion conditions had enough high temperature and excess oxygen content. The slight changes made little effects on PCDD/Fs formation. As concerned, chlorinated compounds are usual precursors of PCDD/Fs. In the appropriate conditions especially temperature and atmosphere considered, several CBzs and CPhs could be catalyzed to generate PCDD/Fs by fly ashes containing metals oxides and chlorinates such as CuCl2, CuO, FeCl3 et al.10,11,13,15–17 In the PCA result of this study CBz concentrations were closely related to PCDDs and PCDFs, while PAHs and CPhs were located far away from PCDD/Fs, different from the previous finds that CPhs play a more sensitive role in formation of PCDD/Fs than CBzs.15,16 Besides, the key factors influencing dioxin formation, including temperature, moisture, metal catalyst, chlorine source and oxygen concentration, were investigated to have similar effect on CBzs formation,60 which might be the main reason or mechanism of the great correlation between CBzs and PCDD/Fs. In the PCA result it was also be found CBzs related more closely to PCDFs than PCDDs, which was also proved in correlation coefficient calculation.To get further analysis, PCA and cluster analysis were performed using the homologue concentrations. Hierarchical cluster analysis was conducted with Ward's method and the data were standardized by z-score. Dibenzo(a,h)anthracene (DBA), benzo(g,h,i)perylene (BghiP), indeno(1,2,3-cd)pyrene (IPY), HCBz, tetrachlorophenol (TeCPs) and pentachlorophenol (PCP) were extracted from the cluster analysis because these compounds were located far away from other target compounds in the PCA analysis result (Fig. 2). In the dendrogram (Fig. 3) cluster one is comprised of parts of PAHs. Cluster two contains CBzs, PCDD/Fs and other parts of PAHs while cluster three contains most CPhs and naphthalene (NaP).
Although the total PAHs and total CPh concentrations were far away from PCDD and PCDF (shown in Fig. 1), BkF, BaP and BbF concentrations were related to TCDF concentration, more close than CBzs, and in the cluster analysis dendrogram there was the shortest rescaled distance between TCDF and BbF, which showed BbF had the best correlation with TCDF concentration. Among CBz homologues, HCBz revealed poor correlation with all PCDD/Fs. PCBz, DCBzs, TrCBzs, TeCBzs and most of PCDD/F homologues were located closely in the bottom in Fig. 2, and PCBz had the most close relationship with PCDD/Fs especially with PeCDD and TCDD.
The detailed correlations between PAHs, SO2 and 17 toxic PCDD/F isomers were also analyzed using Parson coefficients calculated. 2378-TCDF has rather significant correlation with most of PAHs at 0.05 level, even at 0.01 level for benzo(a)anthracene (BaA), CHR, BbF, BkF and BaP. CHR, BbF, BkF and BaP had good correlations with most of toxic PCDD/F isomers. However the correlation with the most toxic 2378-TCDD (TEF = 1) was much poor. And the correlation with 23478-PeCDF (TEF = 0.5), which occupies the major TEQ contribution, was also not good. That may be the reason why the correlation between indicators with TEQ values was poorer compared with the results of concentrations. SO2 had significant correlations with 123678-HxCDD, 1234678-HpCDD, OCDD (at 0.05 level) and 2378-TCDF (at 0.01 level), especially 2378-TCDF, which was consistent with the studies on effects of SO2 on PCDD/F formation.70,72 Detailed results could be found in S3.†
4. Conclusion
An extensive investigation and analysis of pollutants emitted from two typical fluidized bed municipal solid waste incinerators in China and their relationships were performed. These useful correlations help achieve a comprehensive study on MSWIs, monitor and diagnose the operation condition and cleanup systems.More suitable and careful pretreatments were used to obtain more accurate data. CBzs, PAHs and CPhs were common indicators for monitoring PCDD/F emissions. Among all CPhs and PAHs were much more sensitive to operation conditions and affected more easily by changes. While CBzs were revealed rather perfect correlations with PCDD/Fs, correlation coefficients nearly all up to 0.8 and significant at 0.05 level (two-tailed). 123-TrCBz, 1234-TrCBz, and PCBz revealed the best positive linear correlation (R2 > 0.9). CBzs were more proper indicators for near online monitoring PCDD/Fs.
In this study most of CPhs didn't perform enough good indicative value. Only 2-MCP revealed a negative linear correlation, of which the R2 value was 0.70 with PCDD/F concentrations and R2 value was up to 0.85 with TEQ. More than half of PAHs reveled a adequate correlation with PCDD/F concentration (R2 > 0.6). Apart from the correlations with total PCDD/Fs, the correlations with PCDDs and PCDFs were also investigated respectively. PCDFs revealed to be the best target for indication due to the similar formation variation trend to that of these precursors. Unary linear regression equations with high coefficients of determination between several CBz, PAHs and PCDD/Fs, TEQ and PCDFs were established for later online monitoring application. Furthermore, the multiple linear regression analysis was also performed to obtain advanced correlation models.
The relationships among PCDD/F homologues, isomers and other compounds and their formation mechanism were discussed. It was found that CBzs related more closely to PCDFs than PCDDs. PCBz had the most close relationship with PCDD/Fs especially with PeCDD and TCDD. BkF, BaP and BbF concentrations were related to TCDF concentration, more close than CBzs. 2378-TCDF had rather significant correlation with most of PAHs. CHR, BbF, BkF and BaP had good correlations with most of toxic PCDD/F isomers. SO2 had significant correlations with 123678-HxCDD, 1234678-HpCDD, OCDD (at 0.05 level) and 2378-TCDF (at 0.01 level), especially 2378-TCDF.
Conflicts of interest
There are no conflicts of interest to declare.Abbreviation
| MCBz | Monochlorobenzene |
| DCBz | Dichlorobenzene |
| TrCBz | Trichorobenzene |
| TeCBz | Tetrachlorbenzene |
| PCBz | Pentachlorobenzene |
| HCBz | Hexachlorobenzene |
| MCP | Monochlorphenol |
| DCP | Dichlorophenol |
| TrCP | Trichlorophenol |
| TeCP | Tetrachlorophenol |
| PCP | Pentachlorophenol |
| NAP | Naphthalene |
| ANY | Acenaphthylene |
| ANA | Acenaphthene |
| FLU | Fluorene |
| PHE | Phenanthrene |
| ANT | Anthracene |
| FLT | Fluoranthene |
| PYR | Pyrene |
| BaA | Benzo(a)anthracene |
| CHR | Chrysene |
| BbF | Benzo(b)fluoranthene |
| BkF | Benzo(k)fluoranthene |
| BaP | Benzo(a)pyrene |
| IPY | Indeno(1,2,3-cd)pyrene |
| DBA | Dibenzo(a,h)anthracene |
| BghiP | Benzo(g,h,i)perylene |
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC51476138).References
- G. McKay, Chem. Eng. J., 2002, 86, 343–368 CrossRef CAS.
- B. Stanmore, Combust. Flame, 2004, 136, 398–427 CrossRef CAS.
- H. Zhou, A. Meng, Y. Long, Q. Li and Y. Zhang, Waste Manag., 2015, 36, 106–118 CrossRef CAS PubMed.
- I. Vermeulen, J. Van Caneghem and C. Vandecasteele, J. Mater. Cycles Waste Manage., 2014, 16, 167–171 CrossRef CAS.
- R. Addink and K. Olie, Environ. Sci. Technol., 1995, 29, 1425–1435 CrossRef CAS PubMed.
- W. Pan, D. Zhang, Z. Han, J. Zhan and C. Liu, Environ. Sci. Technol., 2013, 47, 8489–8498 CAS.
- F. Xu, W. Yu, R. Gao, Q. Zhou, Q. Zhang and W. Wang, Environ. Sci. Technol., 2010, 44, 6745–6751 CrossRef CAS PubMed.
- L. Khachatryan, A. Burcat and B. Dellinger, Combust. Flame, 2003, 132, 406–421 CrossRef CAS.
- D. H. Kim, J. A. Mulholland and J.-Y. Ryu, Chemosphere, 2007, 67, S135–S143 CrossRef CAS PubMed.
- S. Lomnicki and B. Dellinger, Proc. Combust. Inst., 2002, 29, 2463–2468 CrossRef CAS.
- S. Nganai, S. Lomnicki and B. Dellinger, Chemosphere, 2012, 88, 371–376 CrossRef CAS PubMed.
- J. A. Mulholland and J.-Y. Ryu, Combust. Sci. Technol., 2001, 169, 107–126 CrossRef CAS.
- K. Hell, L. Stieglitz, E. Altwicker, R. Addink and R. Will, Chemosphere, 2001, 42, 697–702 CrossRef CAS PubMed.
- E. Wikström, M. Tysklind and S. Marklund, Environ. Sci. Technol., 1999, 33, 4263–4269 CrossRef.
- G. Yin, C. Tong, Y. A. N. Jianhua, L. I. Xiaodong, L. U. Shengyong and C. E. N. Kefa, J. Combust. Sci. Technol., 2006, 12, 253–256 Search PubMed.
- S. B. Ghorishi and E. R. Altwicker, Chemosphere, 1996, 32, 133–144 CrossRef CAS.
- Z. H. Du Yongguang, C. Jiping, F. Yun and N. Yuwen, Environ. Sci., 2010, 31, 2774–2779 Search PubMed.
- F. Iino, T. Imagawa, M. Takeuchi and M. Sadakata, Environ. Sci. Technol., 1999, 33, 1038–1043 CrossRef CAS.
- A. Fullana and S. S. Sidhu, J. Anal. Appl. Pyrolysis, 2005, 74, 479–485 CrossRef CAS.
- M. H. Schoonenboom and K. Olie, Environ. Sci. Technol., 1995, 29, 2005–2009 CrossRef CAS PubMed.
- F. Iino, T. Imagawa, M. Takeuchi, M. Sadakata and R. Weber, Chemosphere, 1999, 39, 2749–2756 CrossRef CAS.
- R. Weber, F. Iino, T. Imagawa, M. Takeuchi, T. Sakurai and M. Sadakata, Chemosphere, 2001, 44, 1429–1438 CrossRef CAS PubMed.
- S. Kuribayashi, H. Yamakoshi, M. Danno, S. Sakai, S. Tsuruga, H. Futami and S. Morii, Anal. Chem., 2005, 77, 1007–1012 CrossRef CAS PubMed.
- B. Gullett, Opt. Soc. Am., 2004 Search PubMed.
- L. Oudejans and B. K. Gullett, Opt. Soc. Am., 2004 Search PubMed.
- B. K. Gullett, A. Touati, L. Oudejans and S. P. Ryan, Atmos. Environ., 2006, 40, 4037–4047 CrossRef CAS.
- L. Oudejans, A. Touati and B. K. Gullett, Anal. Chem., 2004, 76, 2517–2524 CrossRef CAS PubMed.
- B. Gullett, A. Touati and L. Oudejans, Atmos. Environ., 2008, 42, 2117–2128 CrossRef CAS.
- H. Heger, R. Zimmermann, M. Blumenstock and A. Kettrup, Chemosphere, 2001, 42, 691–696 CrossRef CAS PubMed.
- B. K. Gullett, L. Oudejans, D. Tabor, A. Touati and S. Ryan, Environ. Sci. Technol., 2011, 46, 923–928 CrossRef PubMed.
- N. Watanabe, K. Kawamoto, S. Asada, H. Fujiyoshi, H. Miyata, G. Watanabe and S. Suzuki, J. Mater. Cycles Waste Manage., 2010, 12, 254–263 CrossRef CAS.
- M. Yan, X.-D. Li, X.-X. Zhang, K. Liu, J.-H. Yan and K.-F. Cen, J. Zhejiang Univ., Eng. Sci., 2010, 6, 017 Search PubMed.
- L. X.-D. Y. Xue-Feng, L. S.-Y. G. Yue-Ling, Y. J.-H. N. Ming-Jiang and C. Ke-Fa, J. Eng. Thermophys., 2006, 4, 049 Search PubMed.
- K. Yoneda, T. Ikeguchi, Y. Yagi, Y. Tamade and K. Omori, Chemosphere, 2002, 46, 1309–1319 CrossRef CAS PubMed.
- H. Oser, R. Thanner and H.-H. Grotheer, Chemosphere, 1998, 37, 2361–2374 CrossRef CAS.
- T. Streibel, H. Nordsieck, K. Neuer-Etscheidt, J. Schnelle-Kreis and R. Zimmermann, Chemosphere, 2007, 67, S155–S163 CrossRef CAS PubMed.
- R. Zimmermann, H. J. Heger, M. Blumenstock, R. Dorfner, K. W. Schramm, U. Boesl and A. Kettrup, Rapid Commun. Mass Spectrom., 1999, 13, 307–314 CrossRef CAS.
- R. Weber, T. Sakurai, S. Ueno and J. Nishino, Chemosphere, 2002, 49, 127–134 CrossRef CAS PubMed.
- K. Tuppurainen, M. Aatamila, P. Ruokojärvi, I. Halonen and J. Ruuskanen, Chemosphere, 1999, 38, 2205–2217 CrossRef CAS PubMed.
- M. Yamada, I. Waki, M. Sakairi, M. Sakamoto and T. Imai, Chemosphere, 2004, 54, 1475–1480 CrossRef CAS PubMed.
- M. Kato, K. Urano and T. Tasaki, Environ. Sci. Technol., 2000, 34, 4071–4075 CrossRef CAS.
- M. Kato and K. Urano, Waste Manag., 2001, 21, 55–62 CrossRef CAS PubMed.
- F. Iino, T. Takasuga, A. Touati and B. K. Gullett, Waste Manag., 2003, 23, 729–736 CrossRef CAS PubMed.
- B. K. Gullett and E. Wikström, Chemosphere, 2000, 40, 1015–1019 CrossRef CAS PubMed.
- J.-E. Oh, A. Touati, B. K. Gullett and J. A. Mulholland, Environ. Sci. Technol., 2004, 38, 4694–4700 CrossRef CAS PubMed.
- P. Lemieux and E. Stewart, Environ. Eng. Sci., 2004, 21, 3–9 CrossRef CAS.
- A. Kaune, D. Lenoir, K.-W. Schramm, R. Zimmermann, A. Kettrup, K. Jaeger, H. Rückel and F. Frank, Environ. Eng. Sci., 1998, 15, 85–95 CrossRef CAS.
- J. Yan, T. Chen, X. Li, J. Zhang, S. Lu, M. Ni and K. Cen, J. Hazard. Mater., 2006, 135, 47–51 CrossRef CAS PubMed.
- S.-Y. Lu, J.-H. Yan, X.-D. Li, M.-J. Ni and K.-F. Cen, J. Environ. Sci., 2007, 19, 762–767 CrossRef CAS.
- B. K. Gullett, J. E. Dunn and K. Raghunathan, Environ. Sci. Technol., 2000, 34, 282–290 CrossRef CAS.
- US EPA, Method 23: Determination of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from municipal waste combustors, U.S. EPA, Washington, DC, 1995 Search PubMed.
- K. Neuer-Etscheidt, H. O. Nordsieck, Y. Liu, A. Kettrup and R. Zimmermann, Environ. Sci. Technol., 2006, 40, 342–349 CrossRef CAS PubMed.
- J.-E. Oh, B. Gullett, S. Ryan and A. Touati, Environ. Sci. Technol., 2007, 41, 4705–4710 CrossRef CAS PubMed.
- T. Chen, J. Yan, S. Lu, X. Li, Y. Gu, H. Dai, M. Ni and K. Cen, J. Hazard. Mater., 2008, 150, 510–514 CrossRef CAS PubMed.
- M. Yan, Inhibition of PCDD/Fs Formation during Medical Waste incineration and Research of Environmental Impact of Incinerator, Doctoral Dissertation, Zhejiang University, 2012.
- T. Chen, M.-X. Zhan, X.-Q. Lin, Y.-Q. Li, J. Zhang, X.-D. Li, J.-H. Yan and A. Buekens, Environ. Sci. Pollut. Res., 2016, 1–10 Search PubMed.
- Y. Li, Y. Yang, G. Yu, J. Huang, B. Wang, S. Deng and Y. Wang, Chemosphere, 2016, 158, 17–23 CrossRef CAS PubMed.
- Y. Ni, H. Zhang, S. Fan, X. Zhang, Q. Zhang and J. Chen, Chemosphere, 2009, 75, 1153–1158 CrossRef CAS PubMed.
- M. Takaoka, P. Liao, N. Takeda, T. Fujiwara and K. Oshita, Chemosphere, 2003, 53, 153–161 CrossRef CAS PubMed.
- M. Yan, X. Li, T. Chen, S. Lu, J. Yan and K. Cen, J. Environ. Sci., 2010, 22, 1637–1642 CrossRef CAS.
- M. Yan, Z. F. Qi, X. D. Li, T. Chen, S. Y. Lu, A. G. Buekens, K. Olie and J. H. Yan, Environ. Eng. Sci., 2012, 29, 890–896 CrossRef CAS.
- J. Vehlow, Dioxins in Waste Combustion–Conclusions from 20 Years of Research, 2005 Search PubMed.
- B. Stanmore, Chemosphere, 2002, 47, 565–573 CrossRef CAS PubMed.
- M. Blumenstock, R. Zimmermann, K.-W. Schramm and A. Kettrup, Chemosphere, 2000, 40, 987–993 CrossRef CAS PubMed.
- R. Zimmermann, M. Blumenstock, H. Heger, K.-W. Schramm and A. Kettrup, Environ. Sci. Technol., 2001, 35, 1019–1030 CrossRef CAS PubMed.
- H. Tejima, M. Nishigaki, Y. Fujita, A. Matsumoto, N. Takeda and M. Takaoka, Chemosphere, 2007, 66, 1123–1130 CrossRef CAS PubMed.
- H. C. Wang, J. F. Hwang, K. H. Chi and M. B. Chang, Chemosphere, 2007, 67, S177–S184 CrossRef CAS PubMed.
- L.-C. Wang, H.-C. Hsi, J.-E. Chang, X.-Y. Yang, G.-P. Chang-Chien and W.-S. Lee, Chemosphere, 2007, 67, 1346–1353 CrossRef CAS PubMed.
- G. Ying, C. Tong, Y. Jie, C. Xuan, L. Shengyong and L. Xiaodong, Chin. J. Environ. Eng., 2014, 3524–3529 Search PubMed.
- Y. Hajizadeh, J. A. Onwudili and P. T. Williams, Waste Manag., 2012, 32, 1378–1386 CrossRef CAS PubMed.
- H. Hunsinger, H. Seifert and K. Jay, Environ. Eng. Sci., 2007, 24, 1145–1159 CrossRef CAS.
- S. P. Ryan, X.-D. Li, B. K. Gullett, C. Lee, M. Clayton and A. Touati, Environ. Sci. Technol., 2006, 40, 7040–7047 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04168c |
| This journal is © The Royal Society of Chemistry 2017 |