Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of highly substituted dihydro-2-oxopyrroles using Fe3O4@nano-cellulose–OPO3H as a novel bio-based magnetic nanocatalyst†
Naeimeh Salehi and
Bi Bi Fatameh Mirjalili *
*
Department of Chemistry, Faculty of Sciences, Yazd University, P.O. Box 89195-741, Yazd, Islamic Republic of Iran. E-mail: fmirjalili@yazd.ac.ir; Fax: +98 38210644; Tel: +98 3531232672
First published on 12th June 2017
Abstract
A bio-based magnetic nanocatalyst (Fe3O4@nano-cellulose–OPO3H) has been made via immobilization of –OPO3H groups on a Fe3O4@nano-cellulose surface. Fe3O4@nano-cellulose was synthesized by co-precipitation of Fe3+ and Fe2+ salts in an aqueous suspension of nano-cellulose. The catalyst was characterized by FT-IR, FESEM, XRD, TGA, VSM, EDX, XRF and BET. It has been proved that such a heterogeneous catalyst shows high efficiency for the synthesis of dihydro-2-oxopyrrole derivatives via four-component reactions of amines, dialkyl acetylenedicarboxylates and aldehydes under mild reaction conditions. The present procedure is a green and environmental friendly approach that offers many advantages including high yield, easy work-up, simple recovery and reusability of the catalyst.
Introduction
The implementation of chemical reactions through greener pathways is one of the main challenges in today's organic chemistry. In this regard, chemists are focusing on the development of new synthetic methodologies using bio-based solvents such as water or ethanol and eco-friendly heterogeneous catalysts.1–3 On the other hand, multicomponent reactions (MCRs) as a powerful tool play an important role in the development of molecular designs. Such processes promote combinatorial chemistry by forming several bonds in a single operation without the need for time-consuming and expensive purification procedures.4–7 Due to such benefits, utilizing multicomponent routes is an alternative strategy to develop a simple and green synthesis of organic compounds.2-Oxodihydropyrroles as an important class of heterocycle compounds have wide biological activities such as antitumor and anticancer,8 antibiotic,9 anti-HIV,10 DNA polymerase inhibition,11 herbicidal12 and inhibition of human cytomegalovirus protease.13 Also, its derivatives are found in the structural cores of many natural bioactive products like bilirubins,14 pyrrocidine A,15 oteromycin,16 talaroconvolutin A,17 ypaoamide,18 thiomarinol A4,19 (Z) pulchellalactam,20 PI-091 (ref. 21) and Jatropham.22 Recently, a few protocols to synthesise polyfunctionalized dihydro-2-oxopyrroles via the four-component reaction of dialkylacetylenedicarboxylate, aldehyde, and amines have been developed. TiO2 nanopowder,23 I2,24 AcOH,25 Cu(OAc)2·H2O,26 [n-Bu4N][HSO4],27 trityl chloride (Ph3CCl),28 nano-TiCl4/SiO2,29 BF3/nano-sawdust,30 UiO-66-SO3H,31 CoFe2O4@SiO2@IRMOF32 and 2,6-pyridinedicarboxylic acid33 are applied for synthesis of dihydro-2-oxopyrroles as catalysts. Despite the remarkable achievements, some of these catalysts have many imperfections such as lack of catalyst recyclability, production of large amounts of toxic chemical waste and difficulties in catalyst recovery. Hence, there is a need to prepare an easily recyclable catalyst for the synthesis of dihydro-2-oxopyrroles.
Economically importance and environmentally benign features of magnetic nanoparticles (MNPs) have put them under chemical spotlight. They have several important advantages such as easy preparation and functionalization, high catalytic activity, simple separation using an external magnet and a high degree of chemical stability.34–36 Among the various magnetic nanoparticles, Fe3O4 nanoparticles have been more extensively studied as the core magnetic support due to their stronger magnetic properties, chemical stability, readily available, effortless preparation via co-precipitation and low toxicity.37 It should be noted that pure Fe3O4 NPs, with the high surface area to volume ratio, are highly chemically active and suffer from an inherent instability. They are very sensitive to oxidation and tend to aggregate spontaneously when exposed to acids and aqueous solutions. To overcome the above inherent limitations, the surface of nanoparticles can be coated by a suitable protective coating such as polymers, silica or carbon.38 Cellulose, as a renewable and naturally abundant biopolymer, is one of the most ideal coating layers for Fe3O4 NPs because it not only stabilizes the nanoparticles in solution but also enjoys free OH groups for functionalization purposes.39,40
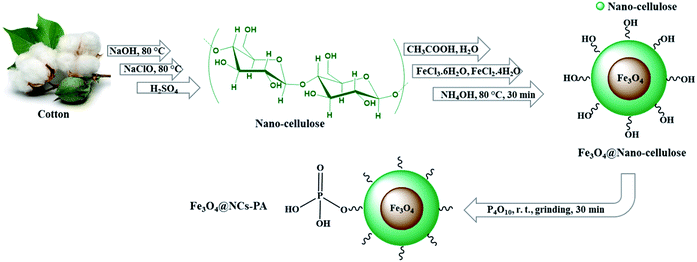
In this study, Fe3O4@nano-cellulose–OPO3H (Fe3O4@NCs–PA) was synthesized as a new magnetic bio-based nanocatalyst and then it was successfully applied to the synthesis of 2-oxo dihydropyrrole derivatives via the four-component reaction of dialkylacetylenedicarboxylate, aldehyde, and amines.
Results and discussion
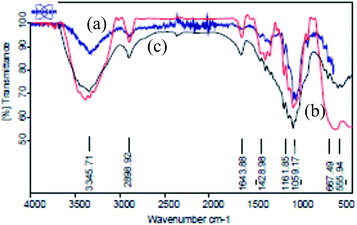
The sequential step for the preparation of Fe3O4@NCs–PA has been shown in Scheme 1. At first, nano-cellulose was prepared from cotton using the previously reported method.41 Then, magnetic core–shell nanoparticles, Fe3O4@nano-cellulose (Fe3O4@NCs), were obtained simply through in situ co-precipitation of ferric and ferrous ions with ammonium hydroxide in an aqueous solution containing nano-cellulose.41 At the end, the Fe3O4@nano-cellulose served as a magnetic support for the immobilization of –OPO3H groups by simple grinding with P2O5 at room temperature (Scheme 1). The characterization of Fe3O4@NCs–PA structure was performed by FT-IR, FESEM, XRD, TGA, VSM, EDX, XRF and BET analyses.Fig. 1 shows the FT-IR (ATR) spectra of nano-cellulose, Fe3O4@NCs and Fe3O4@NCs–PA. In the FT-IR spectrum of nano-cellulose (Fig. 1(a)), the signals related to the O–H and C–O stretching vibrations appeared at 3000–3600 cm−1 and 1027–1157 cm−1, respectively. In the FT-IR spectrum of Fe3O4@NCs (Fig. 1(b)), in addition to the above mentioned bands, a broad band at around 550–660 cm−1 shows Fe–O stretching vibrations reflecting the formation of a nano-cellulose shell around the Fe3O4 nano-particles. Successful –OPO3H groups functionalization on Fe3O4@NCs was also confirmed by the appearance of new bands at 2650–2700, 1150–1220 and 940–1100 cm−1 attributed to the stretching vibrations of PO–H, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P–OH bonds, respectively (Fig. 1(c)).
O, P–OH bonds, respectively (Fig. 1(c)).
Fig. 2 represents the result of field emission scanning electron microscopy (FESEM) of Fe3O4@NCs–PA to investigate its particle size and surface morphology. This image indicates that Fe3O4@NCs–PA nanoparticles have a quasi-spherical shape with an average size about 60 nm.
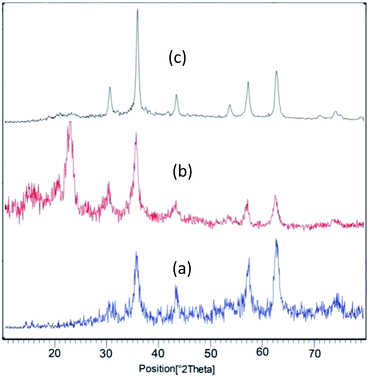
The comparison between Fe3O4, Fe3O4@NCs and Fe3O4@NCs–PA, XRD patterns in a range of 5–70° was shown in Fig. 3. In Fe3O4@NCs XRD pattern, in addition to all peaks of naked Fe3O4 (2θ = 30°, 35°, 43°, 53°, 57°, 63°, 71° and 73°), 2θ = 23° confirmed the existence of cellulose in its structure. The difference between XRD patterns of Fe3O4@NCs and Fe3O4@NCs–PA shows the additional weak diffraction peaks at 2θ = 21°, 32° and 42° in Fe3O4@NCs–PA, which seems to be linked to –PO3H on the surface of Fe3O4@NCs (Fig. 3(c)).
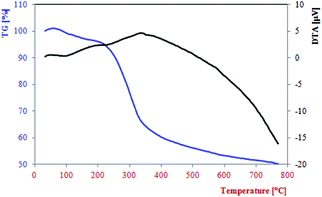
TGA-DTA analysis was performed to estimate thermal stability of the Fe3O4@NCs–PA in the temperature range of 32–770 °C (Fig. 4). The first decrease of weight was assigned to the catalyst moisture removal (endothermic effect at 100–200 °C, 4% weight loss) while the second decrease showed the decomposition and burning of cellulose in the nanocomposite (exothermal effect 200–330 °C, 32% weight loss). The char yield of the catalyst in 770 °C is 50.16%.
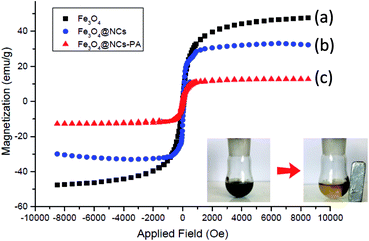
The magnetic properties of Fe3O4, Fe3O4@NCs, and Fe3O4@NCs–PA were characterized at RT (300 K) by a vibrating sample magnetometer (VSM) and their hysteresis curves are presented in Fig. 5. According to this image, the zero coercivity and remanence of the hysteresis loops of these magnetic nanoparticles confirm superparamagnetic property at room temperature. The amount of specific saturation magnetization (Ms) for Fe3O4 nanoparticles was about 47 emu g−1, which decreased to 32 emu g−1 after coating the Fe3O4 with cellulose and to 12 emu g−1 after the immobilization of –PO3H on the surface of Fe3O4@NCs. Despite this significant decrease, the saturated magnetization of these magnetic nanoparticles is sufficient for magnetic separation.
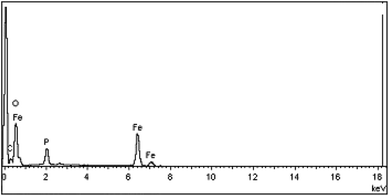
The existence of the expected elements in the structure of the Fe3O4@NCs–PA was approved by energy-dispersive X-ray spectroscopy EDS (EDX) analysis (Fig. 6). The EDS results clearly confirm the presence of Fe, O, P, C elements in the catalyst. According to this data, the elemental compositions of Fe3O4@NCs–PA were found to be 11.75, 54.42, 3.69 and 30.14% for Fe, O, P and C, respectively. The weight percentages of Fe, O, P and C are 32.75, 43.46, 5.72 and 18.07, respectively.
In addition to EDX analysis, X-ray fluorescence (XRF) analysis of Fe3O4@NCs–PA also indicates that the ratio of Fe2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5
P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 is equal to 4.76
CO2 is equal to 4.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.6
15.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78.9%. And so, inductively coupled plasma (ICP) analysis of catalyst shows 4.95% P and 21.1% of Fe.
78.9%. And so, inductively coupled plasma (ICP) analysis of catalyst shows 4.95% P and 21.1% of Fe.
The specific surface area of catalyst was measured by Brunauer–Emmett–Teller (BET) theory. The single point surface area at P/P0 = 0.989 is 2.85 m2 g−1, while the mean pore diameter is 12.612 nm and the total pore volume is 9.0032 cm3 g−1. The N2 adsorption isotherm of catalyst is depicted in Fig. 7. The total acid capacity was found to be in the range of 0.50 mmol g−1, which was determined through the neutralization titration.
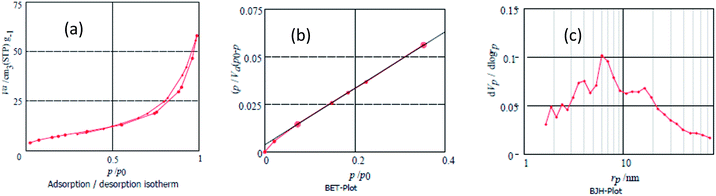 | ||
| Fig. 7 (a) BET (Brunauer–Emmett–Teller), (b) adsorption/desorption isotherm and (c) BJH (Barrett–Joyner–Halenda) plots of Fe3O4@NCs–PA. | ||
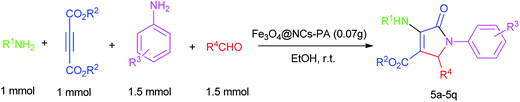
The catalytic activity of Fe3O4@NCs–PA was investigated for the synthesis of different 2-oxo dihydropyrroles derivatives via one-pot reaction of amines, aldehydes and dialkylacetylenedicarboxylates in two steps. As a model reaction, the reaction between dimethyl acetylenedicarboxylate, 4-chloroaniline and formaldehyde was investigated under various conditions (Table 1). As can be seen from Table 1, the maximum yield of methyl-1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate was obtained in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (1
1.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) by using 0.07 g catalyst at room temperature after 3.5 h (Table 1, entry 15). The previously reported protocol was modified by changing the priority of substance addition to reaction vessel. In the first step, in two separated vessels, dimethyl acetylenedicarboxylate with 4-chloroaniline (molar ratio 1
4) by using 0.07 g catalyst at room temperature after 3.5 h (Table 1, entry 15). The previously reported protocol was modified by changing the priority of substance addition to reaction vessel. In the first step, in two separated vessels, dimethyl acetylenedicarboxylate with 4-chloroaniline (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vessel A) and formaldehyde, 4-chloroaniline (molar ratio 1.5
1, vessel A) and formaldehyde, 4-chloroaniline (molar ratio 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and 0.07 g of catalyst (vessel B) were charged and mixed at room temperature. In the second step, the resulting mixture in vessel A was added to vessel B and mixed at room temperature for 3.5 h. Using the optimal reaction conditions, the scope and the versatility of this catalytic protocol were explored for the synthesis of various 2-oxo dihydropyrroles (Table 2). The obtained results indicate that the reactions can proceed well enough with a relatively wide range of aromatic amines containing electron-donating and electron-withdrawing groups (Table 2, entries 1–15). Additionally, benzylamine acts as a good reactant in this method and the corresponding products are formed in excellent yields (Table 2, entries 16, 17). As shown in Table 2, entries 14 and 15, the reactions are carried out very well with benzaldehyde and 4-methyl benzaldehyde.
1.5) and 0.07 g of catalyst (vessel B) were charged and mixed at room temperature. In the second step, the resulting mixture in vessel A was added to vessel B and mixed at room temperature for 3.5 h. Using the optimal reaction conditions, the scope and the versatility of this catalytic protocol were explored for the synthesis of various 2-oxo dihydropyrroles (Table 2). The obtained results indicate that the reactions can proceed well enough with a relatively wide range of aromatic amines containing electron-donating and electron-withdrawing groups (Table 2, entries 1–15). Additionally, benzylamine acts as a good reactant in this method and the corresponding products are formed in excellent yields (Table 2, entries 16, 17). As shown in Table 2, entries 14 and 15, the reactions are carried out very well with benzaldehyde and 4-methyl benzaldehyde.
| Entry | Conditions | Time (h) | Yieldb (%) | |||
|---|---|---|---|---|---|---|
| Catalyst (g) | Solvent | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (mmol) 4 (mmol) |
Temperature (°C) | |||
| a The molar ratios are 1 (1 mmol), 2 (1 mmol), 3 (1–1.5 mmol) and 4 (1–1.5 mmol).b Isolated yield. | ||||||
| 1 | Fe3O4@NCs–PA (0.05) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 3 | 35 |
| 2 | Fe3O4@NCs–PA (0.05) | MeOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 | 3 | 54 |
| 3 | Fe3O4@NCs–PA (0.05) | CHCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 3 | 35 |
| 4 | Fe3O4@NCs–PA (0.05) | n-Hexane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68 | 3 | 40 |
| 5 | Fe3O4@NCs–PA (0.05) | H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 3 | 15 |
| 6 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 | 3 | 56 |
| 7 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | 3 | 54 |
| 8 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
78 | 3 | 82 |
| 9 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
78 | 3 | 87 |
| 10 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 83 |
| 11 | Fe3O4 MNPs (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 38 |
| 12 | Fe3O4@NCs–PA (0.05) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 35 |
| 13 | — | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 5 | 5 |
| 14 | Fe3O4@NCs–PA (0.03) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 78 |
| 15 | Fe3O4@NCs–PA (0.07) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 88 |
| 16 | Fe3O4@NCs–PA (0.09) | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 3.5 | 88 |
| Entry | R1 | R2 | R3 | R4 | Product | Time (h) | Yieldb (%) | Mp (°C) (ref.) |
|---|---|---|---|---|---|---|---|---|
a The ratio of 1 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (mmol) 2 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (mmol) 3 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (mmol) 4 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4@NCs–PA (g) is 1 Fe3O4@NCs–PA (g) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07.b Isolated yields after recrystallization from ethanol. 0.07.b Isolated yields after recrystallization from ethanol. |
||||||||
| 1 | 4-NO2–C6H4 | Et | 4-NO2 | H | 5a | 4 | 72 | 207–208 (30) |
| 2 | 3-NO2–C6H4 | Et | 3-NO2 | H | 5b | 4 | 78 | 190–191 (30) |
| 3 | 3-NO2–C6H4 | Me | 3-NO2 | H | 5c | 4.5 | 75 | 204–206 (30) |
| 4 | 4-Br–C6H4 | Et | 4-Br | H | 5d | 3.5 | 89 | 164–166 (26) |
| 5 | 4-Br–C6H4 | Me | 4-Br | H | 5e | 3.5 | 90 | 181–183 (26) |
| 6 | 4-Cl–C6H4 | Et | 4-Cl | H | 5f | 4 | 91 | 165–167 (27) |
| 7 | 4-Cl–C6H4 | Me | 4-Cl | H | 5g | 3.5 | 88 | 172–174 (26) |
| 8 | 4-OMe–C6H4 | Et | 4-OMe | H | 5h | 3.5 | 88 | 153–154 (30) |
| 9 | 4-OMe–C6H4 | Me | 4-OMe | H | 5i | 3.5 | 85 | 160–162 (25) |
| 10 | 4-Me–C6H4 | Et | 4-Me | H | 5j | 3 | 89 | 128–129 (25) |
| 11 | 4-Me–C6H4 | Me | 4-Me | H | 5k | 3.5 | 86 | 176–177 (30) |
| 12 | 4-Et–C6H4 | Et | 4-Et | H | 5l | 3 | 85 | 102–104 (30) |
| 13 | 4-Et–C6H4 | Me | 4-Et | H | 5m | 3 | 84 | 124–125 (24) |
| 14 | 4-Cl–C6H4 | Me | 4-Cl | C6H4 | 5n | 3 | 88 | 176–177 (26) |
| 15 | 4-Cl–C6H4 | Me | 4-Cl | 4-Me–C6H4 | 5o | 4 | 87 | 150–151 (30) |
| 16 | PhCH2 | Me | 4-Br | Ph | 5p | 3.5 | 90 | 152–154 (26) |
| 17 | PhCH2 | Et | H | H | 5q | 3.5 | 89 | 139–141 (30) |
The structures of these products were characterized by physical and spectroscopic data such as mp, FT-IR, 1H NMR, 13C NMR and MS.
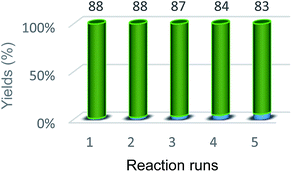
In order to investigate the reusability of the catalyst, it was separated by an external magnet following the completion of the reaction and was washed several times with methanol and ethylacetate. The separated catalyst was then dried at room temperature to be used in the subsequent run of the reaction with fresh reactants under similar conditions. It was observed that the recovered magnetite nanoparticles could be used at least five times with marginal decrease in their catalytic activity (Fig. 8). Meanwhile, FT-IR, XRD, VSM spectra and ICP analysis of recovered catalyst were identical with the original catalyst spectra that indicating no considerable leaching of catalyst in reaction medium.
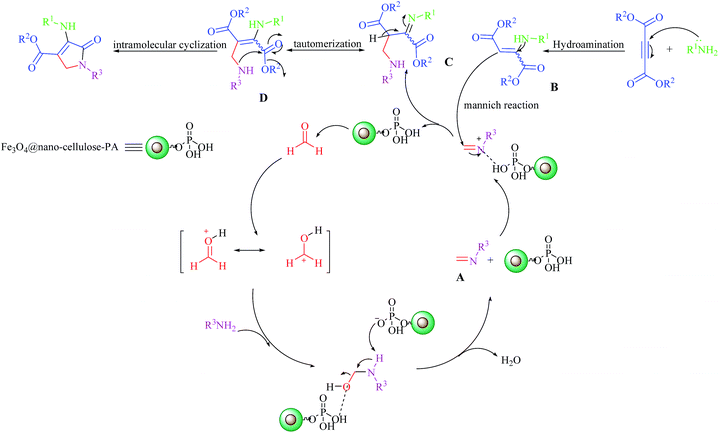
The proposed mechanism for the formation of dihydro-2-oxopyrroles is illustrated in Scheme 2.28,29 Initially, the amine reacts with formaldehyde in the presence of Fe3O4@NCs–PA to form imine A. Also, the Michael reaction between amine and dialkylacetylenedicarboxylate gives enamine B. Activated imine A undergoes a Mannich type reaction with enamine B to generate intermediate C, which converts to more stable tautomeric form D. The intramolecular cyclization in intermediate D forms the desired dihydro-2-oxypyrrole derivatives.
The comparison of data between the efficiency of Fe3O4@NCs–PA with other previously reported catalysts for the synthesis of methyl-1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate (5 g) are shown in Table 3. From environmental friendly and simplicity of protocol viewpoints, the present report is one of the successful methods and is comparable with others. Meanwhile, magnetic property of the applied catalyst in this work cause simpler workup and recovery of catalyst than each other reported procedure.
| Entry | Catalyst (mol% or g) | Solvent | Conditions | Molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Time (h) | Yield (%)ref. |
|---|---|---|---|---|---|---|
| 1 | Al(H2PO4)3 (0.1 g) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
6 | 81 (ref. 44) |
| 2 | I2 (10 mol%) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
1 | 81 (ref. 24) |
| 3 | [n-Bu4N][HSO4] (10 mol%) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
4 | 86 (ref. 27) |
| 4 | Cl3CCO2H (10 mol%) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
4 | 84 (ref. 43) |
| 5 | Cu(OAc)2·H2O (15 mol%) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
6 | 81 (ref. 26) |
| 6 | InCl3 (20 mol%) | Methanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
3 | 79 (ref. 42) |
| 7 | Nano-TiCl4/SiO2 (0.08 g) | Ethanol | 70 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 95 (ref. 29) |
| 8 | Ph3CCl (10 mol%) | Ethanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
4 | 83 (ref. 28) |
| 9 | BF3/nano-sawdust (0.08 g) | Ethanol | Reflux | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
3.5 | 92 (ref. 30) |
| 10 | Fe3O4@NCs–PA (0.07 g) | Ethanol | r.t. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
3.5 | 88present work |
Conclusions
In summary, we have developed the four-component reaction between primary amines, dialkylacetylenedicarboxylates and aldehydes for the synthesis of highly functionalize dihydro-2-oxypyrroles using Fe3O4@NCs–PA as a novel bio-based magnetically recyclable catalyst. The present methodology could produce all desired products in good to excellent yields without the need for time-consuming purification procedures. Mild reaction conditions, use of green solvent, high atom-economy and the lack of by-product are among the other advantages of this method.Experimental
General
All compounds were purchased from Merck, Aldrich and Fluka chemical companies and used without any additional purification. A refrigerated centrifuge (Appendorf Centrifuge 5417R) was used for the preparation of nano-cellulose. FT-IR spectra were run on a Bruker, Equinox 55 spectrometer. A Bruker (DRX-400 Avance) NMR was used to record the 1H-NMR and 13C-NMR spectra. Melting points were determined by a Buchi melting point B-540 B.V.CHI apparatus and were uncorrected. The X-ray diffraction (XRD) pattern was obtained by a Philips Xpert MPD diffractometer equipped with a Cu Kα anode (k = 1.54 Å) in the 2θ range from 10 to 80°. Field Emission Scanning Electron Microscopy (FESEM) was obtained on a Mira 3-XMU. XRF analysis was done with Bruker, S4 Explorer instrument. VSM measurements were performed by using a vibrating sample magnetometer (MeghnatisDaghighKavir Co. Kashan Kavir, Iran).Preparation of Fe3O4@NCs–PA
A mixture of synthesized Fe3O4@NCs (1 g) and P2O5 (2 g) was ground in a mortar at room temperature for 30 minutes to obtain a glue-like residue. After cooling the reaction mixture, 5 mL of ethanol was added and the obtained catalyst was filtered off, washed with ethanol and dried at room temperature.General procedure for synthesis of dihydro-2-oxopyrroles
As the first step, a mixture of primary amine (1 mmol), dialkylacetylenedicarboxylate (1 mmol) and absolute ethanol (3 mL) was poured into a 50 mL round-bottom flask (vessel A) and stirred for 15 min at room temperature. Then, the other flask (vessel B) was charged with a mixture of other amine (1.5 mmol), formaldehyde 37% (1.5 mmol), Fe3O4@NCs–PA (0.07 g) and absolute ethanol (3 mL). The mixture in vessel B was stirred at room temperature for 30 minutes. In the second step, the contents of vessel A were added to vessel B and the final mixture was stirred at room temperature for appropriate time (monitored by TLC, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (80
EtOAc (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20)). Then the catalyst was separated by an external magnet and the residue mixture was concentrated to obtain crystalline product.
20)). Then the catalyst was separated by an external magnet and the residue mixture was concentrated to obtain crystalline product.
Acknowledgements
The Research Council of Yazd University gratefully acknowledged for the financial support for this work.References
- J. H. Clark, Green Chem., 1999, 1, 1–8 RSC.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS PubMed.
- Y. Gu, Green Chem., 2012, 14, 2091 RSC.
- N. Isambert, M. Sanchez Duque Mdel, J. C. Plaquevent, Y. Genisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347–1357 RSC.
- P. Slobbe, E. Ruijter and R. V. A. Orru, MedChemComm, 2012, 3, 1189 RSC.
- B. Li, M. P. Lyle, G. Chen, J. Li, K. Hu, L. Tang, M. A. Alaoui-Jamali and J. Webster, Bioorg. Med. Chem., 2007, 15, 4601–4608 CrossRef CAS PubMed.
- R. Shiraki, A. Sumino, K.-i. Tadano and S. Ogawa, Tetrahedron Lett., 1995, 36, 5551–5554 CrossRef CAS.
- T. Kawasuji, M. Fuji, T. Yoshinaga, A. Sato, T. Fujiwara and R. Kiyama, Bioorg. Med. Chem., 2007, 15, 5487–5492 CrossRef CAS PubMed.
- Y. Mizushina, S. Kobayashi, K. Kuramochi, S. Nagata, F. Sugawara and K. Sakaguchi, Biochem. Biophys. Res. Commun., 2000, 273, 784–788 CrossRef CAS PubMed.
- L. Zhang, Y. Tan, N. X. Wang, Q. Y. Wu, Z. Xi and G. F. Yang, Bioorg. Med. Chem., 2010, 18, 7948–7956 CrossRef CAS PubMed.
- A. D. Borthwick, A. J. Crame, P. F. Ertl, A. M. Exall, T. M. Haley, G. J. Hart, A. M. Mason, A. M. K. Pennell, O. M. P. Singh, G. G. Weingarten and J. M. Woolven, J. Med. Chem., 2002, 45, 1–18 CrossRef CAS PubMed.
- Q. Chen, M. T. Huggins, D. A. Lightner, W. Norona and A. F. McDonagh, J. Am. Chem. Soc., 1999, 121, 9253–9264 CrossRef CAS.
- H. He, H. Y. Yang, R. Bigelis, E. H. Solum, M. Greenstein and G. T. Carter, Tetrahedron Lett., 2002, 43, 1633–1636 CrossRef CAS.
- H. Uchiro, N. Shionozaki, R. Tanaka, H. Kitano, N. Iwamura and K. Makino, Tetrahedron Lett., 2013, 54, 506–511 CrossRef CAS.
- S. Suzuki, T. Hosoe, K. Nozawa, K.-i. Kawai, T. Yaguchi and S.-I. Udagawa, J. Nat. Prod., 2000, 63, 768–772 CrossRef CAS.
- D. G. Nagle, V. J. Paul and M. Ann Roberts, Tetrahedron Lett., 1996, 37, 6263–6266 CrossRef CAS.
- H. Shiozawa and S. Takahashi, J. Antibiot., 1994, 47, 851–853 CrossRef CAS PubMed.
- W.-R. Li, S. T. Lin, N.-M. Hsu and M.-S. Chern, J. Org. Chem., 2002, 67, 4702–4706 CrossRef CAS PubMed.
- R. Shiraki, A. Sumino, K.-i. Tadano and S. Ogawa, J. Org. Chem., 1996, 61, 2845–2852 CrossRef CAS PubMed.
- R. M. Wiedhopf, E. R. Trumbull and J. R. Cole, J. Pharm. Sci., 1973, 62, 1206–1207 CrossRef CAS PubMed.
- S. Rana, M. Brown, A. Dutta, A. Bhaumik and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 1371–1379 CrossRef CAS.
- A. Khan, A. Ghosh and M. M. Khan, Tetrahedron Lett., 2012, 53, 2622–2626 CrossRef CAS.
- Q. Zhu, H. Jiang, J. Li, S. Liu, C. Xia and M. Zhang, J. Comb. Chem., 2009, 11, 685–696 CrossRef CAS PubMed.
- L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu and S. Liu, ACS Comb. Sci., 2013, 15, 183–192 CrossRef CAS PubMed.
- S. S. Sajadikhah and N. Hazeri, Res. Chem. Intermed., 2013, 40, 737–748 CrossRef.
- S. S. Sajadikhah and M. T. Maghsoodlou, RSC Adv., 2014, 4, 43454–43459 RSC.
- A. Bamoniri, B. B. F. Mirjlili and R. Tarazian, Monatsh. Chem., 2015, 146, 2107–2115 CrossRef CAS.
- B. B. F. Mirjalili and R. ZareReshquiyea, RSC Adv., 2015, 5, 15566–15571 RSC.
- R. Ghorbani-Vaghei, D. Azarifar, S. Daliran and A. R. Oveisi, RSC Adv., 2016, 6, 29182–29189 RSC.
- J.-N. Zhang, X.-H. Yang, W.-J. Guo, B. Wang and Z.-H. Zhang, Synlett, 2017, 6, 734–737 Search PubMed.
- M. M. Khan, S. Khan, S. Iqbal, Saigal and R. Yousuf, New J. Chem., 2016, 40, 7504–7512 RSC.
- R. B. Baig and R. S. Varma, Chem. Commun., 2013, 49, 752–770 RSC.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- L. M. Rossi, N. J. S. Costa, F. P. Silva and R. Wojcieszak, Green Chem., 2014, 16, 2906 RSC.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- A. Maleki and M. Kamalzare, Catal. Commun., 2014, 53, 67–71 CrossRef CAS.
- L. Edjlali, R. H. Khanamiri and J. Abolhasani, Monatsh. Chem., 2014, 146, 1339–1342 CrossRef.
- S. Azad and B. F. Mirjalili, RSC Adv., 2016, 6, 96928–96934 RSC.
- S. S. Sajadikhah, M. T. Maghsoodlou and N. Hazeri, Chin. Chem. Lett., 2014, 25, 58–60 CrossRef CAS.
- N. Hazeri, M. T. Maghsoodlou, S. Mohamadian-Souri, M. Lashkari and M. Ghashang, Iran. J. Catal., 2015, 5, 9–14 Search PubMed.
- S. S. Sajadikhah, N. Hazeri, M. T. Maghsoodlou, S. M. Habibi-Khorassani, A. Beigbabaei and A. C. Willis, J. Iran. Chem. Soc., 2013, 10, 863–871 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04101b |
| This journal is © The Royal Society of Chemistry 2017 |