Open Access Article
Open Access ArticleDesign, synthesis and evaluation of novel angiotensin II receptor 1 antagonists with antihypertensive activities†
Xiao-Lu Bao a,
Wei-Bo Zhub,
Tian-Li Shanb,
Zhuo Wub,
Rui-Jing Zhangb,
Ping-Yong Liaob,
Mei-Zhen Zhengc,
He-Sheng Tangc,
Yi-Jia Yan*c and
Zhi-Long Chen*ab
a,
Wei-Bo Zhub,
Tian-Li Shanb,
Zhuo Wub,
Rui-Jing Zhangb,
Ping-Yong Liaob,
Mei-Zhen Zhengc,
He-Sheng Tangc,
Yi-Jia Yan*c and
Zhi-Long Chen*ab
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, 2999 North Renmin Road, Shanghai 201620, P. R. China. E-mail: zlchen1967@qq.com
bDepartment of Pharmaceutical Science and Technology, College of Chemistry and Biology, Donghua University, Shanghai 201600, China
cShanghai Xianhui Pharmaceutical Co., Ltd, 2399 Guangfulin Road, Shanghai 200433, China. E-mail: shxh2006@outlook.com
First published on 17th May 2017
Abstract
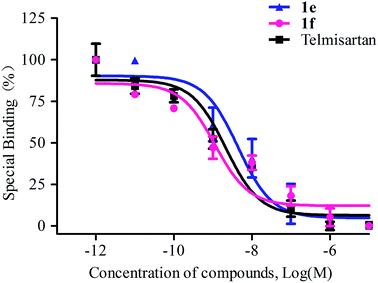
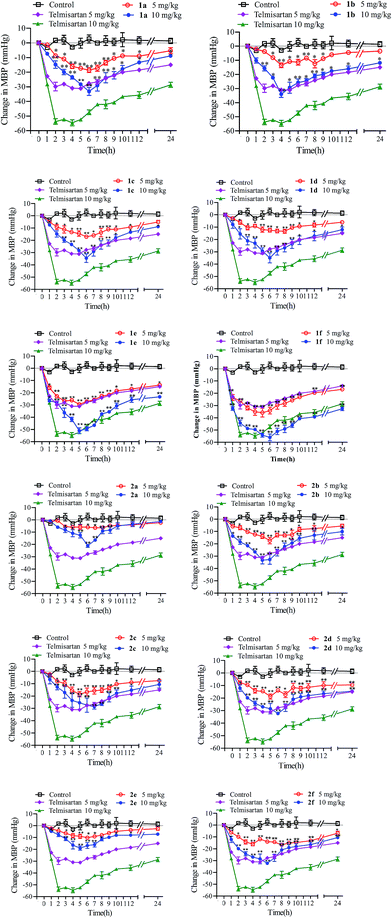
A series of novel angiotensin II receptor 1 antagonists (1a–f, 2a–f) were designed, synthesized and evaluated. Radioligand binding assays showed that all these prepared compounds displayed nanomolar affinity for angiotensin II type 1 receptor, among which compound 1f was more affinitive than telmisartan at the same order of magnitude with an IC50 value of 1.13 ± 1.68 nM. The antihypertensive effects showed that all these compounds could decrease blood pressure in a dose dependent manner on spontaneously hypertensive rats. And compound 2-(4-((2-butyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl) benzoic acid (1f), showed efficient and long-lasting effects in reducing blood pressure, with a maximal response lowered 55.98 ± 4.74 mmHg at 10 mg kg−1 and 35.82 ± 6.20 mmHg at 5 mg kg−1, the antihypertensive effect of it lasted beyond 24 h which was better than telmisartan. In the single-dose pharmacokinetic experiments, compound 1f was absorbed efficiently and metabolized smoothly in Wistar rats. The values of Cmax, Tmax, AUC0–72, MRT0–72 and T1/2 were 17.92 ± 10.85 ng mL−1, 2.60 ± 3.05 h, 252.85 ± 144.59 ng mL−1 h, 18.75 ± 0.43 h and 17.16 ± 4.24 h respectively. Compound 1f was distributed into tissues rapidly and extensively after oral administration and the level of it was the highest in the liver, followed by in the kidney, and the lowest in brain. The acute toxicity assays in ICR rats of 1f showed that it had low acute toxicity with an LD50 value of 1459.89 mg kg−1. These encouraging results make 1f an efficient, long-acting and safe antihypertensive drug candidate and deserving of further investigation.
Introduction
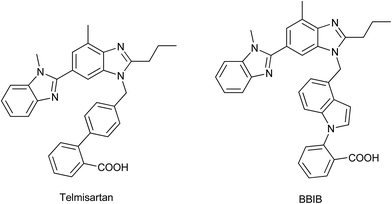
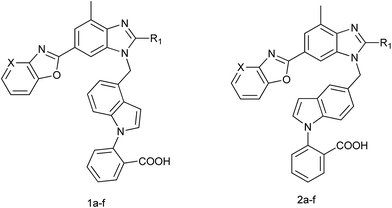
Hypertension is a growing undesired symptom and usually associated with many complications, such as coronary heart disease, stroke, myocardial infarction and renal disease. The renin-angiotensin aldosterone system (RAAS) plays a critical role in blood pressure regulation and electrolyte/fluid homeostasis.1 The octapeptide angiotensin II is the main pressor component of the RAAS and angiotensin II (Ang II) receptor antagonists are widely accepted as novel antihypertensive drugs because of the small side effects and good therapeutic profiles.2 The first nonpeptidic Ang II receptor antagonists, N-benzylimidazole-5-acetic acid derivatives, were firstly reported by the Takeda laboratories. Since then, this imidazole lead has been developed into a series of potent, selective and orally active AT1 receptor antagonists. Losartan (Fig. 1) is the most widely used drug of this series, and numerous modifications to its chemical structure have generated a large number of Ang II antagonists including valsartan, irbesartan, telmisartan, candesartan, olmesartan and so on.3,4 All these drugs contain common structural features represented by a biphenyl fragment bearing an acidic moiety (i.e., tetrazole, carboxylic group) that is linked to heterocycles by means of a methylene group.5 Telmisartan (Fig. 1) is the most recently marketed drug as an AT1 receptor antagonist. Besides, indole derivatives have been extensively studied as a pharmacophore group because of their wide potential bioactivities.6–9In our previous work, a potential new compound 2-[4-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)benzimidazole-1-yl]methyl]-1H-indol-1-yl]benzoicacid (BBIB, Fig. 1) was found reducing blood pressure more effectively than losartan and telmisartan.10 Based on the analysis of the chemical structure of telmisartan and BBIB, herein, a series of novel potent non-peptide AT1 receptor antagonists were designed and synthesized. Their pharmacological activities were evaluated, including the affinity to AT1 receptor in vitro and antihypertensive effect in vivo. The plasma pharmacokinetics, tissue distribution in Wistar rats and acute toxicity in ICRs of the most potential compound were further investigated.
Results and discussion
Chemistry
We developed a general and useful synthesis of indole derivatives 1a–f, 2a–f with a variety of substituents. In short, this procedure comprised of two coupling parts, one gave the chlorine and the other gave the heterocyclic amine. We initially synthesized the halide component which was coupled to the heterocyclic fragment to construct the target molecule. And the preparation of new compounds was performed by means of a multistep procedure described in Scheme 1 and 2.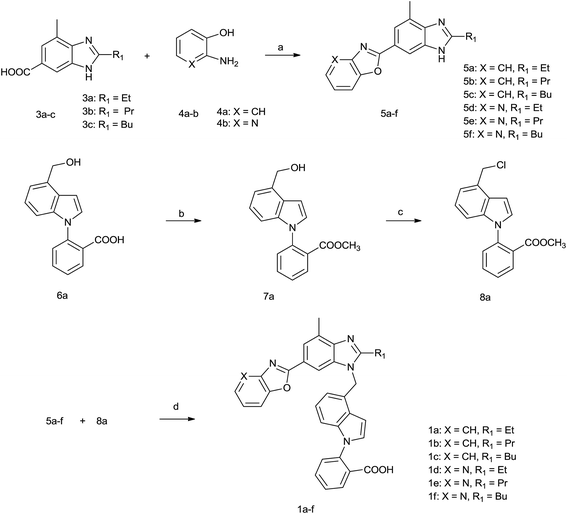 | ||
| Scheme 1 Reagents and reaction conditions: (a) PPA, 150 °C, 12 h, 40–51%; (b) CH3I, KHCO3, DMF, 5 h, 95%; (c) HCl–EtOH, DCM, 2 h, 82%; (d) NaH, DMF, 2 h and then NaOH, H2O, 4 h, 50–60%. | ||
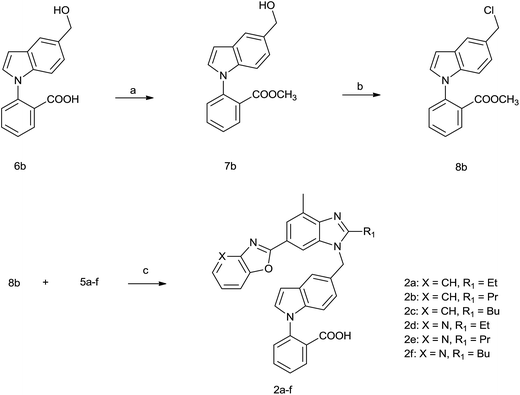 | ||
| Scheme 2 Reagents and reaction conditions: (a) CH3I, KHCO3, DMF, 5 h, 81%; (b) HCl–EtOH, DCM, 2 h, 82%; (c) NaH, DMF, 2 h and then NaOH, H2O, 40–49%. | ||
The synthesis of 8a, 5a–f and 1a–f were outlined in Scheme 1. 4-Chlorinemtehyl-N-phenyl indole compound (8a) was obtained by chlorinating the ester of ethyl benzoate compound (7a) which was achieved through esterification of benzoic acid compound (6a). 6-Substituted benzimidazole compounds (5a–f) were obtained by the condensation reaction of 6-benzimidazole carboxylic acid compounds (3a–c) with different ortho amino phenol compounds (4a–b). They were reacted with 8a to get the designed carboxylic acid compounds (1a–f).
The synthesis of 5-chlorinemethyl-N-phenyl indole compound (8b) was similar to that of 8a which was outlined in Scheme 2. Compound 8b reacted with 5a–f to get the designed carboxylic acid compounds (2a–f).
Biological evaluation
| Compounds | X | R1 | IC50 ± SEM (nM) | Ki ± SEM (nM) |
|---|---|---|---|---|
| 1a | CH | Et | 5.61 ± 6.45 | 4.06 ± 4.60 |
| 1b | CH | Pr | 5.86 ± 7.49 | 4.04 ± 5.43 |
| 1c | CH | Bu | 6.08 ± 5.50 | 4.40 ± 3.99 |
| 1d | N | Et | 14.25 ± 6.34 | 10.32 ± 4.60 |
| 1e | N | Pr | 4.37 ± 1.63 | 3.17 ± 1.18 |
| 1f | N | Bu | 1.13 ± 1.68 | 0.82 ± 0.12 |
| 2a | CH | Et | 5.77 ± 1.65 | 4.18 ± 1.20 |
| 2b | CH | Pr | 9.07 ± 4.67 | 6.57 ± 3.38 |
| 2c | CH | Bu | 6.40 ± 6.67 | 4.63 ± 4.83 |
| 2d | N | Et | 10.24 ± 5.27 | 7.42 ± 3.82 |
| 2e | N | Pr | 5.20 ± 6.70 | 3.77 ± 4.85 |
| 2f | N | Bu | 12.63 ± 6.52 | 9.15 ± 5.23 |
| Telmisartan | 2.12 ± 0.14 | 1.53 ± 0.10 | ||
| Cmax (ng mL−1) | Tmax (h) | T1/2 (h) | AUC(0–72) (ng mL−1 h) | AUC(0–∞) (ng mL−1 h) | MRT(0–72) (h) | MRT(0–∞) (h) |
|---|---|---|---|---|---|---|
| 17.92 ± 10.85 | 2.60 ± 3.05 | 17.16 ± 4.24 | 252.85 ± 144.59 | 268.28 ± 155.94 | 18.75 ± 0.43 | 29.66 ± 5.21 |
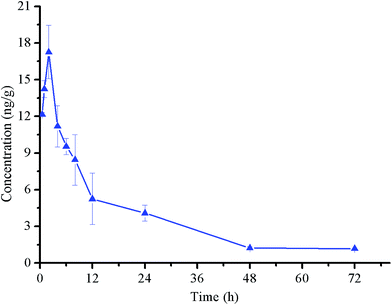 | ||
| Fig. 4 Observed plasma concentrations of 1f in rats after signal oral administration at 10 mg kg−1. Data were average values of 8 experiments (mean ± SD). | ||
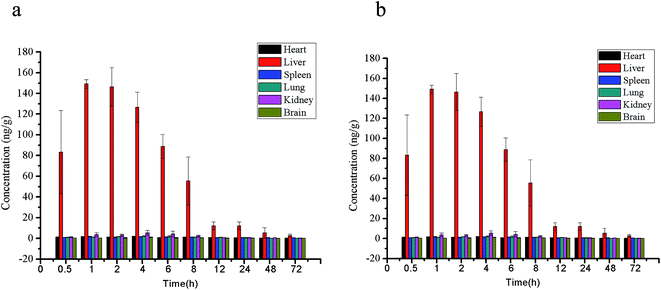
The compound levels in heart, liver, spleen, kidney and brain of Wistar rats at 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 72 h post oral administration of compound 1f at 10 mg kg−1 were shown in Fig. 5 and the pharmacokinetic parameters were shown in Table 3. It was found that 1f was distributed extensively in rat tissues. And it was accumulated especially in liver which exhibited the highest concentration of 152.87 ± 10.10 ng g−1 followed by kidney of 5.84 ± 2.14 ng g−1 and the lowest in brain of 1.31 ± 0.59 ng g−1. These results indicate that liver and renal transporters might play an important role in the disposition of 1f. Since the highest concentration of telmisartan after oral administration was also detected in liver and followed by in kidney,11 the distribution of 1f appeared to have some similarity to telmisartan. Information on the likelihood of crossing the blood brain barrier (BBB) is important for targets related to central nervous system (CNS). 1f was detectable in brain which means it could penetrate the BBB. Telmisartan was also able to cross the BBB after oral administration, but other sartans such as candesartan, losartan, and valsartan were poorly penetrated to BBB.12 Thus, 1f may have effect on dilating cerebral blood vessels, improving cerebral circulation and hence could be useful for the potential treatment of ischemic cerebrovascular disease.13,14
| Tissue | Cmax (ng g−1) | Tmax (h) | T1/2 (h) | AUC(0–72) (ng g−1 h) | AUC(0–∞) (ng g−1 h) | AUCtissue/AUCplasma |
|---|---|---|---|---|---|---|
| Heart | 2.26 ± 0.16 | 3.25 ± 1.50 | 16.87 ± 7.91 | 31.16 ± 8.34 | 33.51 ± 6.37 | 0.12 |
| Liver | 152.87 ± 10.10 | 1.33 ± 0.58 | 22.81 ± 3.60 | 1444.65 ± 273.97 | 1541.40 ± 241.08 | 5.71 |
| Spleen | 1.92 ± 0.23 | 2.00 ± 1.73 | 23.12 ± 1.23 | 25.70 ± 7.38 | 29.68 ± 8.33 | 0.10 |
| Lung | 2.12 ± 0.61 | 4.00 ± 2.00 | 13.02 ± 3.59 | 37.14 ± 8.74 | 38.05 ± 8.43 | 0.15 |
| Kidney | 5.84 ± 2.14 | 3.00 ± 1.73 | 19.15 ± 3.12 | 66.78 ± 21.53 | 71.61 ± 21.07 | 0.26 |
| Brain | 1.31 ± 0.59 | 3.33 ± 1.16 | 17.83 ± 1.35 | 15.47 ± 7.94 | 16.40 ± 8.87 | 0.06 |
These results also showed that compound 1f was absorbed and distributed efficiently and metabolized gradually in plasma and in tissues of Wistar rats.
| Dose (mg kg−1) | Log(dose) | Mortality (%) | LD50 (mg kg−1) and 95% confidence interval (mg kg−1) |
|---|---|---|---|
| 632.81 | 2.80 | 0 | 1459.89 (1246.98–1717.49) |
| 843.75 | 2.93 | 10 | |
| 1125.00 | 3.05 | 20 | |
| 1500.00 | 3.18 | 40 | |
| 2000.00 | 3.30 | 90 | |
| 2666.67 | 3.43 | 100 |
Conclusions
A series of novel 6-substituted benzimidazole with 1,4-disubstituted or 1,5-disubstituted indole derivatives were designed, synthesized and evaluated. The Ang II antagonistic activities in vitro and antihypertensive activities in vivo of them were investigated to assess their antihypertensive effect. All the prepared compounds displayed nanomolar affinity for angiotensin II type 1 receptor and could course obvious reduction in blood pressure. Among these compounds, 1f showed more affinitive to AT1 receptor with an IC50 value of 1.13 ± 1.68 nM respectively than telmisartan (IC50 = 2.12 ± 0.14 nM). Also, 1f had significant antihypertensive effects in SHRs with the maximal response of 55.98 ± 4.74 mmHg at 10 mg kg−1 and 35.82 ± 6.20 mmHg at 5 mg kg−1, the significant antihypertensive effect of it lasted beyond 24 h which was better than telmisartan. The pharmacokinetic experiments showed that 1f could be absorbed efficiently and metabolized smoothly in Wistar rats after oral administration. With single-dose 1f at 10 mg kg−1, the Cmax, Tmax, AUC0–72, and T1/2 value were 17.92 ± 10.85 ng mL−1, 2.60 ± 3.05 h, 252.85 ± 144.59 ng mL−1 h and 17.16 ± 4.24 h respectively. It was well distributed in tissues including in brain which suggested that 1f could cross the blood–brain barrier (BBB) and might play important role in the control of central nervous system blood pressure. The acute toxicity assay of 1f showed that it had low acute toxicity with no significant changes in the weight and no obvious untoward reactions. The LD50 value of it was 1459.89 mg kg−1. These encouraging results make 1f an effective and durable anti-hypertension drug candidate deserving for further investigation.Material and methods
Chemistry
All chemical reagents were purchased from commercial suppliers and were used without further purification. Yields of the purified products were not optimized. Melting points (MP) were measured on an electro thermal melting point apparatus and were not corrected. 1H NMR spectra were measured on a Bruker 400 MHz spectrometer using TMS (Me4Si) as internal standard. ESI-MS spectra were recorded on a Micromass triple quadrupole mass spectrometer. Column chromatography was performed using silica gel H (300–400 meshes).Methyl 2-ethyl-4-methyl-benzo[d]imidazole-6-carboxylate (3a). The above general procedure was followed by using propionyl chloride. The residue was purified by column chromatography to give the product as yellow oil. Yield: 50%. 1H NMR (400 MHz, CDCl3): δ ppm 8.10 (s, 1H), 7.96 (s, 1H), 3.96 (s, 3H), 2.70 (s, 3H), 2.63 (s, 3H). MS (ESI) m/z: 205.1 [M + H]+.
Methyl 2-propyl-4-methyl-1H-benzo[d]imidazole-6-carboxylate (3b). The above general procedure was followed by using butyryl chloride. The residue was purified by column chromatography to give the product as yellow oil. Yield: 52%. 1H NMR (400 MHz, CDCl3): δ ppm 8.15 (s, 1H), 7.88 (s, 1H), 3.93 (s, 3H), 2.81 (q, 2H), 2.63 (s, 3H), 1.45 (t, 3H). MS (ESI) m/z: 219.1 [M + H]+.
Methyl 2-butyl-4-methyl-1H-benzo[d]imidazole-6-carboxylate (3c). The above general procedure was followed by using valeryl chloride. The residue was purified by column chromatography to give the product as yellow oil. Yield: 56%. 1H NMR (400 MHz, CDCl3): δ ppm 8.15 (s, 1H), 7.88 (s, 1H), 3.93 (s, 3H), 2.81 (q, 2H), 2.63 (s, 3H), 1.45 (t, 3H). MS (ESI) m/z: 232.1 [M + H]+.
2-Ethyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole (5a). The filter solution was concentrated in vacuo and the residue was purified by column chromatography to give brown solid compound 5a. Yield: 50%. Mp: 190–193 °C. 1H NMR (400 MHz, CDCl3): δ ppm 8.21 (s, 1H), 7.87 (s, 1H), 7.78 (d, 1H), 7.48–7.42 (m, 1H), 7.23–7.18 (m, 1H), 6.85–6.79 (m, 1H), 2.93 (q, 2H), 2.67 (s, 3H), 1.38 (t, 3H). MS (ESI) m/z: 278.2 [M + H]+.
2-Propyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole (5b). The synthesis of compound 5b was similar to that of compound 5a, obtaining as yellow solid. Mp: 191–193 °C. Yield: 54%. 1H NMR (400 MHz, CDCl3): δ ppm 8.22 (s, 1H), 7.89 (s, 1H), 7.78 (d, 1H), 7.48–7.45 (m, 1H), 7.23–7.18 (m, 1H), 6.85–6.79 (m, 1H), 2.88 (t, 2H), 2.67 (s, 3H), 1.88–1.79 (m, 2H), 0.99 (t, 3H). MS (ESI) m/z: 292.2 [M + H]+.
2-Butyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole (5c). The synthesis of compound 5c was similar to that of compound 5a, obtaining as yellow solid. Yield: 48%. Mp: 192–195 °C. 1H NMR (400 MHz, CDCl3): δ ppm 8.22 (s, 1H), 7.89 (s, 1H), 7.78 (d, 1H), 7.48–7.44 (m, 1H), 7.23–7.17 (m, 1H), 6.85–6.79 (m, 1H), 2.88 (t, 2H), 2.67 (s, 3H), 1.83–1.77 (m, 2H), 1.40–1.35 (m, 2H), 0.90 (t, 3H). MS (ESI) m/z: 306.2 [M + H]+.
2-Ethyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole (5d). The synthesis of compound 5d was similar to that of compound 5a, obtaining as yellow solid. Yield: 48%. Mp: 193–195 °C. 1H NMR (400 MHz, CDCl3): δ ppm 8.82 (s, 1H), 8.55 (d, J = 4.9 Hz, 1H), 8.03 (s, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.37 (dd, J = 7.7, 5.4 Hz, 1H), 3.20 (q, J = 7.6 Hz, 2H), 2.74 (s, 3H), 1.62 (t, J = 7.4 Hz, 3H). MS (ESI) m/z: 279.3 [M + H]+.
2-Propyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole (5e). The synthesis of compound 5e was similar to that of compound 5a, obtaining as yellow solid. Yield: 40%. Mp: 194–197 °C. 1H NMR (400 MHz, CDCl3): δ ppm 8.82 (s, 1H), 8.55 (d, J = 4.9 Hz, 1H), 8.03 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.37 (dd, J = 7.7, 5.4 Hz, 1H), 3.10 (t, J = 7.7 Hz, 2H), 2.76 (s, 3H), 2.06–1.96 (m, 2H), 1.12 (t, J = 7.3 Hz, 3H). MS (ESI) m/z: 293.3 [M + H]+.
2-Butyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole (5f). The synthesis of compound 5f was similar to that of compound 5a, obtaining as yellow solid. Yield: 51%. Mp: 195–198 °C. 1H NMR (400 MHz, CDCl3): δ ppm 8.81 (s, 1H), 8.55 (d, J = 4.7 Hz, 1H), 8.03 (s, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.37 (dd, J = 7.7, 5.4 Hz, 1H), 3.15 (t, J = 7.8 Hz, 2H), 2.76 (s, 3H), 2.04–1.94 (m, 2H), 1.64–1.56 (m, 2H), 1.12 (t, J = 7.4 Hz, 3H). MS (ESI) m/z: 307.3 [M + H]+.
Methyl 2-(4-(hydroxymethyl)-1H-indol-1-yl)benzoate (7a). The residue was purified by column chromatography to give the product as yellow oil. Yield: 95%. 1H NMR (400 MHz, CDCl3): δ ppm 8.02 (dd, 1H), 7.72–7.68 (m, 1H), 7.57–7.51 (m, 2H), 7.31 (d, 1H), 7.21–7.11 (m, 3H), 6.86 (d, 1H), 5.77 (s, 2H), 3.47 (s, 3H). MS (ESI) m/z: 282.1 [M + H]+.
Methyl 2-(5-(hydroxymethyl)-1H-indol-1-yl)benzoate (7b). The synthesis of compound 7b was similar with 7a, obtaining as yellow oil. Yield: 81%. 1H NMR (400 MHz, CDCl3): δ ppm 7.88 (d, 1H), 7.78 (t, 1H), 7.72 (s, 1H), 7.64 (d, 1H), 7.54 (t, 1H), 7.45 (d, 1H), 7.36–7.29 (m, 2H), 6.79 (d, 1H), 4.82 (s, 2H), 3.51 (s, 3H). MS (ESI) m/z: 282.1 [M + H]+.
Methyl 2-(5-(chloromethyl)-1H-indol-1-yl)benzoate (8a). The above general procedure get brown solid or brown oil compound (8a). Yield: 82%. 1H NMR (400 MHz, CDCl3): δ ppm 8.03 (dd, 1H), 7.72–7.68 (m, 1H), 7.57–7.51 (m, 2H), 7.31 (d, 1H), 7.21–7.11 (m, 3H), 6.86 (d, 1H), 4.96 (s, 2H), 3.48 (s, 3H). MS (ESI) m/z: 302.1 [M + H]+.
Methyl 2-(5-(chloromethyl)-1H-indol-1-yl)benzoate (8b). The synthesis of compound 8b was similar to that of compound 8a, obtaining brown yellow oil product. Yield: 82%. 1H NMR (400 MHz, CDCl3): δ ppm 7.88 (d, 1H), 7.78 (t, 1H), 7.72 (s, 1H), 7.64 (d, 1H), 7.54 (t, 1H), 7.45 (d, 1H), 7.36–7.29 (m, 2H), 6.79 (d, 1H), 4.64 (s, 2H), 3.48 (s, 3H). MS (ESI) m/z: 302.1 [M + H]+.
2-(4-((2-Ethyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (1a). The above general procedure was followed by purified by column chromatography to give the product 1a as white solid. Yield: 60%. Mp: 261–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.93 (s, 1H), 8.17 (s, 1H), 7.93 (m, 2H), 7.80–7.69 (m, 3H), 7.64–7.48 (m, 3H), 7.39–7.37 (m, 2H), 7.04–7.00 (m, 2H), 6.73 (d, J = 3.2 Hz, 1H), 6.33 (d, J = 5.8 Hz, 1H), 5.95 (s, 2H), 2.91 (q, J = 7.5 Hz, 2H), 2.69 (s, 3H), 1.31 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.7, 163.9, 159.2, 150.6, 145.0, 142.2, 137.7, 137.2, 136.0, 132.9, 131.1, 130.6, 130.6, 129.5, 128.9, 128.9, 128.5, 126.5, 125.4, 125.2, 122.5, 121.5, 120.1, 119.8, 116.6, 111.1, 109.8, 107.9, 101.1, 45.2, 20.8, 16.9, 12.0. HRMS (ESI): m/z calcd for C33H27N4O3 [M + H]+: 527.2083; found 527.2080.
2-(4-((2-Propyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (1b). The synthesis of compound 1b was similar to that of compound 1a, obtaining as white solid. Yield: 58%. Mp: 262–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.89 (s, 1H), 8.14 (s, 1H), 7.93 (m, 2H), 7.74–7.70 (m, 3H), 7.66–7.45 (m, 3H), 7.44–7.32 (m, 2H), 7.05–6.98 (m, 2H), 6.75 (s, 1H), 6.33 (d, J = 6.3 Hz, 1H), 5.95 (s, 2H), 2.88 (t, J = 7.5 Hz, 2H), 2.68 (s, 3H), 1.85–1.71 (m, 2H), 0.96 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.7, 163.9, 158.2, 150.6, 145.0, 142.2, 137.6, 137.2, 135.9, 132.8, 131.2, 131.0, 130.6, 129.5, 128.9, 128.8, 128.4, 126.5, 125.4, 125.2, 122.5, 121.5, 120.1, 119.8, 116.6, 111.1, 109.8, 108.0, 101.1, 45.2, 29.3, 20.9, 16.9, 14.3. HRMS (ESI): m/z calcd for C34H29N4O3 [M + H]+: 541.2240; found 541.2234.
2-(4-((2-Butyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (1c). The synthesis of compound 1c was similar to that of compound 1a, obtaining as white solid. Yield: 56%. Mp: 264–266 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.90 (s, 1H), 8.15 (s, 1H), 7.98–7.88 (m, 2H), 7.79–7.68 (m, 3H), 7.62–7.52 (m, 3H), 7.43–7.33 (m, 2H), 7.09–6.95 (m, 2H), 6.74 (d, J = 3.3 Hz, 1H), 6.34 (dd, J = 5.6, 2.3 Hz, 1H), 5.95 (s, 2H), 2.89 (t, 2H), 2.67 (s, 3H), 1.76–1.68 (m, 2H), 1.41–1.31 (m, 2H), 0.85 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.6, 163.9, 158.3, 150.6, 145.0, 142.2, 137.8, 137.2, 135.9, 133.2, 131.2, 130.5, 130.3, 129.5, 129.0, 129.0, 128.5, 126.5, 125.4, 125.2, 122.5, 121.5, 120.1, 119.8, 116.7, 111.1, 109.8, 108.0, 101.2, 45.2, 29.6, 27.1, 22.4, 16.9, 14.2. HRMS (ESI): m/z calcd for C35H31N4O3 [M + H]+: 555.2396; found 555.2392.
2-(4-((2-Ethyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (1d). The synthesis of compound 1d was similar to that of compound 1a, obtaining as white solid. Yield: 61%. Mp: 260–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.97 (s, 1H), 8.50 (m, 1H), 8.24 (s, 1H), 8.17 (m, 1H), 7.97 (s, 1H), 7.92 (m, 1H), 7.73 (m, 1H), 7.55 (m, 3H), 7.45–7.36 (m, 1H), 7.02 (m, 2H), 6.72 (d, J = 3.3 Hz, 1H), 6.39–6.29 (m, 1H), 5.96 (s, 2H), 2.92 (q, J = 7.5 Hz, 2H), 2.70 (s, 3H), 1.31 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.7, 166.5, 159.7, 156.4, 146.7, 145.6, 143.2, 137.6, 137.2, 136.1, 132.9, 131.1, 130.6, 130.6, 129.7, 128.9, 128.8, 128.5, 126.5, 122.5, 121.8, 120.7, 119.5, 119.1, 116.7, 109.8, 108.5, 101.1, 45.2, 20.9, 16.9, 12.0. HRMS (ESI): m/z calcd for C32H26N5O3 [M + H]+: 528.2036; found 528.2031.
2-(4-((2-Propyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid(1e). The synthesis of compound 1e was similar to that of compound 1a, obtaining as white solid. Yield: 50%. Mp: 262–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.97 (s, 1H), 8.50 (dd, J = 4.9, 1.3 Hz, 1H), 8.22–8.15 (m, 2H), 7.96 (s, 1H), 7.89 (m, 1H), 7.69 (m, 1H), 7.58–7.49 (m, 3H), 7.41 (m, 1H), 7.05–7.00 (m, 2H), 6.73 (d, J = 3.3 Hz, 1H), 6.32 (d, J = 6.5 Hz, 1H), 5.96 (s, 2H), 2.89 (t, J = 7.6 Hz, 2H), 2.69 (s, 3H), 1.79–1.74 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.6, 166.5, 158.6, 156.4, 146.7, 145.7, 143.2, 137.5, 137.2, 135.9, 132.5, 130.9, 130.6, 130.6, 129.6, 128.9, 128.8, 128.4, 126.5, 122.4, 121.8, 120.7, 119.5, 119.1, 116.6, 109.9, 108.6, 101.0, 45.3, 29.3, 20.9, 16.9, 14.3. HRMS (ESI): m/z calcd for C33H28N5O3 [M + H]+: 542.2192; found 542.2186.
2-(4-((2-Butyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (1f). The synthesis of compound 1f was similar to that of compound 1a, obtaining as white solid. Yield: 54%. Mp: 264–266 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 13.00 (s, 1H), 8.50 (m, 1H), 8.23 (s, 1H), 8.17 (m, 1H), 7.96 (s, 1H), 7.91 (m, 1H), 7.71 (m, 1H), 7.63–7.47 (m, 3H), 7.46–7.37 (m, 1H), 7.05–7.00 (m, 2H), 6.73 (d, J = 3.2 Hz, 1H), 6.35 (d, J = 6.7 Hz, 1H), 5.96 (s, 2H), 2.90 (t, 2H), 2.69 (s, 3H), 1.72 (m, 2H), 1.36 (m, 2H), 0.85 (t, J = 4.5 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.7, 166.5, 158.8, 156.4, 146.7, 145.6, 143.2, 137.5, 137.2, 135.9, 132.9, 131.0, 130.6, 130.6, 129.6, 128.8, 128.8, 128.4, 126.5, 122.5, 121.8, 120.7, 119.4, 119.1, 116.7, 109.9, 108.6, 101.1, 45.3, 29.6, 27.1, 22.4, 16.9, 14.2. HRMS (ESI): m/z calcd for C34H30N5O3 [M + H]+: 556.2349; found 556.2345.
2-(5-((2-Ethyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2a). The synthesis of compound 2a was similar to that of compound 1a, obtaining as white solid. Yield: 46%. Mp: 261–264 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.84 (s, 1H), 8.20 (s, 1H), 7.91 (d, J = 5.6 Hz, 2H), 7.78–7.73 (m, 2H), 7.70 (d, J = 7.6 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 3.2 Hz, 1H), 7.42–7.35 (m, 2H), 7.30 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 6.99 (d, J = 8.6 Hz, 1H), 6.59 (d, J = 3.0 Hz, 1H), 5.70 (s, 2H), 2.97 (q, J = 7.4 Hz, 2H), 2.66 (s, 3H), 1.35 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.1, 163.5, 158.5, 150.2, 144.6, 141.8, 137.3, 136.1, 135.3, 132.6, 130.7, 130.5, 129.0, 128.6, 128.4, 128.3, 127.9, 125.0, 124.7, 121.0, 121.0, 120.6, 119.6, 119.4, 118.1, 110.7, 110.3, 107.5, 102.8, 46.5, 20.4, 16.5, 11.7. HRMS (ESI): m/z calcd for C33H27N4O3 [M + H]+: 527.2083; found 527.2078.
2-(5-((2-Propyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2b). The synthesis of compound 2b was similar to that of compound 1a, obtaining as white solid. Yield: 46%. Mp: 262–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.91 (s, 1H), 8.18 (s, 1H), 7.94–7.84 (m, 2H), 7.79–7.72 (m, 2H), 7.68 (m, 1H), 7.55 (m, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.43 (d, J = 3.2 Hz, 1H), 7.41–7.35 (m, 2H), 7.28 (s, 1H), 7.09 (d, J = 8.5 Hz, 1H), 6.98 (d, J = 8.6 Hz, 1H), 6.57 (d, J = 3.2 Hz, 1H), 5.71 (s, 2H), 2.93 (t, J = 7.6 Hz, 2H), 2.65 (s, 3H), 1.93–1.74 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.8, 163.9, 157.9, 150.6, 145.1, 142.2, 137.6, 136.5, 135.6, 132.8, 131.0, 130.9, 129.4, 129.0, 128.7, 128.7, 128.3, 125.4, 125.2, 121.5, 121.5, 120.9, 120.0, 119.8, 118.4, 111.2, 110.8, 108.0, 103.1, 46.9, 29.3, 21.0, 16.9, 14.3. HRMS (ESI): m/z calcd for C34H29N4O3 [M + H]+: 541.2240; found 541.2239.
2-(5-((2-Butyl-4-methyl-6-(benzoxazol-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2c). The synthesis of compound 2c was similar to that of compound 1a, obtaining as white solid. Yield: 47%. Mp: 263–266 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.97 (s, 1H), 8.18 (s, 1H), 7.90 (s, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.80–7.71 (m, 2H), 7.66 (m, 1H), 7.53 (m, 1H), 7.44 (m, 2H), 7.42–7.33 (m, 2H), 7.28 (s, 1H), 7.10 (d, J = 8.5 Hz, 1H), 6.97 (d, J = 8.6 Hz, 1H), 6.56 (d, J = 3.2 Hz, 1H), 5.70 (s, 2H), 2.94 (t, 2H), 2.65 (s, 3H), 1.82–1.68 (m, 2H), 1.44–1.37 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.8, 163.9, 158.1, 150.7, 145.1, 142.2, 137.5, 136.5, 135.7, 132.5, 131.0, 130.9, 129.4, 129.0, 128.7, 128.6, 128.2, 125.4, 125.2, 121.5, 121.4, 120.9, 120.0, 119.8, 118.4, 111.2, 110.8, 108.0, 103.1, 47.0, 29.7, 27.1, 22.4, 16.9, 14.2. HRMS (ESI): m/z calcd for C35H31N4O3 [M + H]+: 555.2396; found 555.2396.
2-(5-((2-Ethyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2d). The synthesis of compound 2d was similar to that of compound 1a, obtaining as white solid. Yield: 44%. Mp: 261–264 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.82 (s, 1H), 8.51 (d, J = 4.8 Hz, 1H), 8.27 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.95–7.88 (m, 2H), 7.70 (m, 1H), 7.56 (m, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.45–7.39 (m, 2H), 7.31 (s, 1H), 7.08 (d, J = 8.5 Hz, 1H), 7.00 (d, J = 8.4 Hz, 1H), 6.59 (d, J = 3.1 Hz, 1H), 5.71 (s, 2H), 2.98 (q, J = 7.5 Hz, 2H), 2.67 (s, 3H), 1.35 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.6, 166.5, 159.4, 156.4, 146.7, 145.6, 143.2, 137.8, 136.5, 135.8, 133.1, 131.1, 130.91, 129.6, 129.0, 128.8, 128.7, 128.4, 128.4, 121.7, 121.1, 120.7, 119.4, 119.1, 118.6, 110.7, 108.6, 103.2, 47.0, 20.9, 16.9, 12.1. HRMS (ESI): m/z calcd for C32H26N5O3 [M + H]+: 528.2036; found 528.2038.
2-(5-((2-Propyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2e). The synthesis of compound 2e was similar to that of compound 1a, obtaining as white solid. Yield: 45%. Mp: 263–265 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.91 (s, 1H), 8.50 (d, J = 4.8 Hz, 1H), 8.24 (s, 1H), 8.17 (d, J = 8.1 Hz, 1H), 7.94 (s, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.66 (m, 1H), 7.53 (m, 1H), 7.47–7.38 (m, 3H), 7.29 (s, 1H), 7.10 (d, J = 8.5 Hz, 1H), 6.99 (d, J = 8.5 Hz, 1H), 6.57 (d, J = 3.0 Hz, 1H), 5.72 (s, 2H), 2.94 (t, J = 7.6 Hz, 2H), 2.66 (s, 3H), 1.88–1.74 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.4, 166.1, 157.9, 155.9, 146.3, 145.2, 142.7, 137.0, 136.0, 135.2, 132.1, 130.5, 130.5, 129.1, 128.6, 128.6, 128.2, 128.1, 127.8, 121.3, 120.5, 120.2, 119.0, 118.6, 118.0, 110.4, 108.2, 102.6, 46.6, 28.8, 20.5, 16.4, 13.9. HRMS (ESI): m/z calcd for C33H28N5O3 [M + H]+: 542.2192; found 542.2190.
2-(5-((2-Butyl-4-methyl-6-(oxazolo[4,5-b]pyridine-2-yl)benzimidazole-1-yl)methyl)-1H-indol-1-yl)benzoic acid (2f). The synthesis of compound 2f was similar to that of compound 1a, obtaining as white solid. Yield: 49%. Mp: 264–266 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 12.89 (s, 1H), 8.51 (m, 1H), 8.26 (s, 1H), 8.18 (m, 1H), 7.94 (s, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.67 (m, 1H), 7.56–7.52 (m, 1H), 7.48–7.39 (m, 3H), 7.29 (s, 1H), 7.10 (m, 1H), 6.99 (d, J = 8.6 Hz, 1H), 6.57 (d, J = 3.2 Hz, 1H), 5.72 (s, 2H), 2.96 (t, 2H), 2.66 (s, 3H), 1.88–1.71 (m, 2H), 1.46–1.36 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO): δ ppm 167.3, 166.1, 158.0, 155.9, 146.3, 145.2, 142.7, 137.1, 136.0, 135.2, 132.2, 130.5, 130.5, 129.1, 128.6, 128.6, 128.2, 128.1, 127.8, 121.3, 120.5, 120.2, 118.9, 118.6, 118.0, 110.4, 108.2, 102.7, 46.6, 29.2, 26.7, 22.0, 16.4, 13.7. HRMS (ESI): m/z calcd for C34H30N5O3 [M + H]+: 556.2349; found 556.2349.
Biological evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B (v/v) were 55
B (v/v) were 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 (1–10 min), 30
45 (1–10 min), 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (10–11 min), 55
70 (10–11 min), 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 (11–18 min). LC-MS spectral data of each single sample were collected, Microsoft Excel program, Origin 8.0 and DAS 2.0 software were used to calculate the pharmacokinetic parameters.
45 (11–18 min). LC-MS spectral data of each single sample were collected, Microsoft Excel program, Origin 8.0 and DAS 2.0 software were used to calculate the pharmacokinetic parameters.8 male Wistar rats (180–200 g, Shanghai Slac Laboratory Animal Company, Shanghai, China) were used and administrated with 1f at dose of 10 mg kg−1. Blood samples were collected from each rat by retro-orbital puncture at a predetermined time interval of pre-dose, 0.5, 1, 2, 4, 6, 8, 12, 24, 48 and 72 h into the polypropylene microfuge tubes containing heparin. Plasma was separated by centrifuging the blood samples at 4000 × g at 4 °C for 10 min. 200 μL plasma samples were collected and followed by 100 μL of internal standard. Acetonitrile (200 μL) was added to precipitate proteins and the sample was vortexed for 1 min. Precipitated proteins were separated by centrifugation for 5 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g at 4 °C. The supernatant was filtered by needle filter of 0.22 μm and the resulting 20 μL filtrate was taken to analyze in LC-MS. Linearity for 1f was tested by extracting plasma standards spiked at nominal concentrations of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 100.0 μg mL−1. The calibration line was generated by least squares linear regression of the peak height ratio (PHR) of analyte/internal standard against nominal concentration with a weighting of concentration−2.18,19
000 × g at 4 °C. The supernatant was filtered by needle filter of 0.22 μm and the resulting 20 μL filtrate was taken to analyze in LC-MS. Linearity for 1f was tested by extracting plasma standards spiked at nominal concentrations of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 100.0 μg mL−1. The calibration line was generated by least squares linear regression of the peak height ratio (PHR) of analyte/internal standard against nominal concentration with a weighting of concentration−2.18,19
66 male Wistar rats (180–200 g, Shanghai Slac Laboratory Animal Company, Shanghai, China) were used to investigate the tissue distribution of compound 1f. Animals were orally administrated with 1f at 10 mg kg−1. Heart, liver, spleen, lung, kidney and brain samples were collected at 0 (prior to dosing), 0.5, 1, 2, 4, 6, 8, 12, 24, 48 and 72 h post-dosing (n = 6). 0.25 g samples from different tissues were obtained after being rinsed with normal saline solution and blotted with filter paper. The samples were grinded into tissue fluid (0.25 g/1 mL normal saline solution) with High-speed Homogenizer (PRO200, Bio-gen series, USA). The tissue fluid was added with 4 mL methanol and vortexed for 1 min and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min. 2 mL supernatant was obtained and dried in gentle stream of nitrogen flow at 50 °C. The residue was spun for 2 min after being reconstituted with 100 μL methanol, and was centrifuged at 12
000 × g for 10 min. 2 mL supernatant was obtained and dried in gentle stream of nitrogen flow at 50 °C. The residue was spun for 2 min after being reconstituted with 100 μL methanol, and was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 min to get supernatant sample. 20 μL supernatant samples were taken to analyze through LC-MS.17
000 × g for 5 min to get supernatant sample. 20 μL supernatant samples were taken to analyze through LC-MS.17
Acknowledgements
This work was supported by Science and Technology Commission of Shanghai Municipality (Grant No. 14431906200, 15XD1523400, 15ZR1439900), National Natural Science Foundation of China (No. 21372042), and the Fundamental Research Funds for the Central Universities (Grant No. CUSF-DH-D-2016041).References
- J. L. Wang, J. Zhang, M. Zhou, Z. H. Li, W. Z. Xue, D. Xu, L. P. Hao, X. F. Han, F. Fei, T. Liu and A. H. Liang, Eur. J. Med. Chem., 2012, 49, 183–190 CrossRef CAS PubMed.
- A. Bali, Y. Baansal, M. Sugumaran, J. S. Saggu, P. Balakumar, G. Kaur, G. Bansal, A. Sharma and M. Singh, Bioorg. Med. Chem. Lett., 2005, 15, 3962–3966 CrossRef CAS PubMed.
- G. Bakris, A. Gradman, M. Reif, M. Wofford, M. Munger, S. Harris, J. Vendetti, E. L. Michelson and R. Wang, J. Clin. Hypertens., 2001, 3, 16–31 CrossRef CAS.
- J. A. Brousil and J. M. Burke, Clin. Ther., 2003, 25, 1041–1055 CrossRef CAS PubMed.
- J. Zhang, J. L. Wang, W. F. Yu, Z. M. Zhou, W. C. Tao, Y. C. Wang, W. Z. Xue, D. Xu, L. P. Hao, X. F. Han, F. Fei, T. Liu and A. H. Liang, Eur. J. Med. Chem., 2013, 69, 44–54 CrossRef CAS PubMed.
- K. S. Kumar, B. Rajesham, M. S. Ramulu, B. Bhaskar, S. N. Dash, M. A. Ashfaq, R. Nagarapu, A. A. Khan, S. Lehtonen and M. Pal, RSC Adv., 2016, 6, 100487–100493 RSC.
- U. Herzberg, E. Eliav, G. J. Bennett and I. J. Kopin, Neurosci. Lett., 1997, 221, 157–160 CrossRef CAS PubMed.
- U. Jacquemard, N. Dias, A. Lansiaux, C. Bailly, C. Logé, J. M. Robert, O. Lozach, L. Meijer, J. Y. Mérour and S. Routier, Bioorg. Med. Chem., 2008, 16, 4832–4953 CrossRef PubMed.
- N. Sharaf El-Dina and A. Barseem, J. Pharm. Sci., 2016, 6, 075–083 Search PubMed.
- X. L. Bao, W. B. Zhu, H. Ren, Y. J. Da, D. Wu, F. M. Li, Y. J. Yan, L. Wang and Z. L. Chen, Eur. J. Med. Chem., 2016, 115, 167–178 Search PubMed.
- W. Wienen, M. Entzeroth, J. C. A. Meel, J. Stangier, U. Busch, T. Ebner, J. Schmid, H. Lehmann, K. Matzek, J. Kempthorne-RawsonK, V. Gladigau and N. H. Hauel, Cardiovasc. Drug Rev., 2000, 18, 127–154 CrossRef CAS.
- G. Berellini, G. Cruciani and R. Mannhold, J. Med. Chem., 2005, 48, 4389–4399 CrossRef CAS PubMed.
- A. Justin, M. Sathishkumar, A. Sudheer, S. Shanthakumari and M. Ramanathan, Pharmacol., Biochem. Behav., 2014, 122, 61–73 CrossRef CAS PubMed.
- T. Haraguchi, K. Iwasaki, K. Takasaki, K. Uchida, T. Naito, A. Nogami, K. Kubota, T. Shindo, N. Uchida, S. Katsurabayashi, K. Mishima, R. Nishimura and M. Fujiwara, Brain Res., 2010, 1353, 125–132 CrossRef CAS PubMed.
- C. M. Qi, L. C. Yang, G. X. Zhang and H. Yu, China Pat., CN1623992A, 2005.
- X. L. Bao, W. B. Zhu, H. Ren, P. Y. Liao, W. Zhu, Y. J. Yan, L. Wang and Z. L. Chen, Clin. Exp. Hypertens., 2016, 38, 435–442 CrossRef PubMed.
- W. B. Zhu, X. L. Bao, R. J. Zhang, C. H. Wen, L. Wang, Y. J. Yan, H. S. Tang and Z. L. Chen, Eur. J. Med. Chem., 2016, 123, 115–127 CrossRef PubMed.
- G. Y. Cao, P. Y. Ying, B. Yan, W. Xue, K. X. Li, A. X. Shi, T. H. Sun, J. L. Yan and X. Hua, J. Ethnopharmacol., 2015, 168, 31–36 CrossRef CAS PubMed.
- K. W. Mosure, J. O. Knipe, M. Browning, V. Arora, Y. Z. Shu, T. Phillip, F. Mcphee, P. Scola, A. Balakrishnan, M. G. Soars, K. Santone and M. Sinz, J. Pharm. Sci., 2015, 104, 2813–2823 CrossRef CAS PubMed.
- Y. J. Da, T. Xin, Y. Y. Nie, Y. Ye, Y. J. Yan, L. S. Liang and Z. L. Chen, Bioorg. Med. Chem., 2012, 20, 7101–7111 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03915h |
| This journal is © The Royal Society of Chemistry 2017 |