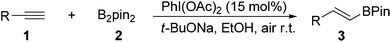Open Access Article
Open Access ArticleTransition-metal-free PhI(OAc)2-promoted highly selective hydroboration of terminal alkynes under air†
Suyuan Chenab,
Lu Yangab,
Dong Yiab,
Qiang Fu ab,
Zhijie Zhangab,
Wu Liangab,
Qiang Zhang*a,
Jianxin Jia and
Wei Wei
ab,
Zhijie Zhangab,
Wu Liangab,
Qiang Zhang*a,
Jianxin Jia and
Wei Wei *c
*c
aChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China. E-mail: zhangqiang@cib.ac.cn
bUniversity of the Chinese Academy of Sciences, Beijing 100049, China
cSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, China. E-mail: weiweiqfnu@163.com
First published on 16th May 2017
Abstract
A new transition-metal-free PhI(OAc)2-promoted hydroboration reaction of terminal alkynes with bis(pinacolato)diboron has been developed at room temperature under air. A series of vinyl boronates could be conveniently and efficiently obtained in moderate to good yields with good regioselectivity and stereoselectivity as well as favorable functional group tolerance. The key I–B intermediates were first demonstrated in the present reaction system that explains the proposed mechanism.
Alkenylboronates are important building blocks in the Suzuki–Miyaura cross-coupling reaction for complex molecule synthesis in organic semiconductors and pharmaceuticals.1 The catalytic hydroboration of alkynes represents one of the most straightforward and powerful tools for the construction of alkenylboronates.2 Over the past several years, considerable efforts have been made in this area and some transition-metal-catalyzed synthetic methods have been successfully developed, such as Cu,3 Pd,4 Ag,5 Ir,4a Pt3a,6 and Au7 catalyzed addition of boron reagents to alkynes. Nevertheless, the cost, toxicity and environmental impact of these metal-catalysts might thereby limit their wide applications on a large scale in the field of synthetic and pharmaceutical chemistry.
Recently, transition-metal-free methods have attracted increasingly attention of chemists for the synthesis of alkenylboronates through direct hydroboration reactions of alkynes.8 For instance, Sun and co-workers reported the hydroboration of terminal alkynes with bis(pinacolato)diboron using N-heterocyclic carbene (NHC) as the catalyst.8a Jin's group presented a carboxylic acid-catalyzed hydroboration of alkynes with pinacolborane at 100 °C under an argon atmosphere.8b In 2016, Deng described a t-BuOLi mediated hydroboration of terminal alkynes in toluene under argon.8c Nevertheless, most of those transition-metal-free methods required relatively harsh reaction conditions, inert atmosphere protection or toxic solvents. Therefore, the development of a new, simple, convenient, and environmentally-benign transition-metal-free method to access alkenylboronates is still high desirable.
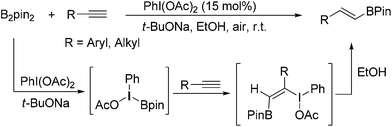
On the other hand, hypervalent iodine reagents have been increasingly employed for various organic transformations due to their ready available, cost-effective and environmentally friendly properties.9 They are generally used as stoichiometric oxidizing reagents and recognized as alternatives to highly toxic heavy-metal oxidants.10 However, to our knowledge, only a few strategies for the reactions using a catalytic amount of hypervalent iodine reagents have been exploited, in which m-CPBA (m-chloroperoxybenzoic acid) was mostly used as a stoichiometric chemical oxidant to accomplish the catalytic cycle.11 As our continued interests in metal-free functionalization of unsaturated hydrocarbons,12 here, we wish to report a new PhI(OAc)2-promoted highly selective hydroboration of terminal alkynes with bis(pinacolato)diboron in EtOH under transition-metal and external-oxidant free conditions. The present protocol provides an attractive and efficient approach to various E-vinyl boronates in moderate to good yields with favorable functional group tolerance, which does not require inert atmosphere (Scheme 1).
Initially, phenylacetylene 1a and B2Pin2 2 were used as the model substrates for the screening of reaction conditions (Table 1). In the absence of the catalyst, only 8% yield of 3a was obtained when the reaction was performed in EtOH using t-BuONa as base at room temperature under air (entry 1). Then, various iodine reagents (15 mol%) such as PhI, TBAI, KIO3, PhI(OTFA)2, DMP, and PhI(OAc)2 were tested (entries 2–7). To our delight, among those iodine reagents examined, PhI(OAc)2 could provide excellent catalytic activity for the formation of product 3a with an exclusive regioselectivity and stereoselectivity (entry 7). Subsequently, the screening of a range of bases showed that the reaction performed in the presence of t-BuONa was significantly better than those promoted by KOH, DBU, MeONa, or t-BuOLi (entries 7–11). None of 3a was obtained when the reaction was performed in the absence of base (entry 12). Notably, the reaction efficiency was obviously low with the decreasing of PhI(OAc)2 loading to 5 mol% or 10 mol% (entries 13 and 14). The further increase of PhI(OAc)2 loading to 20 mol% did not improve the reaction efficiency (entry 15). The optimization of solvents demonstrated that other solvents such as MeOH, n-PrOH, and i-PrOH were all inferior to EtOH (entries 16–18). In addition, the reaction activity was slightly lower when the amount of t-BuONa was decreased to 1.5 equiv. and 1.8 equiv. of t-BuONa was the best choice (entries 19 and 20).
| Entry | Catalyst | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2 (0.75 mmol), iodine reagents (15 mol%), base (2 equiv.), solvent (4 mL), r.t., air, 12 h; TBAI: tetrabutyl ammoniuiodide; DMP: Dess–Martin periodinane; DBU: 1,5-diaza(5,4,0)undec-5-ene.b Isolated yields based on 1a, isomers were not detected in crude 1H NMR.c PhI(OAc)2 (5 mol%).d PhI(OAc)2 (10 mol%).e PhI(OAc)2 (20 mol%).f t-BuONa (1.5 equiv.).g t-BuONa (1.8 equiv.). | ||||
| 1 | — | t-BuONa | EtOH | 8 |
| 2 | PhI | t-BuONa | EtOH | 17 |
| 3 | TBAI | t-BuONa | EtOH | 13 |
| 4 | KIO3 | t-BuONa | EtOH | 35 |
| 5 | PhI(OTFA)2 | t-BuONa | EtOH | 36 |
| 6 | DMP | t-BuONa | EtOH | 12 |
| 7 | PhI(OAc)2 | t-BuONa | EtOH | 90 |
| 8 | PhI(OAc)2 | KOH | EtOH | 31 |
| 9 | PhI(OAc)2 | DBU | EtOH | 7 |
| 10 | PhI(OAc)2 | MeONa | EtOH | 50 |
| 11 | PhI(OAc)2 | t-BuOLi | EtOH | 5 |
| 12 | PhI(OAc)2 | — | EtOH | 0 |
| 13 | PhI(OAc)2 | t-BuONa | EtOH | 42c |
| 14 | PhI(OAc)2 | t-BuONa | EtOH | 73d |
| 15 | PhI(OAc)2 | t-BuONa | EtOH | 90e |
| 16 | PhI(OAc)2 | t-BuONa | MeOH | 40 |
| 17 | PhI(OAc)2 | t-BuONa | n-PrOH | 71 |
| 18 | PhI(OAc)2 | t-BuONa | i-PrOH | 12 |
| 19 | PhI(OAc)2 | t-BuONa | EtOH | 82f |
| 20 | PhI(OAc)2 | t-BuONa | EtOH | 90g |
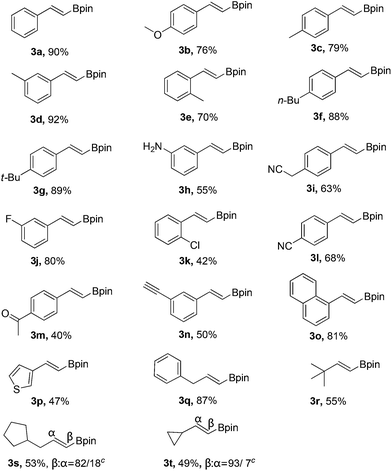
With the optimized reaction conditions in hand (Table 1, entry 20), the substrate scope of hydroboration of alkynes was investigated. As shown in Table 2, a variety of E-vinyl boronates could be conveniently and efficiently obtained by this novel PhI(OAc)2-promoted regioselective hydroboration of terminal alkynes. Generally, both electron-rich and electron-deficient aromatic alkynes were suitable for this process, and the corresponding hydroboration products were obtained in moderate to good yields (3b–3n). It should be noted that various functional groups including unprotected amino, cyano, fluoro, chloro and keto groups were all compatible with this protocol to give the corresponding products (3h–3m), which could be employed for further transformations. In addition, when 1,3-diethynylbenzene was used in this reaction, the monoalkynyl substituted E-vinyl boronate 3n was isolated in 50% yield. Furthermore, 1-ethynylnaphthalene and heterocyclic alkyne such as 3-ethynylthiophene were also tolerated in this reaction to provide the desired products (3o and 3p) in 81% and 47% yields, respectively. Notably, the aliphatic terminal alkynes were all suitable substrates, and generated the corresponding products in good yields (3q–3t).
A radical trapping experiment was conducted to investigate the possible reaction mechanism. When TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) was added into the model reaction of 1a and B2Pin2 2 under air or N2 conditions, the corresponding product 3a was still obtained in 88% or 89% yield, respectively. This result indicated that a radical process might not be involved in the present hydroboration reaction.
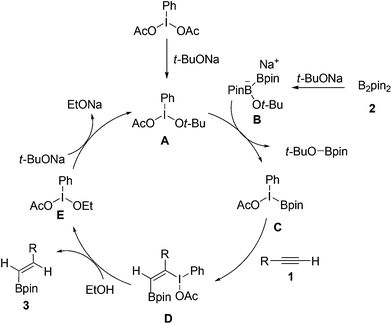
On the basis of our experimental results and previous reports,13 a possible reaction pathway was proposed as shown in Scheme 2. Initially, PhI(OAc)2 reacted with t-BuONa leading to intermediate A. Subsequently, the interaction of intermediate A with ate complex B, which was generated from the reaction of B2Pin2 with t-BuONa, gave the key intermediate C. Then, the selective addition of C to alkyne 1 would lead to the formation of addition intermediate D. Next, ethanol acted as hydrogen donor reagent to provide target product 3 and iodine(III) compound E. Finally, compound E was activated by another t-BuONa to accomplish the catalytic circle.
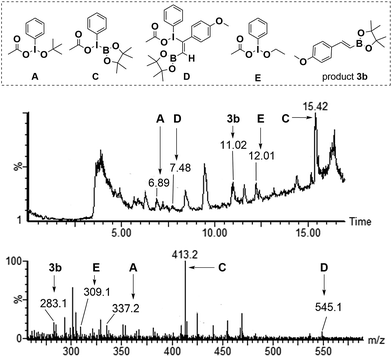
LC-MS analysis technology was employed to investigate the existence of possible intermediates described in Fig. 1. The reaction mixture was detected by LC-MS when the reaction of 4-methoxyphenylacetylene 1b and 2 was conducted under standard conditions for 4h. Fortunately, the [M + H]+ ion peaks (m/z = 337.2, 309.1) for intermediates A and E, and the [M + Na]+ ion peaks (m/z = 413.3, 545.1) for intermediate C and D were detected by LC-MS analysis, respectively (Fig. 1).
In conclusion, we have developed a new transition-metal-free PhI(OAc)2-promoted hydroboration reaction of terminal alkynes with bis(pinacolato)diboron under air. A series of vinyl boronates could be conveniently and efficiently obtained in moderate to good yields with good regioselectivity and stereoselectivity at room temperature. Taking into account the following desirable features including operation simplicity, cheap catalyst, broad functional group tolerance and mild reaction conditions, this novel reaction system provides a highly attractive approach to access E-vinyl boronates. The present protocol also expands the scope of potential applications of hypervalent iodine reagents in the synthetic chemistry. Further investigations on increasing the reaction scope, synthetic applications and mechanistic details are undergoing in our laboratory.
Acknowledgements
We gratefully acknowledge financial support from the Chinese National Natural Science Foundation (No. 21402184, 21572217, and 21302109) and Institute of Drug Innovation, Chinese Academy of Sciences (CASIMM0420152013 and CASIMM0420151009); Strategic Biological Resources Service Network Program of Chinese Academy of Sciences (ZSTH-001).Notes and references
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) J. Takagi, K. Takahashi, T. Ishiyama and N. Miyaura, J. Am. Chem. Soc., 2002, 124, 8001 CrossRef CAS PubMed; (c) T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829 CrossRef CAS PubMed; (d) Boronic Acids. Preparation, Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH, Weinheim, 2005 Search PubMed; (e) G. A. Molander and N. Ellis, Acc. Chem. Res., 2007, 40, 275 CrossRef CAS PubMed; (f) T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829 CrossRef CAS PubMed; (g) T. R. Wu and J. M. Chong, J. Am. Chem. Soc., 2007, 129, 4908 CrossRef CAS PubMed.
- (a) G. J. Irvine, M. J. G. Lesley, T. B. Marder, N. C. Norman, C. R. Rice, E. G. Robins, W. R. Roper, G. R. Whittell and L. J. Wright, Chem. Rev., 1998, 98, 2685 CrossRef CAS PubMed; (b) I. Beletskaya and C. Moberg, Chem. Rev., 2006, 106, 2320 CrossRef CAS PubMed; (c) C. Gunanathan, M. Holscher, F. F. Pan and W. Leitner, J. Am. Chem. Soc., 2012, 134, 14349 CrossRef CAS PubMed.
- (a) A. Grirrane, A. Corma and H. Garcia, Chem.–Eur. J., 2011, 17, 2467 CrossRef CAS PubMed; (b) H. Jang, A. R. Zhugralin, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7859 CrossRef CAS PubMed; (c) A. L. Moure, P. Mauleón, R. G. Arrayás and J. C. Carretero, Org. Lett., 2013, 15, 2054 CrossRef CAS PubMed; (d) P. Liu, Y. Fukui, P. Tian, Z.-T. He, C.-Y. Sun, N.-Y. Wu and G.-Q. Lin, J. Am. Chem. Soc., 2013, 135, 11700 CrossRef CAS PubMed; (e) S. Lee, D. Li and J. Yun, Chem.–Asian J., 2014, 9, 2440 CrossRef CAS PubMed.
- (a) N. Iwadate and M. Suginome, J. Am. Chem. Soc., 2010, 132, 2548 CrossRef CAS PubMed; (b) D. P. Ojha and K. R. Prabhu, Org. Lett., 2016, 18, 432 CrossRef CAS PubMed.
- H. Yoshida, I. Kageyuki and K. Takaki, Org. Lett., 2014, 16, 3512 CrossRef CAS PubMed.
- F. Alonso, Y. Moglie, L. Pastor-Pérez and A. Sepúlveda-Escribano, Chemcatchem, 2014, 6, 857 CrossRef CAS.
- Q. Chen, J. Zhao, Y. Ishikawa, N. Asao, Y. Yamamoto and T. Jin, Org. Lett., 2013, 15, 5766 CrossRef CAS PubMed.
- (a) K. Wen, J. B. Chen, F. Gao, P. S. Bhadury, E. K. Fan and Z. H. Sun, Org. Biomol. Chem., 2013, 11, 6350 RSC; (b) H. E. Ho, N. Asao, Y. Yamamoto and T. N. Jin, Org. Lett., 2014, 16, 4670 CrossRef CAS PubMed; (c) S. Hong, W. Zhang, M. Liu, Z.-J. Yao and W. Deng, Tetrahedron Lett., 2016, 57, 1 CrossRef CAS.
- (a) T. Wirth, Angew. Chem., Int. Ed., 2001, 40, 2812 CrossRef CAS; (b) R. M. Moriarty, J. Org. Chem., 2005, 70, 2893 CrossRef CAS PubMed; (c) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (d) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS PubMed; (e) J. Wen, W. Wei, S. Xue, D. Yang, Y. Lou, C. Gao and H. Wang, J. Org. Chem., 2015, 80, 4966 CrossRef CAS PubMed; (f) C. Liu, W. Wei, D. Yang, Y. Zheng, Y. Bi, M. Chen and H. Wang, Tetrahedron, 2015, 71, 6901 CrossRef CAS; (g) C. Liu, M. Zhu, W. Wei, D. Yang, H. Cui, X. Liu and H. Wang, Org. Chem. Front., 2015, 2, 1356 RSC; (h) E. M. Woerly, S. M. Banik and E. N. Jacobsen, J. Am. Chem. Soc., 2016, 138, 13858 CrossRef CAS PubMed.
- (a) P. J. Stang and V. V. Zhdankin, Chem. Rev., 1996, 96, 1123 CrossRef CAS PubMed; (b) A. Varvoglis, Hypervalent Iodine in Organic Synthesis, 1997 Search PubMed; (c) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS PubMed; (d) T. Wirth, Hypervalent Iodine Chemistry, Springer, Berlin Heidelberg, 2003 Search PubMed; (e) T. Wirth, Angew. Chem., Int. Ed., 2005, 44, 3656 CrossRef CAS PubMed.
- (a) T. Dohi, A. Maruyama, M. Yoshimura, K. Morimoto, H. Tohma and Y. Kita, Angew. Chem., Int. Ed., 2005, 44, 6193 CrossRef CAS PubMed; (b) M. Ochiai, Y. Takeuchi, T. Katayama, T. Sueda and K. Miyamoto, J. Am. Chem. Soc., 2005, 127, 12244 CrossRef CAS PubMed; (c) F. V. Singh and T. Wirth, Chem.–Asian J., 2014, 9, 950 CrossRef CAS PubMed.
- (a) M. S. Li, Q. Zhang, D. Y. Hu, W. W. Zhong, M. Cheng, J. X. Ji and W. Wei, Tetrahedron Lett., 2016, 57, 2642 CrossRef CAS; (b) W. Wei, J. Wen, D. Yang, J. Du, J. You and H. Wang, Green Chem., 2014, 16, 2988 RSC; (c) W. Wei, J. Wen, D. Yang, M. Guo, Y. Wang, J. You and H. Wang, Chem. Commun., 2015, 51, 768 RSC; (d) W. Wei, J. Wen, D. Yang, X. Liu, M. Guo, R. Dong and H. Wang, J. Org. Chem., 2014, 79, 4225 CrossRef CAS PubMed; (e) W. Wei, J. Wen, D. Yang, H. Jing, J. You and H. Wang, RSC Adv., 2015, 5, 4416 RSC; (f) W. Wei, X. Liu, D. Yang, R. Dong, Y. Cui, F. Yuan and H. Wang, Tetrahedron Lett., 2015, 56, 1808 CrossRef CAS; (g) H. Cui, X. Liu, W. Wei, D. Yang, C. He, T. Zhang and H. Wang, J. Org. Chem., 2016, 81, 2252 CrossRef CAS PubMed.
- (a) S. Pietsch, E. C. Neeve, D. C. Apperley, R. Bertermann, F. Mo, D. Qiu, M. S. Cheung, L. Dang, J. Wang, U. Radius, Z. Lin, C. Kleeberg and T. B. Marder, Chem.–Eur. J., 2015, 21, 7082 CrossRef CAS PubMed; (b) D. Q. Dong, S. H. Hao, Z. L. Wang and C. Chen, Org. Biomol. Chem., 2014, 12, 4278 RSC; (c) R. Frei and J. Waser, J. Am. Chem. Soc., 2013, 135, 9620 CrossRef CAS PubMed; (d) M. Ochiai, in Hypervalent Iodine Chemistry, ed. T. Wirth, Springer, Berlin Heidelberg, 2003, vol. 224, ch. 2, pp. 5–68 Search PubMed; (e) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091 CrossRef CAS PubMed; (f) A. B. Cuenca, R. Shishido, H. Ito and E. Fernández, Chem. Soc. Rev., 2017, 46, 415 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03680a |
| This journal is © The Royal Society of Chemistry 2017 |