Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phase evolution and microwave dielectric properties of ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4 for LTCC applications
Xiangyu Dua,
Hua Su *ab,
Huaiwu Zhanga,
Xiuting Liua and
Xiaoli Tanga
*ab,
Huaiwu Zhanga,
Xiuting Liua and
Xiaoli Tanga
aState Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, Chengdu 610054, China. E-mail: uestcsh77@163.com
bInstitute of Electronic and Information Engineering, University of Electronic Science and Technology of China, Dongguan 518105, China
First published on 23rd May 2017
Abstract
Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics were prepared through the conventional solid-state route. A fixed amount of Li2O–MgO–ZnO–B2O3–SiO2 (LMZBS) glass was used as a sintering aid to help lower the sintering temperature to around 900 °C. The effects of part lithium-ion substitution on the phase formation, sintering behaviour, microstructures and microwave dielectric properties of the ceramics were systematically investigated. When x = 0.25, the sample achieved a dense microstructure and exhibited excellent microwave dielectric properties of εr = 6.47, Q × f = 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 GHz and τf = −27.12 ppm °C−1. For its practical application in low-temperature co-fired ceramics (LTCC), large positive τf of CaTiO3 was used to adjust the τf value of the composite ceramic to nearly zero. The composite ceramic of 0.975Li0.5(Zn0.95Co0.05)1.75SiO4–0.025CaTiO3 sintered at 900 °C also presented good microwave dielectric properties of εr = 6.773, Q × f = 69
579 GHz and τf = −27.12 ppm °C−1. For its practical application in low-temperature co-fired ceramics (LTCC), large positive τf of CaTiO3 was used to adjust the τf value of the composite ceramic to nearly zero. The composite ceramic of 0.975Li0.5(Zn0.95Co0.05)1.75SiO4–0.025CaTiO3 sintered at 900 °C also presented good microwave dielectric properties of εr = 6.773, Q × f = 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 GHz and τf = −2.45 ppm °C−1.
177 GHz and τf = −2.45 ppm °C−1.
1. Introduction
Microwave dielectric materials are widely investigated for their extensive applications as filters, duplexers and antennas in microwave communication industries.1,2 Materials with low permittivity (εr), high quality factor (Q × f) and near-zero temperature coefficient of resonant frequency (τf) are required to reduce cross-talk, propagation delay time, noise and power dissipation.3–5 Furthermore, these materials should be sintered at temperatures lower than 950 °C or even 900 °C to be used in LTCC multi-layer devices for the miniaturisation and integration of microwave components because of silver (Ag) electrode (around 961 °C), which is typically used as metallic electrode in LTCC materials.6,7 Although a number of materials (such as Al2O3 (ref. 4), MgTiO3 (ref. 8) and Mg2SiO4 (ref. 3)) have low permittivity and dielectric loss, these materials cannot be used in LTCC multi-layer devices because of the high sintering temperature.9 Zn2SiO4 is one of these materials and has a low dielectric constant (6.6), a high quality factor value (219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GHz) and a temperature coefficient of resonant frequency of −61 ppm °C−1. Analogously, the high sintering temperature of 1340 °C limits the application of Zn2SiO4 in LTCC technology.10
000 GHz) and a temperature coefficient of resonant frequency of −61 ppm °C−1. Analogously, the high sintering temperature of 1340 °C limits the application of Zn2SiO4 in LTCC technology.10
Recently, Chen reported that the co-substitution (Zn1−xCox)2SiO4 ceramics lowers the sintering temperature of Zn2SiO4 from 1340 °C to 1300 °C and restrains the formation of ZnO phase.11 Zhou lowered the sintering temperature of (Zn0.95Co0.05)2SiO4 ceramics to 900 °C by adding 1.5 wt% LBBS glass. The ceramics sintered at 900 °C showed εr = 6.16, Q × f = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GHz and τf = −59 ppm °C−1.12 Nonetheless, the ceramics have a large negative τf value, which restricts its potential for practical application. Furthermore, the quality factor is still too low to keep up with the high-speed development of LTCC technology. Although the addition of low softening point glass is the most inexpensive way to lower the sintering temperature of dielectric ceramics, previous studies concluded that a large amount of glass addition leads to either high microwave dielectric loss or crack formation.13 Hence, new strategies combined with low amount of sintering aids should be developed to further lower the sintering temperature and dielectric loss of (Zn0.95Co0.05)2SiO4 ceramics.
000 GHz and τf = −59 ppm °C−1.12 Nonetheless, the ceramics have a large negative τf value, which restricts its potential for practical application. Furthermore, the quality factor is still too low to keep up with the high-speed development of LTCC technology. Although the addition of low softening point glass is the most inexpensive way to lower the sintering temperature of dielectric ceramics, previous studies concluded that a large amount of glass addition leads to either high microwave dielectric loss or crack formation.13 Hence, new strategies combined with low amount of sintering aids should be developed to further lower the sintering temperature and dielectric loss of (Zn0.95Co0.05)2SiO4 ceramics.
The lithium-substitution on Li2CaSiO4,14 LiAlSiO4 (ref. 15) and Li2MgSiO4 (ref. 16) ceramics presented good dielectric properties of low dielectric constant (<10) and high Q × f value. However, the phase evolution, sintering behaviour and optimal lithium-substitution content on (Zn0.95Co0.05)2SiO4 ceramics are not yet reported. Therefore, in this work, the ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4 was synthesised to systematically study the phase formation, sintering behaviour, microstructures and microwave dielectric properties. A fixed amount of LMZBS glass was used as a sintering aid to help lower the sintering temperature at around 900 °C. Moreover, a common modifier with large positive τf of CaTiO3 was used to adjust the τf value of the ceramic, which exhibited the best microwave dielectric property, in the ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4 to nearly zero, thereby making these ceramics promising candidates for LTCC materials.
2. Experimental
2.1. Preparation of ceramic samples
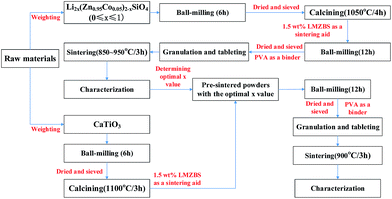
Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics were prepared through the traditional solid-state ceramic route. The schematic plot of the fabrication process of ceramics is shown in Fig. 1. A previous study reported that the cobalt ions substituted for zinc ions in the lattice sites of ZnO exist as Co2+ ions when Co2O3 was used as raw powders.17 Hence, high-purity oxides Li2CO3 (99%), ZnO (99%), SiO2 (99%), Co2O3 (99%), CaCO3 (99%) and TiO2 (99%) were used as the raw materials. These raw powders, which were weighed according to respective stoichiometric ratio of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) and CaTiO3, were ball-milled in nylon jars with zirconia balls in distilled water for 6 h. The resultant slurry was dried, sieved and calcined at 1050 °C for 4 h and 1100 °C for 3 h in air, respectively. A fixed amount of 1.5 wt% LMZBS glass was doped to the pre-sintered Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) powders and then re-milled in distilled water medium for 12 h. With 25 wt% polyvinyl alcohol (PVA) solution as a binder, the powders were dried at 120 °C, well-ground, granulated and pressed into disks under a pressure of 9 MPa and then sintered at 850 °C to 950 °C for 3 h.After determining the optimal x value to obtain the best microwave dielectric properties of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics sintered at 900 °C, different amounts of pre-sintered CaTiO3 powders were added to Li2x(Zn0.95Co0.05)2−xSiO4 (x was determined) powders with 1.5 wt% LMZBS glass to adjust the τf value of Li2x(Zn0.95Co0.05)2−xSiO4 ceramic to nearly zero. The subsequent process was similar to the above-mentioned procedure.
2.2. Preparation of glass
The Li2O–MgO–ZnO–B2O3–SiO2 (LMZBS) glass was synthesised using a quenching method. High-purity grade raw materials (>99%) were mixed, ball-milled and melted at 1350 °C for 1 h using an alumina crucible at the molar ratio of Li2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MgO:ZnO
MgO:ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B2O3
B2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 20
SiO2 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The solution was quickly removed from the furnace and was quenched with cold distilled water to obtain the glass.
20. The solution was quickly removed from the furnace and was quenched with cold distilled water to obtain the glass.
2.3. Sample characterization
The bulk densities of these sintered specimens were measured using the Archimedes method. Relative densities were obtained by using the ratio of the bulk and theoretical densities. The phase compositions of theses sintered samples were determined by X-ray diffraction (XRD:DX-2700) using Cu Kα radiation. The microstructure of the ceramics was examined by scanning electron microscopy (SEM: Hitachi S-3400N). The microwave dielectric properties of the sintered specimens were investigated using an Agilent N5230A network analyser (300 MHz to 20 GHz) in a resonant cavity. The quality factor and relative permittivity were measured by the resonant-cavity method and the Hakki–Coleman method, respectively.18 The temperature coefficient of resonant frequency (τf) was measured from the invar cavity using the following formula in the temperature range of 20–80 °C:19
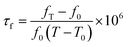 | (1) |
3. Results and discussion
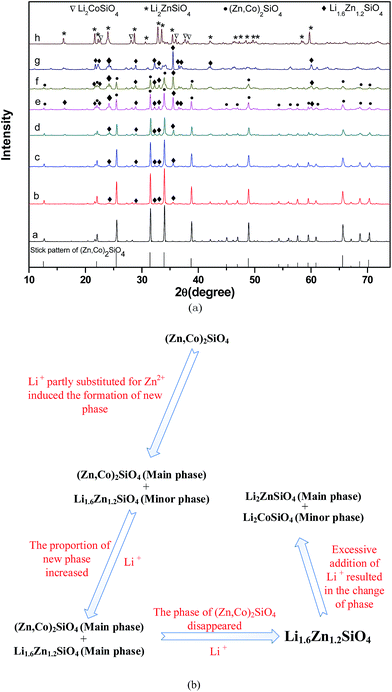
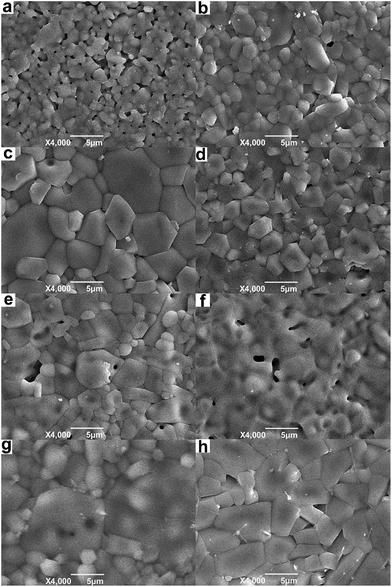
Fig. 2a shows the XRD diffraction patterns of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h in air. Fig. 2b shows the schematic plot of phase evolution of Li2x(Zn0.95Co0.05)2−xSiO4 with various amounts of lithium-ion substitution. Only a rhombohedral structure (Zn,Co)2SiO4 phase (●, PDF #46-1316) appeared in the XRD pattern of the sample with no lithium doped. However, when 0.125 ≤ x ≤ 0.625, the Li1.6Zn1.2SiO4 phase (◆, PDF #24-0676) co-existed with (Zn,Co)2SiO4 phase in the sintered samples. The peak intensities of Li1.6Zn1.2SiO4 phase gradually increased with the increasing x value. When 0.125 ≤ x ≤ 0.375, the crystalline phase of (Zn,Co)2SiO4 was the main phase, and the phase of Li1.6Zn1.2SiO4 was the minor phase. However, both of the crystalline phases became the main phase when x was increased to 0.5. When the x value exceeded 0.625, the phase of (Zn,Co)2SiO4 disappeared (Fig. 2a(g)), and another two crystalline phases of tetragonal structure Li2ZnSiO4 (*, PDF #15-0056) and orthorhombic structure Li2CoSiO4 (▽, PDF #24-0611) appeared in the sintered sample when x = 1 (Fig. 2a(h)). Fig. 2 shows that various amounts of lithium-ion substitution induce different phase-formations in Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics.Fig. 3 illustrates the SEM images of samples doped with 1.5 wt% LMZBS glass and sintered at 900 °C with various x values. Generally, the microstructures present considerable densification that might be due to the sufficient liquid phase, which resulted from the glass addition. In detail, with the increasing x value to 0.25, the pores decreased, and the grain was enlarged (see Fig. 2a–c). Combined with the XRD patterns shown in Fig. 2a, this finding explained that a moderate amount of Li substitution might reduce the sintering temperature of the ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4 via synthesising Li1.6Zn1.2SiO4. The sintering temperature of (Zn0.95Co0.05)2SiO4 was 1300 °C, which is almost similar to that of Zn2SiO4.10,12 It was reported that lithium-ions partly substituted for metal cations significantly reduced the sintering temperature compared with that of initial silicate materials.15,16,20 Hence, the sintering temperature of Li1.6Zn1.2SiO4 was lower than that of (Zn0.95Co0.05)2SiO4. However, with the successive addition of lithium, the pores increased (Fig. 3d–f), and a large amount of liquid phase (see Fig. 3f) and evident closed pores in the grains (see Fig. 3e) were observed. These phenomena are attributed to the fast growth of the grains; thus, some pores were trapped in the bodies of the abnormal growth grains.21 In addition, the liquid phase implied that lithium-ion substitution reduces the sintering temperature and thereby resulted in excessive liquid phase. When x = 0.75, a dense microstructure reappeared; however, the abnormal growth grain with closed pores and the liquid phase were still observed in Fig. 3g. When x was increased to 1, the shape of grains became rectangular, and the intergranular pores increased (Fig. 3h). This outcome is attributed to the formation of tetragonal structure of Li2ZnSiO4. The optimal content of Li+ substitution was set at 0.25 to obtain the most compact and uniform microstructure.
Fig. 4 shows the relative density as a function of x value for Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) specimens doped with 1.5 wt% LMZBS glass and sintered at 850–950 °C for 3 h. The relative density was calculated using the formula 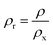 , where ρ and ρx are the bulk and theoretical density values, respectively. The theoretical density ρx was calculated as follows:
, where ρ and ρx are the bulk and theoretical density values, respectively. The theoretical density ρx was calculated as follows:
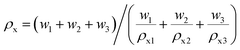 | (2) |
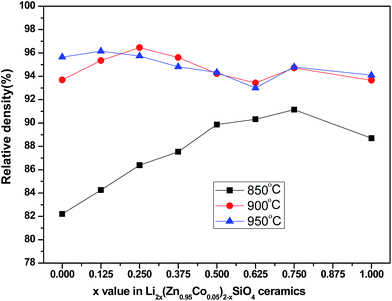 | ||
| Fig. 4 Relative density of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics doped with 1.5 wt% LMZBS glass and sintered at 850–950 °C for 3 h. | ||
The relative density values of samples sintered at 900 °C generally exceed 93%, which implies that 1.5 wt% LMZBS is sufficient to obtain the liquid-phase sintering necessary for the relative densification at 900 °C. When a small amount of lithium was doped to the sample sintered at 900 °C, the relative density increased to 96.46%, which is a fairly high densification. This outcome indicates that a moderate amount of lithium-substitution help facilitate densification. The tendency of relative density of these samples sintered at 900 °C is also consistent with the variations in the microstructure. The relative densities of specimens sintered at 850 °C were significantly lower than those sintered at 900 °C and 950 °C. This phenomenon is attributed to two reasons. One reason is the tight relationship between the sintering temperature and relative density.23 The other reason is that the softening temperature of LMZBS is around 900 °C, which is higher than the sintering temperature of these samples.22 Furthermore, a lower sintering temperature shifts the obtainable maximum relative density to higher lithium doped content. This outcome suggested that the substitution of lithium ions markedly lowers the sintering temperature of the ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4.
Fig. 5 shows the relative permittivity (εr) values of the Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics doped with 1.5 wt% LMZBS glass and sintered at 850–950 °C for 3 h. The variation in relative permittivity of specimens sintered at 850–950 °C is generally in agreement with the trend of the relative density. Densification plays a crucial role in determining the relative permittivity.24 The εr of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics sintered at 900 °C increased with the increasing x value, reached a maximum value (6.47) at x = 0.25 and then decreased to a minimum value (5.84) at x = 1. The relative density of the sample sintered at 900 °C (93.69%) when x = 0 is similar to that of the sample sintered at the same temperature (93.66%) when x = 1, whereas the εr when x = 0 was obviously higher than when x = 1 (6.09 and 5.83, respectively). Combined with the XRD patterns shown in Fig. 2a, this finding is due to the synthesis of Li2ZnSiO4, which exists as the major phase. The εr of Li2ZnSiO4 is 5.8, which is lower than that of (Zn0.95Co0.05)2SiO4 (6.16).12,20
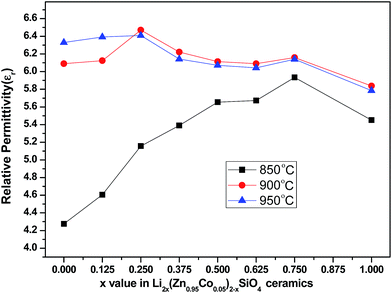 | ||
| Fig. 5 Relative permittivity (εr) of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics doped with 1.5 wt% LMZBS glass and sintered at 850–950 °C for 3 h. | ||
Fig. 6 shows the variations in Q × f values of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics with different x contents doped with 1.5 wt% LMZBS and sintered at 850–950 °C for 3 h. The variations in Q × f values are generally consistent with the variations in the relative density of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics. The Q × f values were all low when the samples were sintered at 850 °C (less than 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GHz), which is mainly attributed to the low relative densities. When the sintering temperature was increased to 900 °C, the Q × f values increased with the increasing x value and achieved the maximum of 131
000 GHz), which is mainly attributed to the low relative densities. When the sintering temperature was increased to 900 °C, the Q × f values increased with the increasing x value and achieved the maximum of 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 GHz at x = 0.25. This outcome might be due to the high densification (96.46%) of the sintered sample when x = 0.25. According to the SEM images shown in Fig. 3c, the large grain resulted in less grain boundary, which indicates less lattice mismatch and lower dielectric loss.25 The Q × f values of the samples sintered at 850–950 °C remarkably decreased to the minimal values of around 6800 GHz with the variation in x from 0.75 to 1. Microwave dielectric loss can be divided into intrinsic and extrinsic losses.26 Intrinsic losses are mainly dependent on the crystal structure and are usually caused by the lattice vibration modes.27 According to the XRD analysis shown in Fig. 2a, tetragonal structure Li2ZnSiO4 (*, PDF #15-0056) was the major phase of the sintered sample when x = 1. In a previous study, Li2ZnSiO4 ceramics doped with 25 wt% ZB glass sintered at 950 °C exhibited a Q × f value of 10
579 GHz at x = 0.25. This outcome might be due to the high densification (96.46%) of the sintered sample when x = 0.25. According to the SEM images shown in Fig. 3c, the large grain resulted in less grain boundary, which indicates less lattice mismatch and lower dielectric loss.25 The Q × f values of the samples sintered at 850–950 °C remarkably decreased to the minimal values of around 6800 GHz with the variation in x from 0.75 to 1. Microwave dielectric loss can be divided into intrinsic and extrinsic losses.26 Intrinsic losses are mainly dependent on the crystal structure and are usually caused by the lattice vibration modes.27 According to the XRD analysis shown in Fig. 2a, tetragonal structure Li2ZnSiO4 (*, PDF #15-0056) was the major phase of the sintered sample when x = 1. In a previous study, Li2ZnSiO4 ceramics doped with 25 wt% ZB glass sintered at 950 °C exhibited a Q × f value of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 GHz, which is approximated to the Q × f value in the present work.20 Hence, in addition to the influence of extrinsic losses caused by lower densification, the high intrinsic losses of Li2ZnSiO4 thereby induced the dramatic decrease of Q × f values.
800 GHz, which is approximated to the Q × f value in the present work.20 Hence, in addition to the influence of extrinsic losses caused by lower densification, the high intrinsic losses of Li2ZnSiO4 thereby induced the dramatic decrease of Q × f values.
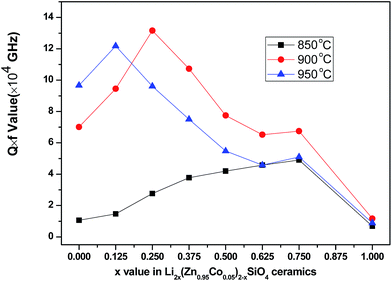 | ||
| Fig. 6 Q × f values of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics with the addition of 1.5 wt% LMZBS glass sintered at 850–950 °C for 3 h. | ||
Fig. 7 shows the τf values of the Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics with different x values doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h. The τf values initially increased and then decreased with the increasing x content. The temperature coefficient of composite ceramics was obtained from the following logarithmic rule:28
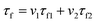 | (3) |
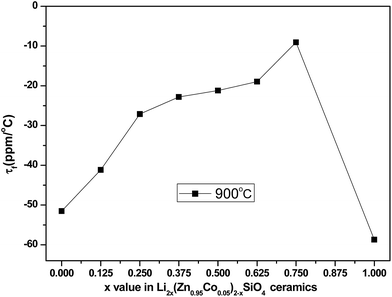 | ||
| Fig. 7 τf values of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics doped with 1.5 wt% LMZBS glass and sintered at 900 °C for 3 h. | ||
Considering the above-mentioned analysis, the optimal x value was set at 0.25 because of the best quality factor of Li0.5(Zn0.95Co0.05)1.75SiO4. One of the most important dielectric properties when considering the practical applications for LTCC is the near-zero temperature coefficient of the resonant frequency (τf).29 Large positive τf of CaTiO3 (+859 ppm °C−1) was used as a modifier and was added into Li0.5(Zn0.95Co0.05)1.75SiO4 powders doped with 1.5 wt% LMZBS to further adjust the τf value (−27.12 ppm °C−1) to around 0 ppm °C−1. The five weight percentages of CaTiO3 were calculated by using the logarithmic rule, where the τf value of Li0.5(Zn0.95Co0.05)1.75SiO4 was set at −15, −21, −27, −33 and −39 ppm °C−1.
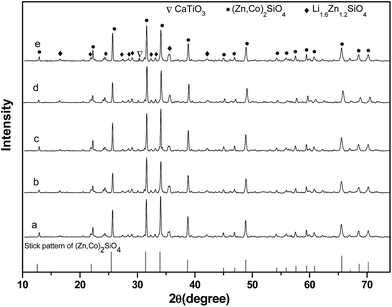
Fig. 8 presents the X-ray diffraction patterns of (1 − x)Li0.5(Zn0.95Co0.05)1.75SiO4-x wt% CaTiO3 ceramics doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h. All the samples exhibited the mixture of (Zn,Co)2SiO4 (●, PDF #46-1316), Li1.6Zn1.2SiO4 (◆, PDF #24-0676) and CaTiO3 (▽, PDF #39-0145) phases, which indicated that the CaTiO3 phase co-exists with the Li0.5(Zn0.95Co0.05)1.75SiO4 ceramic. Furthermore, no other new phase was produced during sintering.
Fig. 9 shows the theoretical and measured τf values of (1 − x)Li0.5(Zn0.95Co0.05)1.75SiO4-x wt% CaTiO3 ceramics doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h. The theoretical τf values of (1 − x)Li0.5(Zn0.95Co0.05)1.75SiO4-x wt% CaTiO3 composite ceramics were calculated using the function (3). As seen in Fig. 9, the theoretical τf value of the sample increased with the increasing CaTiO3 content, which resulted from the high τf value of CaTiO3.23 The change in the measured τf values showed a similar tendency with the theoretical values, which indicated that the addition of CaTiO3 shifts the τf values of Li0.5(Zn0.95Co0.05)1.75SiO4 ceramics to positive values. When the weight percentage of CaTiO3 increased from 1.79 wt% to 4.53 wt%, the measured τf values increased from −14.6 ppm °C−1 to 10.74 ppm °C−1. A near-zero τf value (−2.45 ppm °C−1) was obtained when x = 2.49 wt% and the ceramic was sintered at 900 °C for 3 h.
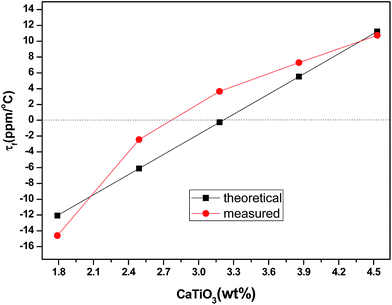 | ||
| Fig. 9 Theoretical and measured τf values of (1 − x)Li0.5(Zn0.95Co0.05)1.75SiO4-x wt% CaTiO3 ceramics doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h. | ||
Table 1 shows the theoretical and measured microwave dielectric properties of (1 − x)Li0.5(Zn0.95Co0.05)1.75SiO4-x wt% CaTiO3 ceramics doped with 1.5 wt% LMZBS and sintered at 900 °C for 3 h. The theoretical permittivities of the composite ceramics were calculated using the function below:30
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε = v1 ε = v1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε1 + v2 ε1 + v2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε2 ε2
| (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 GHz to 41
579 GHz to 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 GHz with the increasing x content. This finding is attributed to the low Q × f value of CaTiO3 (12
344 GHz with the increasing x content. This finding is attributed to the low Q × f value of CaTiO3 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GHz).23 Although CaTiO3 adjusted the τf values of the Li0.5(Zn0.95Co0.05)1.75SiO4 ceramics to nearly zero, the Q × f values sharply decreased when CaTiO3 was added.
000 GHz).23 Although CaTiO3 adjusted the τf values of the Li0.5(Zn0.95Co0.05)1.75SiO4 ceramics to nearly zero, the Q × f values sharply decreased when CaTiO3 was added.
| Compounds | εr (theoretical) | εr (measured) | Q × f values (GHz) |
|---|---|---|---|
| x = 0 | 6.47 | 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 579 |
|
| x = 1.79 | 6.838 | 6.578 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 584 584 |
| x = 2.49 | 6.987 | 6.773 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 177 |
| x = 3.18 | 7.137 | 6.936 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296 |
| x = 3.86 | 7.289 | 7.015 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 782 782 |
| x = 4.53 | 7.441 | 7.152 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 344 |
4. Conclusion
The phase evolution and microwave dielectric properties of ceramics with nominal composition Li2x(Zn0.95Co0.05)2−xSiO4 were investigated. Lithium ions were added to partly substitute for the zinc ions, and a fixed amount of 1.5 wt% LMZBS glass was used as a sintering aid to help lower the sintering temperature of Li2x(Zn0.95Co0.05)2−xSiO4 (0 ≤ x ≤ 1) ceramics of around 900 °C. The XRD patterns indicated that various amounts of lithium-ion substitution induce different production patterns of other phases in the Li2x(Zn0.95Co0.05)2−xSiO4(0 ≤ x ≤ 1) ceramics. A compact and uniform microstructure with few pores, high relative density and Q × f value was obtained when x was set at 0.25. The sample exhibited excellent microwave dielectric properties of εr = 6.47, Q × f = 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 GHz and τf = −27.12 ppm °C−1. In considering the practical application for LTCC, a positive τf of CaTiO3 was used to adjust the τf value of the composite ceramics to nearly zero. The 1.5 wt% LMZBS-doped 0.975 Li0.5(Zn0.95Co0.05)1.75SiO4–0.025CaTiO3 (weight ratio) composite ceramics also presented good dielectric properties of εr = 6.773, Q × f = 69
579 GHz and τf = −27.12 ppm °C−1. In considering the practical application for LTCC, a positive τf of CaTiO3 was used to adjust the τf value of the composite ceramics to nearly zero. The 1.5 wt% LMZBS-doped 0.975 Li0.5(Zn0.95Co0.05)1.75SiO4–0.025CaTiO3 (weight ratio) composite ceramics also presented good dielectric properties of εr = 6.773, Q × f = 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 GHz and τf = −2.45 ppm °C−1.
177 GHz and τf = −2.45 ppm °C−1.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant No. 51372031, 61271038 and 51472042, National High-tech R&D Program of China under Grant No. 2015AA034102, Special Support Program of Guangdong Province under Grant No. 2014TX01C042 and Science and Technology Department of Sichuan Province 2014GZ0015, 2015GZ0227.References
- W. Lei, W.-Z. Lu, D. Liu and J.-H. Zhu, J. Am. Ceram. Soc., 2009, 92, 105–109 CrossRef CAS.
- Y. Zhao and P. Zhang, RSC Adv., 2015, 5, 97746–97754 RSC.
- T. Tsunooka, M. Androu, Y. Higashida, H. Sugiura and H. Ohsato, J. Eur. Ceram. Soc., 2003, 23, 2573–2578 CrossRef CAS.
- O. Yoshihiro, M. Yasuharu, O. Hitoshi and K. Ken-ichi, Jpn. J. Appl. Phys., 2004, 43, L749 CrossRef.
- J. C. Kim, M.-H. Kim, S. Nahm, J.-H. Paik, J.-H. Kim and H.-J. Lee, J. Eur. Ceram. Soc., 2007, 27, 2865–2870 CrossRef CAS.
- H. Li, W. Lu and W. Lei, Mater. Lett., 2012, 71, 148–150 CrossRef CAS.
- J. Guo, D. Zhou, H. Wang and X. Yao, J. Alloys Compd., 2011, 509, 5863–5865 CrossRef CAS.
- V. M. Ferreira, F. Azough, J. L. Baptista and R. Freer, Ferroelectrics, 1992, 133, 127–132 CrossRef CAS.
- Z. Zhang, H. Su, X. L. Tang, H. W. Zhang, T. C. Zhou and Y. L. Jing, Ceram. Int., 2014, 40, 1613–1617 CrossRef CAS.
- Y. Guo, H. Ohsato and K.-i. Kakimoto, J. Eur. Ceram. Soc., 2006, 26, 1827–1830 CrossRef CAS.
- H. W. Chen, H. Su, H. W. Zhang, T. C. Zhou, B. W. Zhang, J. F. Zhang and X. L. Tang, Ceram. Int., 2014, 40, 14655–14659 CrossRef CAS.
- Z. H. Zhou, H. Su, X. L. Tang, H. W. Zhang, F. Xu, S. Zhang and Y. L. Jing, Ceram. Int., 2016, 42, 11161–11164 CrossRef CAS.
- J.-j. Bian, D.-W. Kim and K. S. Hong, Mater. Res. Bull., 2005, 40, 2120–2129 CrossRef CAS.
- S. George, M. T. Sebastian, S. Raman and P. Mohanan, Int. J. Appl. Ceram. Technol., 2011, 8, 172–179 CrossRef CAS.
- S.-H. Kweon, M.-R. Joung, J.-S. Kim, B.-Y. Kim, S. Nahm, J.-H. Paik, Y.-S. Kim and T.-H. Sung, J. Am. Ceram. Soc., 2011, 94, 1995–1998 CrossRef CAS.
- S. George, P. S. Anjana, V. N. Deepu, P. Mohanan and M. T. Sebastian, J. Am. Ceram. Soc., 2009, 92, 1244–1249 CrossRef CAS.
- E. D. Kim, C. H. Kim and M. H. Oh, J. Appl. Phys., 1985, 58, 3231–3235 CrossRef CAS.
- X. C. Fan, X. M. Chen and X. Q. Liu, IEEE Trans. Microwave Theory Tech., 2005, 53, 3130–3134 CrossRef.
- Z. Q. Yuan, B. Liu, X. Q. Liu and X. M. Chen, RSC Adv., 2016, 6, 96229–96236 RSC.
- G. Dou, D. Zhou, S. Gong and M. Guo, J. Mater. Sci.: Mater. Electron., 2012, 24, 1601–1607 CrossRef.
- Q. Zeng, W. Li, J.-l. Shi, J.-k. Guo, M.-w. Zuo and W.-j. Wu, J. Am. Ceram. Soc., 2006, 89, 1733–1735 CrossRef CAS.
- P. S. Anjana and M. T. Sebastian, J. Am. Ceram. Soc., 2009, 92, 96–104 CrossRef CAS.
- C.-H. Hsu, P.-S. Tsai, C.-F. Tseng, S.-H. Hung and I. C. Huang, J. Alloys Compd., 2014, 582, 355–359 CrossRef CAS.
- H. L. Pan, Q. Q. Liu, Y. H. Zhang and H. T. Wu, RSC Adv., 2016, 6, 86889–86903 RSC.
- X. Lu, Y. Zheng, B. Zhou, Z. Dong and P. Cheng, Ceram. Int., 2013, 39, 9829–9833 CrossRef CAS.
- H.-H. Xi, D. Zhou, B. He, H.-D. Xie and N. Alford, J. Am. Ceram. Soc., 2014, 97, 1375–1378 CrossRef CAS.
- C. Tian, Z. Yue and Y. Zhou, Mater. Sci. Eng., B, 2013, 178, 178–182 CrossRef CAS.
- B. W. Hakki and P. D. Coleman, IRE Trans. Microwave Theory Tech., 1960, 8, 402–410 CrossRef.
- M. Guo, G. Dou, S. Gong and D. Zhou, J. Eur. Ceram. Soc., 2012, 32, 883–890 CrossRef CAS.
- Y. Wu, X. Zhao, F. Li and Z. Fan, J. Electroceram., 2003, 11, 227–239 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |