Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design and discovery of novel thiazole derivatives as potential MMP inhibitors to protect against acute lung injury in sepsis rats via attenuation of inflammation and apoptotic oxidative stress
Lingqing Gea,
Qiaozhen Hub,
Mengrao Shia,
Huiyun Yanga and
Guoji Zhu *c
*c
aNeonate Department, Soochow University Affiliated Children's Hospital, Suzhou, Jiangsu 215003, P. R. China
bObstetrical Department, Suzhou Hospital of Traditional Chinese Medicine, Suzhou, Jiangsu 215003, P. R. China
cDepartment of Internal Medicine, Soochow University Affiliated Children's Hospital, Suzhou, Jiangsu 215003, P. R. China. E-mail: zhuguoji189@hotmail.com; Fax: +86-512-6522-3820; Tel: +86-512-6522-3820
First published on 28th June 2017
Abstract
Acute lung injury (ALI) is considered to be an inflammatory syndrome of the airway system that is initiated by failure of the respiratory system. In this study, we evaluated the possible benefits of some novel thiazole derivatives against ALI. These derivatives were synthesised and evaluated for the inhibition of MMP-8 and MMP-2. Most of the tested compounds had better inhibitory activity for MMP-8 than for MMP-2, with compound 26 being the most potent analogue among the tested series. Thus, compound 26 was further investigated to determine its beneficial effects in an ALI model of rats with sepsis. In vivo results suggested that compound 26 significantly reduced the protein concentration together with a decline in enhanced leukocytes compared with those in ALI induced by cecal ligation and puncture. The effect of compound 26 on myeloperoxidase activity was also quantified, which was found to be significantly reduced at the maximum tested dose of 20 mg kg−1. The protective effect of compound 26 against ALI was also established to occur via the significant modulation of various biomarkers; for example, the malondialdehyde level was found to be reduced, while there were increased levels of superoxide dismutase and glutathione. Thus, it is proposed that compound 26 exerts a protective effect against ALI via modulation of the antioxidant status. Furthermore, the compounds tested caused significant attenuation of the levels of tumour necrosis factor-α, interleukin-1β, and interleukin-6, and protected the lung through the modulation of systemic inflammatory mediators in septic rats. In conclusion, we identified a novel series of thiazoles, which potentially exert protective effects against ALI via the inhibition of numerous pathways.
Introduction
Sepsis is classified as a general inflammatory response induced by infection during burns, trauma, or hypoxia, and after surgery, which often leads to multi-organ dysfunction and death.1–3 Despite recent advances in the modalities of clinical approaches for the management of sepsis, there are still difficulties in managing patients with this condition.4,5 Various studies and statistical analyses have revealed that, each year, more than 18 million people suffer from sepsis.6 The number of septic infections is rising at an annual rate of 1.5–8.0%, with mortality ranging from 25% to 50%.7,8 Among cases of sepsis, the lung is classified as the most affected organ and lung damage is largely responsible for the resulting mortality.9 Lung function is greatly hindered by acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS).10 It is considered to be an inflammatory syndrome of the airway system initiated by failure of the respiratory system, resulting from direct/indirect damage to the vasculature of the lung.11,12 This damage leads to increased permeability of leukocytes, together with an enhanced inflammatory response and oxidative stress in the lungs.13,14 Novel agents rather than just ventilation are urgently needed to treat sepsis-induced ALI/ARDS and attenuate the associated pathological changes. Because of the involvement of septic shock in multi-organ failure,15 various studies have focused on the management and attenuation of the inflammatory response, which is thought to be critically involved in the induction of sepsis.16,17 It has also been confirmed that metalloproteinases (MMPs) play a key role in the regulation of the septic inflammatory response.18,19 Apart from degradation of the extracellular matrix (ECM), MMPs are thought to be responsible for modifying the diverse group of non-ECM proteins, which ultimately interfere with the inflammatory process.20 Various rodent studies of sepsis have confirmed that the inhibition of MMPs has a significant influence on the expression of inflammatory markers, together with improved levels of mortality in the experimental animals.21The non-selective inhibition of MMPs often leads to an irrelevant pharmacological response because of its involvement in many regulatory processes.22 Thus, the selective targeting of specific MMPs can overcome the unwanted side effects.23 Among the family of MMPs, MMP-8, a neutrophil-derived collagenase, is believed to be involved in damage to type I collagen.24 It has also been shown to be present in a diverse range of cell types, such as macrophages, fibroblasts, epithelial cells, and other immune cells, and can regulate pro-inflammatory chemokines.25 Among the heterocyclic compounds, the thiazoles and their derivatives are important bioactive molecules due to their excellent pharmacological activity.26 The significance of thiazoles is emphasised by the fact that various drugs originate from them, such as antineoplastic agents (tiazofurin and dasatinib),27 an anti-HIV drug (ritonavir),28 an antifungal agent (ravuconazole),29 an antiparasitic agent (nitazoxanide),30 and an antiulcer agent (nizatidine).31 Thiazoles have also been reported to exhibit anti-inflammatory activity32 and are included among clinically relevant drugs, such as fanetizole, meloxicam, and fentiazac.33 Özdemir et al. reported the MMP inhibitory activity of some thiazoles.34 Thus, in this study, we attempted to determine the effect of some novel thiazole derivatives against MMP-8 and subsequently investigated their beneficial effects in an ALI model of rats with sepsis.
Result
Chemistry
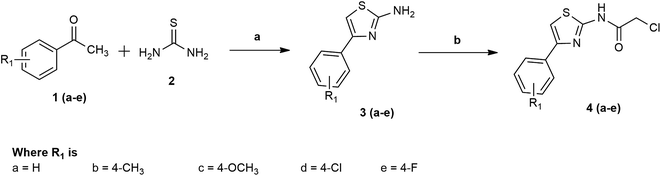
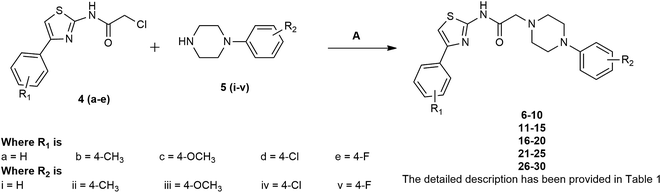
The title compounds were synthesised with an excellent yield via the facile synthetic route shown in Schemes 1 and 2. In the first stage, various substituted phenyl thiazoles 3(a–e) were obtained by the reaction of acetophenone 1(a–e) with thiourea (2) using ammonia solution as a base. In the second stage, 2-chloro-N-[4-(p-substituted phenyl)-thiazol-2-yl]-acetamides 4(a–e) were obtained by the reaction of substituted phenyl thiazole 3(a–e) with chloroacetyl chloride, as shown in Scheme 1. Finally, the title compounds 6–30 were obtained by the reaction of 2-chloro-N-[4-(p-substituted phenyl)-thiazol-2-yl]-acetamides 4(a–e) with various phenyl piperazine derivatives 5(i–v) in ethanol, as shown in Scheme 2.The structures of all synthesised compounds were ascertained by mass spectrometry, 1H-NMR, 12C-NMR, FT-IR, and elemental analysis. The FT-IR spectra of all synthesised derivatives 6–30 were characterised by the appearance of strong bands at 3398–3387 cm−1, which confirmed the presence of a secondary amine group. There was another strong band at 3143–3142 cm−1, which was attributed to stretching vibrations of the C–H group of the aromatic ring. Another strong band at 1198–1187 cm−1 confirmed the presence of an aromatic F group. The thiazole C–H group appeared at 2859–2848 cm−1. Another strong band at 1712–1723 cm−1 confirmed the presence of the CO group. The aromatic chloro group appeared at 798–786 cm−1. The strong band at 662–647 cm−1 was attributed to stretching vibrations of the C–S group of the thiazole ring. The 1H NMR spectra of the synthesised derivatives 6–30 revealed a doublet corresponding to the aromatic ring at 8.15–6.61 ppm. Furthermore, the resonance at 7.58–7.51 ppm confirmed the presence of a phenyl thiazole proton as a singlet peak. The side chain methylene proton appeared at 3.19–3.14 ppm as a single peak. The phenyl thiazole side chain NH proton appeared at 9.16–9.13 as a singlet peak. Furthermore, the resonance at 3.42–2.46 ppm confirmed the presence of a methylene proton, with a doublet peak of the piperazine ring. Finally, the structure of all of the synthesised derivatives 6–30 was confirmed by mass spectrometry and elemental analysis.
Table 1 presents the MMP-2 and MMP-8 inhibitory activities of the designed analogues in the form of comparative inhibitory profiles determined via residual enzyme activity, by means of continuous fluorometric assays in the presence of the fluorescent substrate QF 24. The results suggest that the majority of molecules had a considerable inhibitory profile, with some exceptions where molecules showed less or no activity. In particular, molecules developed in step 1 displayed no inhibitory activity, whereas the introduction of an extended substituted side chain resulted in an improvement in activity, with the exception of compounds 6–13. The introduction of chloro and fluoro groups resulted in compounds being considerably more active against both MMP-2 and MMP-9. Compounds containing electron-donating substituents (17 and 18) showed mild to moderate activity, with no inhibition in the case of compound 16. The presence of an electron withdrawing group in the R2 position resulted in enhanced activity. The next series of compounds (21–30) showed excellent inhibitory activity compared to other analogues, which could be easily understood by the presence of an electron withdrawing group. The comparison of inhibitory activity indicated that molecules containing an electron withdrawing group at both sites exhibited more activity than non-halogen congeners. From these results, it was suggested that fluoro groups had a more prominent influence on the inhibitory profile, with less potency in the case of chloro-containing compounds. These results suggested that most of the compounds had better inhibitory activity for MMP-8 than for MMP-2, with compound 26 being the most potent analogue among the tested series.
| Compound | R1 | R2 | IC50 (in μM) | |
|---|---|---|---|---|
| MMP-2 | MMP-8 | |||
| 4a | H | — | ND | ND |
| 4b | 4-CH3 | — | ND | ND |
| 4c | 4-OCH3 | — | ND | ND |
| 4d | 4-Cl | — | ND | ND |
| 4e | 4-F | — | ND | ND |
| 6 | H | H | >100 | >100 |
| 7 | H | 4-CH3 | >100 | >100 |
| 8 | H | 4-OCH3 | >100 | >100 |
| 9 | H | 4-Cl | >100 | >100 |
| 10 | H | 4-F | >100 | >100 |
| 11 | 4-CH3 | H | >100 | >100 |
| 12 | 4-CH3 | 4-CH3 | >100 | >100 |
| 13 | 4-CH3 | 4-OCH3 | >100 | >100 |
| 14 | 4-CH3 | 4-Cl | 34.31 | 29.23 |
| 15 | 4-CH3 | 4-F | 32.66 | 35.05 |
| 16 | 4-OCH3 | H | >100 | >100 |
| 17 | 4-OCH3 | 4-CH3 | 24.40 | 23.37 |
| 18 | 4-OCH3 | 4-OCH3 | 23.28 | 21.45 |
| 19 | 4-OCH3 | 4-Cl | 18.52 | 19.62 |
| 20 | 4-OCH3 | 4-F | 18.04 | 18.21 |
| 21 | 4-Cl | H | 14.34 | 16.27 |
| 22 | 4-Cl | 4-CH3 | 16.73 | 14.44 |
| 23 | 4-Cl | 4-OCH3 | 15.31 | 13.02 |
| 24 | 4-Cl | 4-Cl | 14.34 | 11.95 |
| 25 | 4-Cl | 4-F | 13.25 | 11.03 |
| 26 | 4-F | H | 4.22 | 2.34 |
| 27 | 4-F | 4-CH3 | 9.21 | 7.30 |
| 28 | 4-F | 4-OCH3 | 13.34 | 9.45 |
| 29 | 4-F | 4-Cl | 14.15 | 11.84 |
| 30 | 4-F | 4-F | 7.23 | 5.45 |
In vivo pharmacological activity
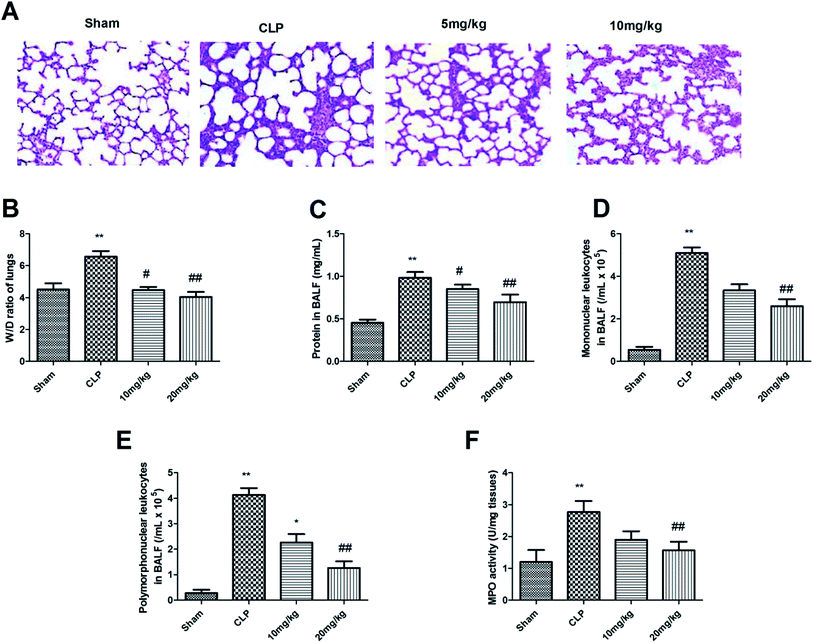
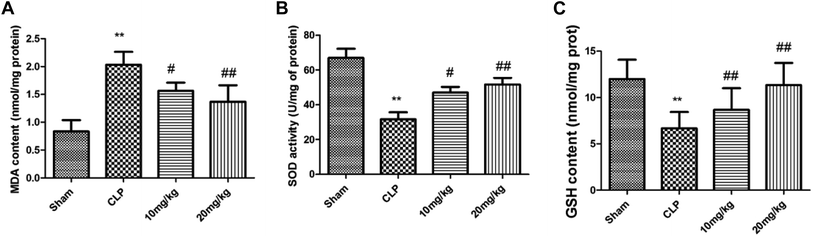
Various studies have confirmed the role of MMP-8 in ALI, against which our designed compounds showed excellent inhibitory activity. After noting the exceptionally potent MMP-8 inhibitory activity of compound 26, we then explored the effects of this compound on the in vivo sepsis-induced lung injury of rats. As shown in Fig. 1A, it was found that compound 26 resulted in improvements following the histopathological examination of the lung tissues of experimental animals exposed to different treatments after staining with H&E. The sham-treated group showed no abnormal architecture, whereas the cecal ligation and puncture (CLP) group had an abnormal micro-structure, with disruption to the vasculature system, increased permeability of inflammatory cells into the alveolar sac, and damage to the alveolar structure. These changes were significantly reversed to normal in the treatment group receiving compound 26 at the maximum dose of 20 mg kg−1. The weight ratio of wet against dry lungs was estimated to confirm the effect of compound 26 on pulmonary oedema, the results of which are presented in Fig. 2B. It was observed that the CLP-treated rats showed a significant increase in the lung wet/dry weight ratio compared with the sham group. However, the ratio was significantly improved only in the group receiving compound 26. The effect of compound 26 was further assayed with regard to the intensity of the inflammatory process. The aim of the next phase of the study was to determine the total protein content and level of leukocytes infiltrated into the BALF after 24 h in the CLP group (Fig. 1C–E). The CLP-treated rats showed increased levels of total protein and leukocytes (mononuclear and polymorphonuclear) in the BALF compared with the sham-treated group. The administration of compound 26 resulted in significant inhibition of the total protein concentration together with a decline in enhanced leukocytes (mononuclear and polymorphonuclear) compared with the CLP-treated group. In addition, the effect of compound 26 regarding MPO activity was also quantified, which is considered to be an important biomarker for the activation of neutrophils and their infiltration. The results of this study are presented in Fig. 1F. It was found that the CLP group had an enhanced level of MPO activity, which was found to be significantly reduced by the administration of compound 26 at the maximum dose of 20 mg kg−1.Oxidative stress is a major determinant of ALI, which has been reported to be associated with an inflammatory response. This releases the reactive oxygen free radicals and is deemed to be toxic for cellular systems. Under normal circumstances, the cellular system counteracts these free radicals by maintaining an optimal balance through the endogenous antioxidant system. The antioxidant defence system comprises MDA (a biomarker for oxidative stress-mediated lipid peroxidation), SOD (a superoxide scavenger), and GSH (a nonenzymatic antioxidant system). Thus, the effect of compound 26 on these biomarkers was investigated to determine its effect on the antioxidant defence system. As shown in Fig. 2, the lungs of rats in the CLP group had an enhanced level of MDA compared with the level in the control group. Moreover, the levels of SOD and GSH were found to be reduced in the CLP-treated lungs. The administration of compound 26 resulted in significant attenuation of these biomarkers; that is, the MDA level was found to be reduced, while the levels of SOD and GSH were increased. Thus, it was suggested that compound 26 exerts a protective effect against ALI via modulation of the antioxidant status.
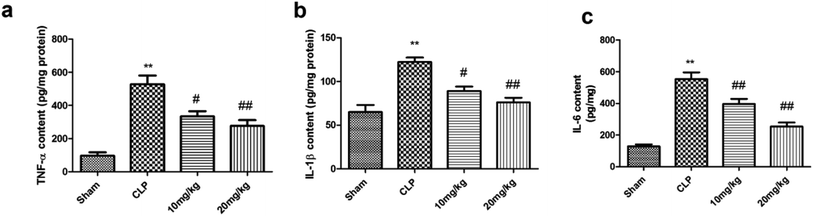
Inflammation plays a critical role in the progression of septic ALI, particularly when it occurs in the airways. It was found that the levels of TNF-α and IL-β were elevated in the serum of septic patients. Moreover, agents reducing the levels of these inflammatory markers have a significant effect on overall survival in animal models. Thus, the anti-inflammatory effect of compound 26 was assessed in terms of both systemic and pulmonary local inflammatory responses, namely, TNF-α, IL-β, and IL-6. The results presented in Fig. 3 suggest that compound 26 caused significant reductions of the levels of TNF-α, IL-β, and IL-6 and could protect the lung through modulation of systemic inflammatory mediators in septic rats.
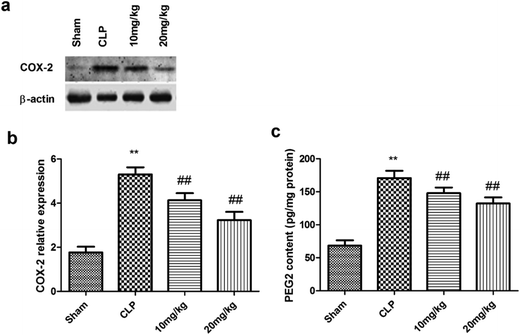
The levels of proinflammatory enzymes, that is, COX-2 and PGE2, are known to be elevated in ALI and other inflammatory diseases. The next stage of the study thus aimed at determining the effect of compound 26 on the expression of COX-2 and PGE2. As shown in Fig. 4, compound 26 caused significant dose-dependent inhibition of both COX-2 and PGE2 compared with their levels in the CLP-treated group.
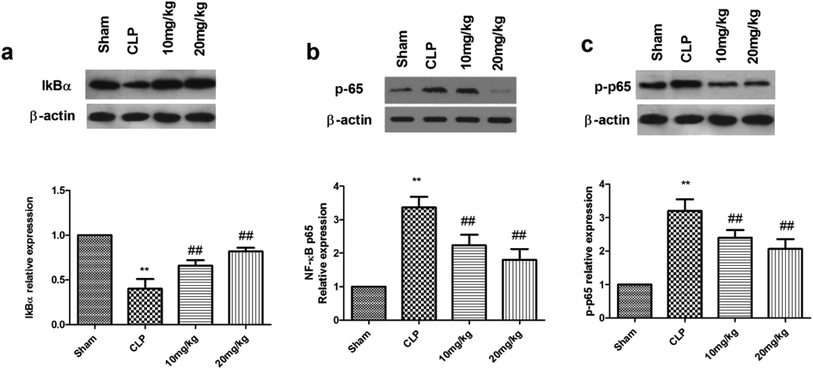
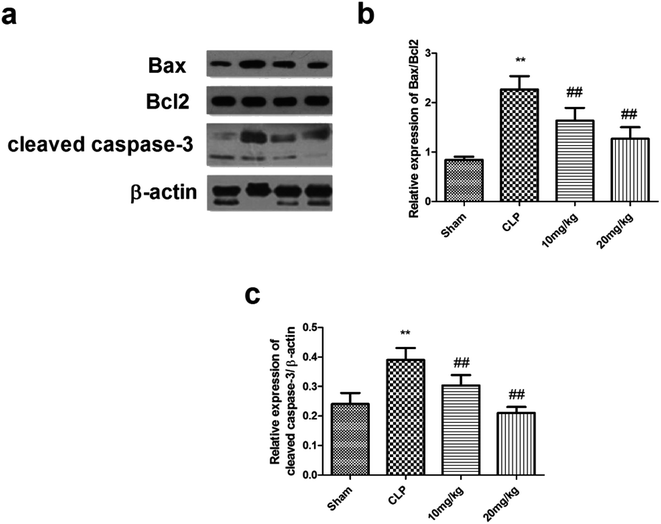
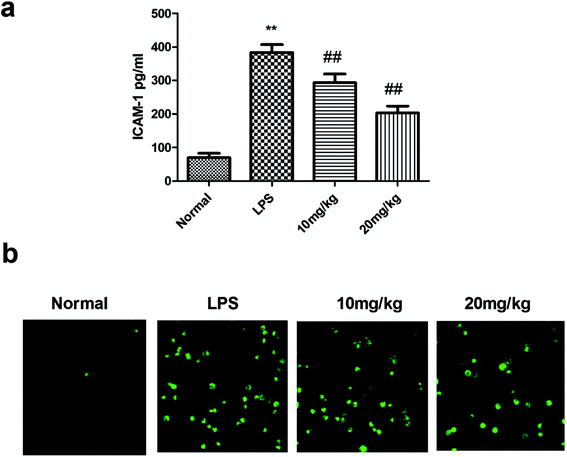
Transcription of the genes encoding many inflammatory proteins is mediated via NF-κB; thus, its inhibition offers a selective advantage in sepsis therapy. Under normal conditions, NF-κB is stored in the cytoplasm by IkB proteins. In the canonical pathway, IkB proteins are phosphorylated and degraded by stimulus, resulting in the rapid translocation of active NF-κB complexes into the nucleus, where they initiate target gene expression or repression. The results have been shown in Fig. 5. Compound 26 had a strong capacity to reduce the decrease of IκBα and increase NF-κB DNA binding activation. Thus, it was suggested that compound 26 had a protective effect on sepsis-induced ALI, possibly because of NF-κB inhibition. The effect of compound 26 on apoptotic markers was also quantified, the results of which are presented in Fig. 6. It was found that compound 26 caused downregulation of the expression levels of caspase-3 and Bax proteins. Intercellular adhesion molecule-1 (ICAM-1) is considered to be a vital adhesion molecule, inducing the migration of neutrophils and infiltration during sepsis. In a recent study, it was concluded that ICAM-1, results in the inhibition of sepsis-induced death and lung injury. Thus, it was imperative to determine the effect of compound 26 on ICAM-1, the results for which are presented in Fig. 7a. It was found that compound 26 caused significant inhibition of ICAM-1 expression in A549 cells compared with that in the lipopolysaccharide (LPS)-treated group. It was also found that the adherence of THP-1 cells to LPS-stimulated A549 cells was inhibited, as shown in Fig. 7b.
Experimental
Chemicals
The starting materials and chemicals used in the processes of synthesis were obtained from Sigma Aldrich (St. Louis, MO, USA). Avance 400 and Avance 100 spectrometers (Billerica, MA, USA) were used for the determination of 1H NMR and 13C NMR spectral data, respectively, in ppm for chemical shifts and hertz (Hz) for the coupling constant. The mass spectrum was obtained using a liquid chromatography/mass spectrometry (LC/MS) system (Waters ZQ; Waters, Milford, MA, USA) and an elemental analysis was conducted for C, H, and O using a Vario elemental analyser (Elementar, Langenselbold, Germany).General procedure for synthesis of 4-((4-substituted phenyl)thiazole-2-yl)amine (3a–e)
A mixture of substituted acetophenone 1(a–e) (110 mmol) and thiourea (2) (220 mmol) in 100 mL of ethanol and then bromine (110 mmol) was added in small fractions (0.5 mL) over 3 h, with continuous stirring, and then heated on a steam bath for 8 h. A sufficient quantity of water was then added and the solution was heated until all of the solid had dissolved. The solution was filtered while hot. The filtrate was then cooled and concentrated ammonia solution was added until precipitation ceased. The solution was recrystallised with a pyridine–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture to achieve a pure product 3(a–e).
1) mixture to achieve a pure product 3(a–e).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1139 (Ar–C–C str), 642 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.86 (d, 2H, J = 7.9 Hz, Ar–H), 7.38 (d, 2H, J = 7.2 Hz, Ar–H), 7.31 (d, 1H, J = 1.3 Hz, Ar–H), 7.15 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 150.3, 133.1, 129.2, 128.8, 127.5, 101.8; mass: 177.29 (M + 1); elemental analysis for C9H8N2S: calculated: C, 61.34; H, 4.58; N, 15.90; found: C, 61.39; H, 4.62; N, 15.88.
N str), 1139 (Ar–C–C str), 642 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.86 (d, 2H, J = 7.9 Hz, Ar–H), 7.38 (d, 2H, J = 7.2 Hz, Ar–H), 7.31 (d, 1H, J = 1.3 Hz, Ar–H), 7.15 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 150.3, 133.1, 129.2, 128.8, 127.5, 101.8; mass: 177.29 (M + 1); elemental analysis for C9H8N2S: calculated: C, 61.34; H, 4.58; N, 15.90; found: C, 61.39; H, 4.62; N, 15.88.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 647 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.68 (d, 2H, J = 8.2 Hz, Ar–H), 7.18 (d, 2H, J = 8.1 Hz, Ar–H), 6.98 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2), 2.29 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.9, 150.3, 131.8, 130.2, 129.6, 125.8, 101.9, 21.4; mass: 191.24 (M + 1); elemental analysis for C10H10N2S: calculated: C, 63.13; H, 5.30; N, 14.72; found: C, 63.18; H, 5.27; N, 14.78.
N str), 1128 (Ar–C–C str), 647 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.68 (d, 2H, J = 8.2 Hz, Ar–H), 7.18 (d, 2H, J = 8.1 Hz, Ar–H), 6.98 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2), 2.29 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.9, 150.3, 131.8, 130.2, 129.6, 125.8, 101.9, 21.4; mass: 191.24 (M + 1); elemental analysis for C10H10N2S: calculated: C, 63.13; H, 5.30; N, 14.72; found: C, 63.18; H, 5.27; N, 14.78.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1518 (C
C str), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1129 (Ar–C–C str), 649 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.64 (d, 2H, J = 8.8 Hz, Ar–H), 7.12 (s, 1H, thiazole-H), 7.05 (d, 2H, J = 8.4 Hz, Ar–H), 6.96 (s, 2H, NH2), 3.84 (s, 3H, OCH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 160.6, 150.2, 128.5, 125.3, 114.8, 101.2, 55.8; mass: 207.29 (M + 1); elemental analysis for C10H10N2OS: calculated: C, 58.23; H, 4.89; N, 13.58; found: C, 58.28; H, 4.93; N, 13.63.
N str), 1129 (Ar–C–C str), 649 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.64 (d, 2H, J = 8.8 Hz, Ar–H), 7.12 (s, 1H, thiazole-H), 7.05 (d, 2H, J = 8.4 Hz, Ar–H), 6.96 (s, 2H, NH2), 3.84 (s, 3H, OCH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 160.6, 150.2, 128.5, 125.3, 114.8, 101.2, 55.8; mass: 207.29 (M + 1); elemental analysis for C10H10N2OS: calculated: C, 58.23; H, 4.89; N, 13.58; found: C, 58.28; H, 4.93; N, 13.63.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1125 (Ar–C–C str), 1094 (Ar–Cl stretch), 648 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.69 (d, 2H, J = 8.9 Hz, Ar–H), 7.58 (d, 2H, J = 8.5 Hz, Ar–H), 7.02 (s, 1H, thiazole-H), 6.95 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.9, 150.4, 134.5, 131.2, 129.2, 128.8, 101.9; mass: 211.73 (M + 1); elemental analysis for C9H7ClN2S: calculated: C, 51.31; H, 3.35; N, 13.30; found: C, 51.26; H, 3.38; N, 13.34.
N str), 1125 (Ar–C–C str), 1094 (Ar–Cl stretch), 648 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 7.69 (d, 2H, J = 8.9 Hz, Ar–H), 7.58 (d, 2H, J = 8.5 Hz, Ar–H), 7.02 (s, 1H, thiazole-H), 6.95 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.9, 150.4, 134.5, 131.2, 129.2, 128.8, 101.9; mass: 211.73 (M + 1); elemental analysis for C9H7ClN2S: calculated: C, 51.31; H, 3.35; N, 13.30; found: C, 51.26; H, 3.38; N, 13.34.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1521 (C
C str), 1521 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1118 (Ar–C–C str), 1156 (Ar–F), 645 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.07 (d, 2H, J = 8.8 Hz, Ar–H), 7.09 (d, 2H, J = 8.2 Hz, Ar–H), 6.98 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 162.8, 150.3, 130.8, 128.6, 116.2, 101.9; mass: 195.28 (M + 1); elemental analysis for C9H7FN2S: calculated: C, 55.65; H, 3.63; N, 14.42; found: C, 55.72; H, 3.68; N, 14.36.
N str), 1118 (Ar–C–C str), 1156 (Ar–F), 645 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.07 (d, 2H, J = 8.8 Hz, Ar–H), 7.09 (d, 2H, J = 8.2 Hz, Ar–H), 6.98 (s, 1H, thiazole-H), 6.94 (s, 2H, NH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 162.8, 150.3, 130.8, 128.6, 116.2, 101.9; mass: 195.28 (M + 1); elemental analysis for C9H7FN2S: calculated: C, 55.65; H, 3.63; N, 14.42; found: C, 55.72; H, 3.68; N, 14.36.General procedure for synthesis of 2-chloro-N-[4-(p-substituted phenyl)-thiazol-2-yl]-acetamides 4(a–e)
Different substituted phenyl–thiazole derivatives 3(a–e) (110 mmol) were dissolved in 20 mL of pyridine, with the dropwise addition of chloroacetyl chloride (112 mmol) to the solution for 30 min with continuous stirring. The mixture was then heated on a water bath for 6 h. The mixture was cooled to room temperature and then poured onto ice. The solid was separated out and washed with distilled water, dried, and recrystallised from ethanol to give 4(a–e).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1628 (C
O str), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 762 (C–Cl str), 649 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 7.83 (d, 2H, J = 7.9 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 7.3 Hz, Ar–H), 7.38 (d, 1H, J = 1.4 Hz, Ar–H), 4.42 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.6, 164.3, 150.2, 133.1, 129.3, 128.8, 127.5, 105.1, 42.8; mass: 253.78 (M + 1); elemental analysis for C11H9ClN2OS: calculated: C, 52.28; H, 3.59; N, 11.08; found: C, 52.31; H, 3.61; N, 11.04.
N str), 1128 (Ar–C–C str), 762 (C–Cl str), 649 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 7.83 (d, 2H, J = 7.9 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 7.3 Hz, Ar–H), 7.38 (d, 1H, J = 1.4 Hz, Ar–H), 4.42 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.6, 164.3, 150.2, 133.1, 129.3, 128.8, 127.5, 105.1, 42.8; mass: 253.78 (M + 1); elemental analysis for C11H9ClN2OS: calculated: C, 52.28; H, 3.59; N, 11.08; found: C, 52.31; H, 3.61; N, 11.04.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1624 (C
O str), 1624 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1126 (Ar–C–C str), 765 (C–Cl str), 652 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.68 (d, 2H, J = 8.2 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.6 Hz, Ar–H), 4.41 (s, 2H, CH2), 2.35 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.5, 164.3, 150.2, 131.8, 130.2, 129.6, 125.9, 105.2, 42.8, 21.4; mass: 267.72 (M + 1); elemental analysis for C12H11ClN2OS: calculated: C, 54.03; H, 4.16; N, 10.50; found: C, 54.09; H, 4.23; N, 10.45.
N str), 1126 (Ar–C–C str), 765 (C–Cl str), 652 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.68 (d, 2H, J = 8.2 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.6 Hz, Ar–H), 4.41 (s, 2H, CH2), 2.35 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.5, 164.3, 150.2, 131.8, 130.2, 129.6, 125.9, 105.2, 42.8, 21.4; mass: 267.72 (M + 1); elemental analysis for C12H11ClN2OS: calculated: C, 54.03; H, 4.16; N, 10.50; found: C, 54.09; H, 4.23; N, 10.45.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1627 (C
O str), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 768 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.08 (s, 1H, –CONH), 7.58 (d, 2H, J = 8.8 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.18 (d, 2H, J = 7.2 Hz, Ar–H), 4.42 (s, 2H, CH2), 3.85 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.7, 164.3, 160.8, 150.4, 128.6, 125.4, 114.8, 105.2, 55.8, 42.9; mass: 283.82 (M + 1); elemental analysis for C12H11ClN2O2S: calculated: C, 50.97; H, 3.92; N, 9.91; found: C, 50.94; H, 3.87; N, 9.98.
N str), 1128 (Ar–C–C str), 768 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.08 (s, 1H, –CONH), 7.58 (d, 2H, J = 8.8 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.18 (d, 2H, J = 7.2 Hz, Ar–H), 4.42 (s, 2H, CH2), 3.85 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.7, 164.3, 160.8, 150.4, 128.6, 125.4, 114.8, 105.2, 55.8, 42.9; mass: 283.82 (M + 1); elemental analysis for C12H11ClN2O2S: calculated: C, 50.97; H, 3.92; N, 9.91; found: C, 50.94; H, 3.87; N, 9.98.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1628 (C
O str), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1514 (C
C str), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1123 (Ar–C–C str), 762 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.12 (s, 1H, –CONH), 7.86 (d, 2H, J = 8.7 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 7.1 Hz, Ar–H), 4.43 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.2, 164.1, 150.2, 134.4, 131.2, 129.3, 128.8, 105.2, 42.6; mass: 288.23 (M + 1); elemental analysis for C11H8Cl2N2OS: calculated: C, 46.01; H, 2.81; N, 9.76; found: C, 46.04; H, 2.85; N, 9.72.
N str), 1123 (Ar–C–C str), 762 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.12 (s, 1H, –CONH), 7.86 (d, 2H, J = 8.7 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 7.1 Hz, Ar–H), 4.43 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.2, 164.1, 150.2, 134.4, 131.2, 129.3, 128.8, 105.2, 42.6; mass: 288.23 (M + 1); elemental analysis for C11H8Cl2N2OS: calculated: C, 46.01; H, 2.81; N, 9.76; found: C, 46.04; H, 2.85; N, 9.72.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1627 (C
O str), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1142 (Ar–F), 1128 (Ar–C–C str), 768 (C–Cl str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.15 (d, 2H, J = 8.9 Hz, Ar–H), 7.51 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.3 Hz, Ar–H), 4.42 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.5, 164.3, 162.8, 150.4, 130.6, 128.6, 116.2, 105.2, 42.8; mass: 271.78 (M + 1); elemental analysis for C11H8ClFN2OS: calculated: C, 48.80; H, 2.98; N, 10.35; found: C, 48.84; H, 2.95; N, 10.42.
N str), 1142 (Ar–F), 1128 (Ar–C–C str), 768 (C–Cl str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.15 (d, 2H, J = 8.9 Hz, Ar–H), 7.51 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.3 Hz, Ar–H), 4.42 (s, 2H, CH2); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.5, 164.3, 162.8, 150.4, 130.6, 128.6, 116.2, 105.2, 42.8; mass: 271.78 (M + 1); elemental analysis for C11H8ClFN2OS: calculated: C, 48.80; H, 2.98; N, 10.35; found: C, 48.84; H, 2.95; N, 10.42.General procedure for synthesis of title compounds 6–30
A mixture of different substituted 2-chloro-N-[4-(p-substituted phenyl)-thiazol-2-yl]-acetamides 4(a–e) (10 mmol) was dissolved in 30 mL of ethanol and different substituted phenyl piperazine derivatives 5(i–v) (10 mmol) were added. The mixture was refluxed for 9 h. Then, the mixture was cooled to room temperature and poured into ice water. The product was precipitated, washed with water, dried, and recrystallised from ethanol to give title compounds 6–30.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1519 (C
C str), 1519 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1124 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 7.85 (d, 2H, J = 7.9 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.2 Hz, Ar–H), 7.29 (d, 1H, J = 1.4 Hz, Ar–H), 7.21 (d, 2H, J = 8.2 Hz, Ar–H), 6.84 (d, 2H, J = 8.1 Hz, Ar–H), 6.72 (d, 1H, J = 1.8 Hz, Ar–H), 3.42 (dd, 4H, J = 10.5 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.56 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.2, 150.4, 149.8, 133.2, 129.8, 129.2, 128.7, 127.5, 121.9, 114.3, 105.2, 63.8, 54.4, 51.6; mass: 379.56 (M + 1); elemental analysis for C21H22N4OS: calculated: C, 66.64; H, 5.86; N, 14.80; found: C, 66.68; H, 5.82; N, 14.78.
N str), 1124 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 7.85 (d, 2H, J = 7.9 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.2 Hz, Ar–H), 7.29 (d, 1H, J = 1.4 Hz, Ar–H), 7.21 (d, 2H, J = 8.2 Hz, Ar–H), 6.84 (d, 2H, J = 8.1 Hz, Ar–H), 6.72 (d, 1H, J = 1.8 Hz, Ar–H), 3.42 (dd, 4H, J = 10.5 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.56 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.2, 150.4, 149.8, 133.2, 129.8, 129.2, 128.7, 127.5, 121.9, 114.3, 105.2, 63.8, 54.4, 51.6; mass: 379.56 (M + 1); elemental analysis for C21H22N4OS: calculated: C, 66.64; H, 5.86; N, 14.80; found: C, 66.68; H, 5.82; N, 14.78.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1638 (C
O str), 1638 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1513 (C
C str), 1513 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1126 (Ar–C–C str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.37 (d, 2H, J = 7.4 Hz, Ar–H), 7.28 (d, 1H, J = 1.2 Hz, Ar–H), 7.08 (d, 2H, J = 8.1 Hz, Ar–H), 6.58 (d, 2H, J = 7.9 Hz, Ar–H), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.54 (dd, 4H, J = 11.4 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 146.8, 133.2, 130.8, 129.8, 129.2, 128.7, 127.5, 112.8, 105.1, 63.6, 54.2, 51.6, 21.3; mass: 393.58 (M + 1); elemental analysis for C22H24N4OS: calculated: C, 67.32; H, 6.16; N, 14.27; found: C, 67.38; H, 6.12; N, 14.28.
N str), 1126 (Ar–C–C str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.37 (d, 2H, J = 7.4 Hz, Ar–H), 7.28 (d, 1H, J = 1.2 Hz, Ar–H), 7.08 (d, 2H, J = 8.1 Hz, Ar–H), 6.58 (d, 2H, J = 7.9 Hz, Ar–H), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.54 (dd, 4H, J = 11.4 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 146.8, 133.2, 130.8, 129.8, 129.2, 128.7, 127.5, 112.8, 105.1, 63.6, 54.2, 51.6, 21.3; mass: 393.58 (M + 1); elemental analysis for C22H24N4OS: calculated: C, 67.32; H, 6.16; N, 14.27; found: C, 67.38; H, 6.12; N, 14.28.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1518 (C
C str), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1124 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.12 (s, 1H, –CONH), 7.83 (d, 2H, J = 7.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.3 Hz, Ar–H), 7.29 (d, 1H, J = 1.2 Hz, Ar–H), 6.87 (d, 2H, J = 8.3 Hz, Ar–H), 6.63 (d, 2H, J = 7.5 Hz, Ar–H), 3.94 (s, 3H, OCH3), 3.42 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.52 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 152.9, 150.2, 146.2, 133.2, 129.4, 128.8, 127.5, 115.3, 115.1, 105.2, 63.8, 55.8, 54.1, 51.8; mass: 409.54 (M + 1); elemental analysis for C22H24N4O2S: calculated: C, 64.68; H, 5.92; N, 13.71; found: C, 64.72; H, 5.89; N, 13.78.
N str), 1124 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.12 (s, 1H, –CONH), 7.83 (d, 2H, J = 7.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.3 Hz, Ar–H), 7.29 (d, 1H, J = 1.2 Hz, Ar–H), 6.87 (d, 2H, J = 8.3 Hz, Ar–H), 6.63 (d, 2H, J = 7.5 Hz, Ar–H), 3.94 (s, 3H, OCH3), 3.42 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.52 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 152.9, 150.2, 146.2, 133.2, 129.4, 128.8, 127.5, 115.3, 115.1, 105.2, 63.8, 55.8, 54.1, 51.8; mass: 409.54 (M + 1); elemental analysis for C22H24N4O2S: calculated: C, 64.68; H, 5.92; N, 13.71; found: C, 64.72; H, 5.89; N, 13.78.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1635 (C
O str), 1635 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 769 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.36 (d, 2H, J = 7.4 Hz, Ar–H), 7.28 (d, 1H, J = 1.2 Hz, Ar–H), 7.25 (d, 2H, J = 8.2 Hz, Ar–H), 6.69 (d, 2H, J = 7.1 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 150.2, 147.9, 133.2, 129.8, 129.1, 128.6, 127.8, 127.2, 115.8, 105.2, 63.8, 54.2, 51.6; mass: 413.96 (M + 1); elemental analysis for C21H21ClN4OS: calculated: C, 61.08; H, 5.13; N, 13.57; found: C, 61.04; H, 5.13; N, 13.56.
N str), 1128 (Ar–C–C str), 769 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.36 (d, 2H, J = 7.4 Hz, Ar–H), 7.28 (d, 1H, J = 1.2 Hz, Ar–H), 7.25 (d, 2H, J = 8.2 Hz, Ar–H), 6.69 (d, 2H, J = 7.1 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 150.2, 147.9, 133.2, 129.8, 129.1, 128.6, 127.8, 127.2, 115.8, 105.2, 63.8, 54.2, 51.6; mass: 413.96 (M + 1); elemental analysis for C21H21ClN4OS: calculated: C, 61.08; H, 5.13; N, 13.57; found: C, 61.04; H, 5.13; N, 13.56.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1514 (C
C str), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1164 (Ar–F), 1123 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.84 (d, 2H, J = 7.9 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.39 (d, 2H, J = 7.5 Hz, Ar–H), 7.29 (d, 1H, J = 1.3 Hz, Ar–H), 7.05 (d, 2H, J = 8.1 Hz, Ar–H), 6.76 (d, 2H, J = 7.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.2, 156.8, 150.2, 145.2, 133.1, 129.4, 128.8, 127.6, 116.5, 115.8, 105.3, 63.6, 54.1, 51.8; mass: 397.51 (M + 1); elemental analysis for C21H21FN4OS: calculated: C, 63.62; H, 5.34; N, 14.13; found: C, 63.61; H, 5.32; N, 14.15.
N str), 1164 (Ar–F), 1123 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.84 (d, 2H, J = 7.9 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.39 (d, 2H, J = 7.5 Hz, Ar–H), 7.29 (d, 1H, J = 1.3 Hz, Ar–H), 7.05 (d, 2H, J = 8.1 Hz, Ar–H), 6.76 (d, 2H, J = 7.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.2, 156.8, 150.2, 145.2, 133.1, 129.4, 128.8, 127.6, 116.5, 115.8, 105.3, 63.6, 54.1, 51.8; mass: 397.51 (M + 1); elemental analysis for C21H21FN4OS: calculated: C, 63.62; H, 5.34; N, 14.13; found: C, 63.61; H, 5.32; N, 14.15.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1634 (C
O str), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.51 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.2 Hz, Ar–H), 7.22 (d, 1H, J = 7.1 Hz, Ar–H), 6.87 (d, 2H, J = 8.2 Hz, Ar–H), 6.72 (d, 2H, J = 1.2 Hz, Ar–H), 3.43 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.3 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.4, 150.3, 149.7, 131.8, 130.2, 129.7, 129.4, 125.8, 121.9, 114.4, 105.2, 63.8, 54.2, 51.8, 21.4; mass: 393.52 (M + 1); elemental analysis for C22H24N4OS: calculated: C, 67.32; H, 6.16; N, 14.27; found: C, 67.31; H, 6.18; N, 14.26.
N str), 1128 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.82 (d, 2H, J = 7.8 Hz, Ar–H), 7.51 (s, 1H, thiazole-H), 7.34 (d, 2H, J = 7.2 Hz, Ar–H), 7.22 (d, 1H, J = 7.1 Hz, Ar–H), 6.87 (d, 2H, J = 8.2 Hz, Ar–H), 6.72 (d, 2H, J = 1.2 Hz, Ar–H), 3.43 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.3 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.4, 150.3, 149.7, 131.8, 130.2, 129.7, 129.4, 125.8, 121.9, 114.4, 105.2, 63.8, 54.2, 51.8, 21.4; mass: 393.52 (M + 1); elemental analysis for C22H24N4OS: calculated: C, 67.32; H, 6.16; N, 14.27; found: C, 67.31; H, 6.18; N, 14.26.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1636 (C
O str), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1514 (C
C str), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1129 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.79 (d, 2H, J = 7.9 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 7.4 Hz, Ar–H), 7.04 (d, 2H, J = 8.4 Hz, Ar–H), 6.68 (d, 2H, J = 1.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.31 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 146.8, 131.8, 130.9, 130.2, 129.8, 129.3, 125.7, 112.8, 105.1, 63.8, 54.1, 51.8, 21.3; mass: 407.55 (M + 1); elemental analysis for C23H26N4OS: calculated: C, 67.95; H, 6.45; N, 13.78; found: C, 67.97; H, 6.44; N, 13.79.
N str), 1129 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.79 (d, 2H, J = 7.9 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 7.4 Hz, Ar–H), 7.04 (d, 2H, J = 8.4 Hz, Ar–H), 6.68 (d, 2H, J = 1.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.31 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 146.8, 131.8, 130.9, 130.2, 129.8, 129.3, 125.7, 112.8, 105.1, 63.8, 54.1, 51.8, 21.3; mass: 407.55 (M + 1); elemental analysis for C23H26N4OS: calculated: C, 67.95; H, 6.45; N, 13.78; found: C, 67.97; H, 6.44; N, 13.79.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1638 (C
O str), 1638 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1124 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.81 (d, 2H, J = 7.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.31 (d, 2H, J = 7.3 Hz, Ar–H), 6.92 (d, 2H, J = 8.2 Hz, Ar–H), 6.65 (d, 2H, J = 1.3 Hz, Ar–H), 3.87 (s, 3H, OCH3) 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.34 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.2, 152.8, 150.3, 146.4, 131.8, 130.1, 129.6, 125.8, 115.4, 115.1, 105.1, 63.8, 54.2, 51.8, 56.8, 21.4; mass: 423.56 (M + 1); elemental analysis for C23H26N4O2S: calculated: C, 65.38; H, 6.20; N, 13.26; found: C, 65.36; H, 6.20; N, 13.28.
N str), 1124 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.81 (d, 2H, J = 7.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.31 (d, 2H, J = 7.3 Hz, Ar–H), 6.92 (d, 2H, J = 8.2 Hz, Ar–H), 6.65 (d, 2H, J = 1.3 Hz, Ar–H), 3.87 (s, 3H, OCH3) 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.34 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.2, 152.8, 150.3, 146.4, 131.8, 130.1, 129.6, 125.8, 115.4, 115.1, 105.1, 63.8, 54.2, 51.8, 56.8, 21.4; mass: 423.56 (M + 1); elemental analysis for C23H26N4O2S: calculated: C, 65.38; H, 6.20; N, 13.26; found: C, 65.36; H, 6.20; N, 13.28.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1518 (C
C str), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1126 (Ar–C–C str), 773 (C–Cl str), 656 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.78 (d, 2H, J = 7.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.30 (d, 2H, J = 7.4 Hz, Ar–H), 7.24 (d, 2H, J = 8.4 Hz, Ar–H), 6.69 (d, 2H, J = 1.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.3 Hz, piperazine-H), 2.32 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 147.8, 131.8, 130.2, 129.8, 129.4, 127.2, 125.8, 115.7, 105.1, 63.8, 54.1, 51.7, 21.4; mass: 427.98 (M + 1); elemental analysis for C22H23ClN4OS: calculated: C, 61.89; H, 5.43; N, 13.12; found: C, 61.91; H, 5.42; N, 13.12.
N str), 1126 (Ar–C–C str), 773 (C–Cl str), 656 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.78 (d, 2H, J = 7.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.30 (d, 2H, J = 7.4 Hz, Ar–H), 7.24 (d, 2H, J = 8.4 Hz, Ar–H), 6.69 (d, 2H, J = 1.4 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.3 Hz, piperazine-H), 2.32 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.2, 147.8, 131.8, 130.2, 129.8, 129.4, 127.2, 125.8, 115.7, 105.1, 63.8, 54.1, 51.7, 21.4; mass: 427.98 (M + 1); elemental analysis for C22H23ClN4OS: calculated: C, 61.89; H, 5.43; N, 13.12; found: C, 61.91; H, 5.42; N, 13.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1636 (C
O str), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1519 (C
C str), 1519 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1148 (Ar–F), 1128 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.76 (d, 2H, J = 7.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.31 (d, 2H, J = 7.3 Hz, Ar–H), 7.04 (d, 2H, J = 8.1 Hz, Ar–H), 6.84 (d, 2H, J = 1.2 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.31 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 156.8, 150.4, 145.2, 131.9, 130.2, 129.6, 125.9, 116.4, 115.9, 105.2, 63.8, 54.1, 51.8, 21.4; mass: 411.52 (M + 1); elemental analysis for C22H23FN4OS: calculated: C, 64.37; H, 5.65; N, 13.65; found: C, 64.39; H, 5.65; N, 13.64.
N str), 1148 (Ar–F), 1128 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.76 (d, 2H, J = 7.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.31 (d, 2H, J = 7.3 Hz, Ar–H), 7.04 (d, 2H, J = 8.1 Hz, Ar–H), 6.84 (d, 2H, J = 1.2 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.31 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 156.8, 150.4, 145.2, 131.9, 130.2, 129.6, 125.9, 116.4, 115.9, 105.2, 63.8, 54.1, 51.8, 21.4; mass: 411.52 (M + 1); elemental analysis for C22H23FN4OS: calculated: C, 64.37; H, 5.65; N, 13.65; found: C, 64.39; H, 5.65; N, 13.64.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1638 (C
O str), 1638 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1517 (C
C str), 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1126 (Ar–C–C str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.56 (d, 2H, J = 8.8 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.27 (d, 2H, J = 7.6 Hz, Ar–H), 7.02 (d, 2H, J = 8.2 Hz, Ar–H), 6.87 (d, 2H, J = 8.1 Hz, Ar–H), 6.74 (d, 1H, J = 1.4 Hz, Ar–H), 3.87 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.8, 150.4, 149.8, 129.8, 128.4, 125.4, 121.9, 114.9, 114.2, 105.2, 63.8, 55.8, 54.2, 51.8; mass: 409.53 (M + 1); elemental analysis for C22H24N4O2S: calculated: C, 64.68; H, 5.92; N, 13.71; found: C, 64.70; H, 5.91; N, 13.72.
N str), 1126 (Ar–C–C str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.56 (d, 2H, J = 8.8 Hz, Ar–H), 7.52 (s, 1H, thiazole-H), 7.27 (d, 2H, J = 7.6 Hz, Ar–H), 7.02 (d, 2H, J = 8.2 Hz, Ar–H), 6.87 (d, 2H, J = 8.1 Hz, Ar–H), 6.74 (d, 1H, J = 1.4 Hz, Ar–H), 3.87 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.8, 150.4, 149.8, 129.8, 128.4, 125.4, 121.9, 114.9, 114.2, 105.2, 63.8, 55.8, 54.2, 51.8; mass: 409.53 (M + 1); elemental analysis for C22H24N4O2S: calculated: C, 64.68; H, 5.92; N, 13.71; found: C, 64.70; H, 5.91; N, 13.72.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1639 (C
O str), 1639 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1129 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.55 (d, 2H, J = 8.7 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.06 (d, 2H, J = 8.2 Hz, Ar–H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.69 (d, 2H, J = 8.4 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.46 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.35 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.4, 160.7, 150.3, 146.6, 130.8, 129.9, 128.6, 125.4, 114.9, 112.8, 105.2, 63.8, 55.8, 54.1, 51.6, 21.3; mass: 423.55 (M + 1); elemental analysis for C23H26N4O2S: calculated: C, 65.38; H, 6.20; N, 13.26; found: C, 65.40; H, 6.18; N, 13.27.
N str), 1129 (Ar–C–C str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.55 (d, 2H, J = 8.7 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.06 (d, 2H, J = 8.2 Hz, Ar–H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.69 (d, 2H, J = 8.4 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.46 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.35 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.4, 160.7, 150.3, 146.6, 130.8, 129.9, 128.6, 125.4, 114.9, 112.8, 105.2, 63.8, 55.8, 54.1, 51.6, 21.3; mass: 423.55 (M + 1); elemental analysis for C23H26N4O2S: calculated: C, 65.38; H, 6.20; N, 13.26; found: C, 65.40; H, 6.18; N, 13.27.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1637 (C
O str), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1518 (C
C str), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1127 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.58 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.87 (d, 2H, J = 8.6 Hz, Ar–H), 6.64 (d, 2H, J = 8.3 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.8, 152.9, 150.3, 146.2, 128.7, 125.4, 115.3, 115.2, 114.9, 105.2, 63.8, 55.8, 54.2, 51.6; mass: 439.54 (M + 1); elemental analysis for C23H26N4O3S: calculated: C, 62.99; H, 5.98; N, 12.78; found: C, 62.96; H, 5.98; N, 12.77.
N str), 1127 (Ar–C–C str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.58 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.87 (d, 2H, J = 8.6 Hz, Ar–H), 6.64 (d, 2H, J = 8.3 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.8, 152.9, 150.3, 146.2, 128.7, 125.4, 115.3, 115.2, 114.9, 105.2, 63.8, 55.8, 54.2, 51.6; mass: 439.54 (M + 1); elemental analysis for C23H26N4O3S: calculated: C, 62.99; H, 5.98; N, 12.78; found: C, 62.96; H, 5.98; N, 12.77.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1630 (C
O str), 1630 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1129 (Ar–C–C str), 783 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.56 (d, 2H, J = 8.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.2 Hz, Ar–H), 7.04 (d, 2H, J = 8.1 Hz, Ar–H), 6.71 (d, 2H, J = 8.5 Hz, Ar–H), 3.83 (s, 3H, OCH3), 3.43 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 160.8, 150.2, 147.7, 129.8, 128.4, 127.2, 125.4, 115.7, 114.8, 105.1, 63.7, 54.1, 51.6, 55.8; mass: 443.97 (M + 1); elemental analysis for C22H23ClN4O2S: calculated: C, 59.65; H, 5.23; N, 12.65; found: C, 59.67; H, 5.23; N, 12.66.
N str), 1129 (Ar–C–C str), 783 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 7.56 (d, 2H, J = 8.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.2 Hz, Ar–H), 7.04 (d, 2H, J = 8.1 Hz, Ar–H), 6.71 (d, 2H, J = 8.5 Hz, Ar–H), 3.83 (s, 3H, OCH3), 3.43 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 160.8, 150.2, 147.7, 129.8, 128.4, 127.2, 125.4, 115.7, 114.8, 105.1, 63.7, 54.1, 51.6, 55.8; mass: 443.97 (M + 1); elemental analysis for C22H23ClN4O2S: calculated: C, 59.65; H, 5.23; N, 12.65; found: C, 59.67; H, 5.23; N, 12.66.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1631 (C
O str), 1631 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1516 (C
C str), 1516 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1168 (Ar–F), 1121 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.54 (d, 2H, J = 8.7 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.09 (d, 2H, J = 8.1 Hz, Ar–H), 7.03 (d, 2H, J = 8.2 Hz, Ar–H), 6.75 (d, 2H, J = 8.4 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.7, 156.8, 150.2, 145.3, 128.6, 125.4, 116.5, 115.8, 114.9, 105.2, 63.7, 55.8, 54.2, 51.8; mass: 427.52 (M + 1); elemental analysis for C22H23FN4O2S: calculated: C, 61.95; H, 5.44; N, 13.14; found: C, 61.97; H, 5.42; N, 13.14.
N str), 1168 (Ar–F), 1121 (Ar–C–C str), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 7.54 (d, 2H, J = 8.7 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.09 (d, 2H, J = 8.1 Hz, Ar–H), 7.03 (d, 2H, J = 8.2 Hz, Ar–H), 6.75 (d, 2H, J = 8.4 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.45 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.48 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 160.7, 156.8, 150.2, 145.3, 128.6, 125.4, 116.5, 115.8, 114.9, 105.2, 63.7, 55.8, 54.2, 51.8; mass: 427.52 (M + 1); elemental analysis for C22H23FN4O2S: calculated: C, 61.95; H, 5.44; N, 13.14; found: C, 61.97; H, 5.42; N, 13.14.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1635 (C
O str), 1635 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1517 (C
C str), 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1125 (Ar–C–C str), 792 (C–Cl str), 652 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.42 (d, 2H, J = 7.8 Hz, Ar–H), 7.23 (d, 2H, J = 8.8 Hz, Ar–H), 6.87 (d, 2H, J = 8.2 Hz, Ar–H), 6.68 (d, 1H, J = 1.3 Hz, Ar–H), 3.43 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 150.4, 149.7, 134.4, 131.2, 129.7, 129.4, 128.8, 121.9, 114.4, 105.2, 63.7, 54.1, 51.7; mass: 413.96 (M + 1); elemental analysis for C21H21ClN4OS: calculated: C, 61.08; H, 5.13; N, 13.57; found: C, 61.06; H, 5.13; N, 13.57.
N str), 1125 (Ar–C–C str), 792 (C–Cl str), 652 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.42 (d, 2H, J = 7.8 Hz, Ar–H), 7.23 (d, 2H, J = 8.8 Hz, Ar–H), 6.87 (d, 2H, J = 8.2 Hz, Ar–H), 6.68 (d, 1H, J = 1.3 Hz, Ar–H), 3.43 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.46 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 150.4, 149.7, 134.4, 131.2, 129.7, 129.4, 128.8, 121.9, 114.4, 105.2, 63.7, 54.1, 51.7; mass: 413.96 (M + 1); elemental analysis for C21H21ClN4OS: calculated: C, 61.08; H, 5.13; N, 13.57; found: C, 61.06; H, 5.13; N, 13.57.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1519 (C
C str), 1519 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1121 (Ar–C–C str), 798 (C–Cl str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 8.03 (d, 2H, J = 8.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 7.9 Hz, Ar–H), 7.04 (d, 2H, J = 8.2 Hz, Ar–H), 6.62 (d, 2H, J = 8.1 Hz, Ar–H), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 150.3, 146.7, 134.4, 131.2, 130.8, 129.8, 129.3, 128.7, 112.9, 105.3, 63.7, 54.3, 51.7, 21.4; mass: 427.98 (M + 1); elemental analysis for C22H23ClN4OS: calculated: C, 61.89; H, 5.43; N, 13.12; found: C, 61.91; H, 5.45; N, 13.12.
N str), 1121 (Ar–C–C str), 798 (C–Cl str), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.16 (s, 1H, –CONH), 8.03 (d, 2H, J = 8.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 7.9 Hz, Ar–H), 7.04 (d, 2H, J = 8.2 Hz, Ar–H), 6.62 (d, 2H, J = 8.1 Hz, Ar–H), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 150.3, 146.7, 134.4, 131.2, 130.8, 129.8, 129.3, 128.7, 112.9, 105.3, 63.7, 54.3, 51.7, 21.4; mass: 427.98 (M + 1); elemental analysis for C22H23ClN4OS: calculated: C, 61.89; H, 5.43; N, 13.12; found: C, 61.91; H, 5.45; N, 13.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1631 (C
O str), 1631 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1517 (C
C str), 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1125 (Ar–C–C str), 794 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.57 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 8.1 Hz, Ar–H), 6.84 (d, 2H, J = 8.3 Hz, Ar–H), 6.58 (d, 2H, J = 8.1 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.44 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 152.9, 150.2, 146.4, 134.4, 131.2, 129.4, 128.8, 115.4, 115.1, 105.2, 63.7, 55.8, 54.2, 51.8; mass: 443.97 (M + 1); elemental analysis for C22H23ClN4O2S: calculated: C, 59.65; H, 5.23; N, 12.65; found: C, 59.67; H, 5.22; N, 12.67.
N str), 1125 (Ar–C–C str), 794 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.57 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 8.1 Hz, Ar–H), 6.84 (d, 2H, J = 8.3 Hz, Ar–H), 6.58 (d, 2H, J = 8.1 Hz, Ar–H), 3.85 (s, 3H, OCH3), 3.44 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 152.9, 150.2, 146.4, 134.4, 131.2, 129.4, 128.8, 115.4, 115.1, 105.2, 63.7, 55.8, 54.2, 51.8; mass: 443.97 (M + 1); elemental analysis for C22H23ClN4O2S: calculated: C, 59.65; H, 5.23; N, 12.65; found: C, 59.67; H, 5.22; N, 12.67.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1634 (C
O str), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1514 (C
C str), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1128 (Ar–C–C str), 792 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 8.03 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 8.2 Hz, Ar–H), 7.28 (d, 2H, J = 8.5 Hz, Ar–H), 6.62 (d, 2H, J = 7.8 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.3, 147.7, 134.4, 131.2, 129.8, 129.2, 128.9, 127.2, 115.7, 105.1, 63.7, 54.2, 51.8; mass: 448.37 (M + 1); elemental analysis for C21H20Cl2N4OS: calculated: C, 56.38; H, 4.51; N, 12.52; found: C, 56.37; H, 4.52; N, 12.51.
N str), 1128 (Ar–C–C str), 792 (C–Cl str), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 8.03 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.46 (d, 2H, J = 8.2 Hz, Ar–H), 7.28 (d, 2H, J = 8.5 Hz, Ar–H), 6.62 (d, 2H, J = 7.8 Hz, Ar–H), 3.43 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.49 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 150.3, 147.7, 134.4, 131.2, 129.8, 129.2, 128.9, 127.2, 115.7, 105.1, 63.7, 54.2, 51.8; mass: 448.37 (M + 1); elemental analysis for C21H20Cl2N4OS: calculated: C, 56.38; H, 4.51; N, 12.52; found: C, 56.37; H, 4.52; N, 12.51.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1632 (C
O str), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1518 (C
C str), 1518 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1174 (Ar–F), 1121 (Ar–C–C str), 798 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 8.4 Hz, Ar–H), 7.04 (d, 2H, J = 8.8 Hz, Ar–H), 6.65 (d, 2H, J = 7.6 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 156.9, 150.3, 145.3, 134.4, 131.2, 129.4, 128.9, 116.5, 115.8, 105.3, 63.7, 54.2, 51.8; mass: 431.95 (M + 1); elemental analysis for C21H20ClFN4OS: calculated: C, 58.53; H, 4.68; N, 13.00; found: C, 58.55; H, 4.69; N, 13.01.
N str), 1174 (Ar–F), 1121 (Ar–C–C str), 798 (C–Cl str), 657 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.02 (d, 2H, J = 8.9 Hz, Ar–H), 7.53 (s, 1H, thiazole-H), 7.48 (d, 2H, J = 8.4 Hz, Ar–H), 7.04 (d, 2H, J = 8.8 Hz, Ar–H), 6.65 (d, 2H, J = 7.6 Hz, Ar–H), 3.45 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.47 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 156.9, 150.3, 145.3, 134.4, 131.2, 129.4, 128.9, 116.5, 115.8, 105.3, 63.7, 54.2, 51.8; mass: 431.95 (M + 1); elemental analysis for C21H20ClFN4OS: calculated: C, 58.53; H, 4.68; N, 13.00; found: C, 58.55; H, 4.69; N, 13.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1639 (C
O str), 1639 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1187 (Ar–F), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.14 (d, 2H, J = 8.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.3 Hz, Ar–H), 7.18 (d, 2H, J = 8.2 Hz, Ar–H), 6.96 (d, 2H, J = 8.1 Hz, Ar–H), 6.72 (d, 1H, J = 1.4 Hz, Ar–H), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.44 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 162.8, 150.2, 149.8, 130.7, 129.8, 128.6, 121.9, 116.2, 114.3, 105.2, 63.7, 54.2, 51.6; mass: 397.49 (M + 1); elemental analysis for C21H21FN4OS: calculated: C, 63.62; H, 5.34; N, 14.13; found: C, 63.63; H, 5.34; N, 14.11.
N str), 1187 (Ar–F), 654 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.14 (d, 2H, J = 8.8 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.3 Hz, Ar–H), 7.18 (d, 2H, J = 8.2 Hz, Ar–H), 6.96 (d, 2H, J = 8.1 Hz, Ar–H), 6.72 (d, 1H, J = 1.4 Hz, Ar–H), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.44 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.7, 164.3, 162.8, 150.2, 149.8, 130.7, 129.8, 128.6, 121.9, 116.2, 114.3, 105.2, 63.7, 54.2, 51.6; mass: 397.49 (M + 1); elemental analysis for C21H21FN4OS: calculated: C, 63.62; H, 5.34; N, 14.13; found: C, 63.63; H, 5.34; N, 14.11.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1643 (C
O str), 1643 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1514 (C
C str), 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1189 (Ar–F), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 8.12 (d, 2H, J = 8.9 Hz, Ar–H), 7.57 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 8.2 Hz, Ar–H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.58 (d, 2H, J = 7.8 Hz, Ar–H), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.42 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 150.2, 146.7, 130.9, 130.5, 129.9, 128.7, 116.2, 112.9, 105.2, 63.7, 54.1, 51.6, 21.5; mass: 411.52 (M + 1); elemental analysis for C22H23FN4OS: calculated: C, 64.37; H, 5.65; N, 13.65; found: C, 64.39; H, 5.67; N, 13.65.
N str), 1189 (Ar–F), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.15 (s, 1H, –CONH), 8.12 (d, 2H, J = 8.9 Hz, Ar–H), 7.57 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 8.2 Hz, Ar–H), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 6.58 (d, 2H, J = 7.8 Hz, Ar–H), 3.45 (dd, 4H, J = 10.4 Hz, piperazine-H), 3.17 (s, 2H, CH2), 2.42 (dd, 4H, J = 11.2 Hz, piperazine-H), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 150.2, 146.7, 130.9, 130.5, 129.9, 128.7, 116.2, 112.9, 105.2, 63.7, 54.1, 51.6, 21.5; mass: 411.52 (M + 1); elemental analysis for C22H23FN4OS: calculated: C, 64.37; H, 5.65; N, 13.65; found: C, 64.39; H, 5.67; N, 13.65.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1648 (C
O str), 1648 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1512 (C
C str), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1193 (Ar–F), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.16 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.3 Hz, Ar–H), 6.83 (d, 2H, J = 8.2 Hz, Ar–H), 6.54 (d, 2H, J = 7.9 Hz, Ar–H), 3.84 (s, 3H, OCH3), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 162.8, 152.8, 150.3, 146.4, 130.8, 128.7, 116.2, 115.6, 115.3, 105.2, 63.7, 56.9, 54.2, 51.8; mass: 427.53 (M + 1); elemental analysis for C22H23FN4O2S: calculated: C, 61.95; H, 5.44; N, 13.14; found: C, 61.97; H, 5.43; N, 13.15.
N str), 1193 (Ar–F), 659 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.16 (d, 2H, J = 8.8 Hz, Ar–H), 7.55 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.3 Hz, Ar–H), 6.83 (d, 2H, J = 8.2 Hz, Ar–H), 6.54 (d, 2H, J = 7.9 Hz, Ar–H), 3.84 (s, 3H, OCH3), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.19 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.1 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.6, 164.3, 162.8, 152.8, 150.3, 146.4, 130.8, 128.7, 116.2, 115.6, 115.3, 105.2, 63.7, 56.9, 54.2, 51.8; mass: 427.53 (M + 1); elemental analysis for C22H23FN4O2S: calculated: C, 61.95; H, 5.44; N, 13.14; found: C, 61.97; H, 5.43; N, 13.15.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1649 (C
O str), 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1517 (C
C str), 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1195 (Ar–F), 796 (C–Cl str), 653 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.14 (d, 2H, J = 8.9 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 8.4 Hz, Ar–H), 7.24 (d, 2H, J = 8.1 Hz, Ar–H), 6.63 (d, 2H, J = 7.8 Hz, Ar–H), 3.48 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.43 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 150.2, 147.8, 130.8, 129.8, 128.7, 127.3, 116.2, 115.8, 105.2, 63.7, 54.1, 51.6; mass: 431.93 (M + 1); elemental analysis for C21H20ClFN4OS: calculated: C, 58.53; H, 4.68; N, 13.00; found: C, 58.55; H, 4.70; N, 13.02.
N str), 1195 (Ar–F), 796 (C–Cl str), 653 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.14 (s, 1H, –CONH), 8.14 (d, 2H, J = 8.9 Hz, Ar–H), 7.56 (s, 1H, thiazole-H), 7.29 (d, 2H, J = 8.4 Hz, Ar–H), 7.24 (d, 2H, J = 8.1 Hz, Ar–H), 6.63 (d, 2H, J = 7.8 Hz, Ar–H), 3.48 (dd, 4H, J = 10.3 Hz, piperazine-H), 3.16 (s, 2H, CH2), 2.43 (dd, 4H, J = 11.2 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 150.2, 147.8, 130.8, 129.8, 128.7, 127.3, 116.2, 115.8, 105.2, 63.7, 54.1, 51.6; mass: 431.93 (M + 1); elemental analysis for C21H20ClFN4OS: calculated: C, 58.53; H, 4.68; N, 13.00; found: C, 58.55; H, 4.70; N, 13.02.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str), 1647 (C
O str), 1647 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1519 (C
C str), 1519 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1193 (Ar–F), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.15 (d, 2H, J = 8.8 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.2 Hz, Ar–H), 7.04 (d, 2H, J = 8.4 Hz, Ar–H), 6.69 (d, 2H, J = 7.5 Hz, Ar–H), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 156.9, 150.3, 145.2, 130.6, 128.7, 116.4, 116.1, 115.9, 105.3, 63.8, 54.3, 51.8; mass: 415.14 (M + 1); elemental analysis for C21H20F2N4OS: calculated: C, 60.85; H, 4.86; N, 13.52; found: C, 60.87; H, 4.86; N, 13.52.
N str), 1193 (Ar–F), 658 (C–S str) cm−1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 9.13 (s, 1H, –CONH), 8.15 (d, 2H, J = 8.8 Hz, Ar–H), 7.54 (s, 1H, thiazole-H), 7.28 (d, 2H, J = 8.2 Hz, Ar–H), 7.04 (d, 2H, J = 8.4 Hz, Ar–H), 6.69 (d, 2H, J = 7.5 Hz, Ar–H), 3.46 (dd, 4H, J = 10.2 Hz, piperazine-H), 3.18 (s, 2H, CH2), 2.45 (dd, 4H, J = 11.3 Hz, piperazine-H); 13C-NMR (100 MHz, CDCl3) δ, ppm: 168.4, 164.3, 162.8, 156.9, 150.3, 145.2, 130.6, 128.7, 116.4, 116.1, 115.9, 105.3, 63.8, 54.3, 51.8; mass: 415.14 (M + 1); elemental analysis for C21H20F2N4OS: calculated: C, 60.85; H, 4.86; N, 13.52; found: C, 60.87; H, 4.86; N, 13.52.MMP-2 and MMP-8 inhibitory activity
Recombinant human pro-MMP-2 and -8 were purchased from R&D Systems (Minneapolis, MN, USA), with catalogue numbers 902-MP and 908-MP, respectively, and with fluorogenic peptide QF24 as a substrate for the in situ determination of MMP. The proenzymes (pro-MMP-2 and -8) were activated immediately before their use with p-aminophenylmercuric acetate (APMA, 1 mM) for 1 h at 37 °C. Briefly, 10−2 mM solutions of the inhibitors were made in 5% MeOH/DMSO and further diluted as required in the assay buffer (50 mM Tris–HCl, 5 mM CaCl2, 0.02% NaN3, 0.05% Brij 35, pH 7.4, for MMP-2; and 50 mM Tris–HCl, 10 mM CaCl2, 150 mM NaCl, pH 7.5, for MMP-8). The activated enzyme together with inhibitor solution was incubated in the assay buffer for 3 h, and the temperature was maintained at 25 °C for MMP-2 and at 37 °C for MMP-8. After the addition of a 0.054 mM DMSO solution of fluorogenic substrate QF24, hydrolysis was monitored by recording the increase in fluorescence (λex 328 and 393 nm) during a 30 min period using a spectrofluorometer. The IC50 values were calculated from control reactions without the inhibitor and performed in triplicate.Pharmacological evaluation
Group 1: sham group (sham),
Group 2: CLP group (CLP),
Group 3: compound 26 (10 mg kg−1),
Group 4: compound 26 (20 mg kg−1).
The CLP-induced sepsis model was established after fasting for 6 h before surgery, under anaesthesia. After sterilisation, a 1.5 cm ventral midline abdominal incision was made and the cecum was then gently isolated and ligated with a 3–0 silk suture, punctured with an 18-gauge needle at three locations, and then repositioned. The abdomen was then closed. Rats in the sham group underwent the same surgery, but the cecum was manipulated without being ligated or perforated. Saline (2 mL/100 g body weight) was given subcutaneously to the rats immediately after the operation for resuscitation. For the compound 26 treatment groups, rats received 10 or 20 mg kg−1 intraperitoneally 30 min before the surgery. Rats in the sham and CLP groups received only the vehicle.
Experimental setup
Histology
The lung tissues were fixed with 4% paraformaldehyde and dehydrated with a graded concentration of alcohols. Tissues were fixed in paraffin and sliced into 5 mm sections. The sections were stained with haematoxylin and eosin (H&E) for 3 min and observed under a light microscope.Measurement of the lung wet/dry weight ratio
The rat lungs were weighed immediately after being harvested and then dried at 80 °C for 72 h to achieve constant weight. The wet lung/dry lung ratio was calculated.Analysis of total protein concentration and cell count of the BALF
The BALF was centrifuged at 1500 rpm for 10 min at 4 °C. The supernatant was collected for total protein analysis using a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Haimen, China), in accordance with the manufacturer's instructions. The cell pellets were resuspended and stained with Wright–Giemsa solution for leukocyte counting using a haemocytometer. The cells were classified as mononuclear and polymorphonuclear based on their normal morphology.Biochemical determination in lung tissues
For the measurement of oxidative states in lung tissues, the activities of myeloperoxidase (MPO) and superoxide dismutase (SOD) and the levels of malondialdehyde (MDA) and glutathione (GSH) in lung homogenates were measured using corresponding commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). For the measurement of inflammatory responses in lung tissues, levels of prostaglandin E2 (PGE2) were measured using a PGE2 Assay Kit (Wanleibio, Shenyang, China). All measurements were performed in accordance with the manufacturer's protocol.Serum cytokines analysis
The whole blood was centrifuged at 1500 rpm for 10 min at 4 °C and the serum was collected. Levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in serum were determined by enzyme-linked immunosorbent assays using commercial kits specific for rats, in accordance with the manufacturer's instructions (USCN Life Science Inc., Wuhan, China). The concentrations are expressed in picograms per milligram protein.Immunohistochemistry
After being dewaxed and hydrated, sections of lung tissue were blocked with phosphate-buffered saline (PBS) containing 10% bovine serum albumin at room temperature and incubated overnight at 4 °C with cyclooxygenase (COX)-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, BA0738; Wanleibio, Shanghai, China) primary antibody. After being incubated in biotinylated goat anti-rabbit secondary antibodies (1
500, BA0738; Wanleibio, Shanghai, China) primary antibody. After being incubated in biotinylated goat anti-rabbit secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200; Beyotime Institute of Biotechnology) for 30 min, sections were then incubated with horseradish peroxidase (HRP)-labelled streptavidin (Beyotime) for 30 min at 37 °C. Each step was followed by three rinses in PBS. The peroxidase was visualized by reaction with diaminobenzidine tetrahydrochloride. Sections were counterstained with haematoxylin and analysed under an optical microscope.
200; Beyotime Institute of Biotechnology) for 30 min, sections were then incubated with horseradish peroxidase (HRP)-labelled streptavidin (Beyotime) for 30 min at 37 °C. Each step was followed by three rinses in PBS. The peroxidase was visualized by reaction with diaminobenzidine tetrahydrochloride. Sections were counterstained with haematoxylin and analysed under an optical microscope.
Protein extraction and western blotting analysis
Lung tissues were homogenised in NP-40 lysis buffer (Beyotime) containing 1% Triton X-100 (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) with 1 mM phenylmethanesulphonyl fluoride (Beyotime Institute of Biotechnology) and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. The supernatants were collected as total protein. Nuclear and cytosolic proteins were extracted using a nuclear and cytoplasmic protein extraction kit (Beyotime Institute of Biotechnology) following the manufacturer's protocol. A commercial bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology) was used for the determination of protein concentration.
000g for 10 min. The supernatants were collected as total protein. Nuclear and cytosolic proteins were extracted using a nuclear and cytoplasmic protein extraction kit (Beyotime Institute of Biotechnology) following the manufacturer's protocol. A commercial bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology) was used for the determination of protein concentration.
For western blot analysis, protein was subjected to 13% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After being blocked with skimmed milk in 1× PBS in 0.05% Tween-20 for 1 h, the membrane was incubated overnight with diluted anti-COX-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, BA0738; Boster, Wuhan, China), inhibitors of NF-κBα (IκBα) (1
1000, BA0738; Boster, Wuhan, China), inhibitors of NF-κBα (IκBα) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs-1287R; Bioss, Beijing, China), p65 antibodies (1
1000, bs-1287R; Bioss, Beijing, China), p65 antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, BA0610; Boster), p-p65ser536 antibodies (1
1000, BA0610; Boster), p-p65ser536 antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs-0982R; Bioss), Bcl-2 antibodies, and Bax antibodies in blocking buffer. The blot was washed with 1× PBS in 0.05% Tween-20 thrice and incubated with HRP-conjugated goat anti-rabbit IgG (1
1000, bs-0982R; Bioss), Bcl-2 antibodies, and Bax antibodies in blocking buffer. The blot was washed with 1× PBS in 0.05% Tween-20 thrice and incubated with HRP-conjugated goat anti-rabbit IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000; Beyotime Institute of Biotechnology) for 1 h at room temperature. β-Actin and lamin A were used as loading controls. The blots were analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The final data were obtained by normalization with a loading control and values of the sham group.
5000; Beyotime Institute of Biotechnology) for 1 h at room temperature. β-Actin and lamin A were used as loading controls. The blots were analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The final data were obtained by normalization with a loading control and values of the sham group.
Compound 26 treatment of A549 cells (Fig. 7)
A549, a human lung epithelial cell line, was obtained from the Bioresource Collection and Research Center in Taiwan. Cells were cultured in F-12 nutrient mixture medium (Life Technologies, Carlsbad, CA, USA), which contained 10% foetal bovine serum (Biological Industries, Haemek, Israel), penicillin (100 units per mL), and streptomycin (100 μg mL−1). Compound 26 was dissolved in DMSO to obtain stock solutions of 10 mg mL−1. In all cell experiments, the DMSO concentration in the culture medium was ≤0.1%. A549 cells were pretreated with compound 26 (1–10 μg mL−1) for 1 h and then stimulated with 1 μg mL−1 LPS for 24 h. The supernatants were collected and assayed with ELISA kits for the specific detection of selected chemokines or cytokines. Moreover, A549 cell viability was tested with MTT reagent. Results were assayed at 570 nm on a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA, USA).Cell adhesion assay
A549 cells were treated with two doses of compound 26 (10 and 20 mg kg−1) and stimulated with LPS for 24 h, as previously described (Liou et al., 2016). A calcein-AM solution (Sigma) was then used to stain acute monocytic leukaemia cells (THP-1 cells). THP-1 cells were co-cultured with the A549 cells to observe THP-1 adhesion. Adherent cells were detected (green fluorescence) with fluorescence microscopy (Olympus, Tokyo, Japan).Conclusion
We identified a novel series of thiazoles, which exert a potential protective effect against ALI via the inhibition/modulation of numerous pathways. This study provides fresh insight that should aid the development of novel therapeutics against sepsis-induced ALI; however, a more diverse range of studies are needed to validate the claims of this study.Conflict of interest
Authors declare no conflict of interest.Acknowledgements
This study was supported by Jiangsu province Natural Science Foundation of China (No. BK20151204).Notes and references
- M. Bosmann and P. A. Ward, Trends Immunol., 2013, 34(3), 129–136 CrossRef CAS PubMed
.
- UPTODATE, sepsis and the systemic inflammatory response syndrome: definitions, epidemiology and prognosis, Progn. Uptodate, 1–11, 2011.
- M. Singer, Virulence, 2014, 5, 66–72 CrossRef PubMed
.
- J. E. Gottsand and M. A. Matthay, BMJ, 2016, 353, i1585 CrossRef PubMed
.
- M. R. Morrell, S. T. Micek and M. H. Kollef, Infect. Dis. Clin., 2009, 23, 485–501 CrossRef PubMed
.
- F. B. Mayr, S. Yende and D. C. Angus, Virulence, 2014, 5, 4–11 CrossRef PubMed
.
- M. Singer, et al., J Am Med Assoc, 2016, 315, 801–810 CrossRef CAS PubMed
.
- J. A. Russel, Minerva Med., 2008, 99, 431–458 CAS
.
- E. Ozturk, et al., Pediatr. Surg. Int., 2008, 24, 899–905 CrossRef PubMed
.
- S. Luh and C. Chiang, J. Zhejiang Univ., Sci., B, 2007, 8, 60–69 CrossRef CAS PubMed
.
- L. Gattinoni, et al., N. Engl. J. Med., 2006, 354, 1775–1786 CrossRef CAS PubMed
.
- A. P. Wheeler and G. R. Bernard, Lancet, 2007, 369, 1553–1564 CrossRef
.
- G. Storz and J. A. Imlayt, Curr. Opin. Microbiol., 1999, 2, 188–194 CrossRef CAS PubMed
.
- N. Bakunina, C. M. Pariante and P. A. Zunszain, Immunology, 2015, 144(3), 365–373 CrossRef CAS PubMed
.
- J. A. Carcillo, Crit. Care Clin., 2003, 19(3), 413–440 CrossRef PubMed
.
- R. S. Hotchkiss, G. Monneret and D. Payen, Nat. Rev. Immunol., 2013, 13, 862–874 CrossRef CAS PubMed
.
- M. Bosmann and P. A. Ward, Trends Immunol., 2013, 34(3), 129–136 CrossRef CAS PubMed
.
- S. Chakraborti, M. Mandal, S. Das, A. Mandal and T. Chakraborti, Mol. Cell. Biochem., 2003, 253(1–2), 269–285 CrossRef CAS PubMed
.
- L. Nissinenand and V. M. Kähäri, Biochim. Biophys. Acta, 2014, 1840(8), 2571–2580 CrossRef PubMed
.
- M. Giannandrea and W. C. Parks, Dis. Models & Mech., 2014, 7, 193–203 CAS
.
- J. Hu, P. E. Van den Steen, Q.-X. a Sang and G. Opdenakker, Nat. Rev. Drug Discovery, 2007, 6, 480–498 CrossRef CAS PubMed
.
- E. E. Creemers, J. P. Cleutjens, J. F. Smits and M. J. Daemen, Circ. Res., 2001, 89, 201–210 CrossRef CAS PubMed
.
- B. Pirard, Drug Discovery Today, 2007, 12(15–16), 640–646 CrossRef CAS PubMed
.
- K. G. Lu and C. M. Stultz, J. Mol. Biol., 2013, 425, 1815–1825 CrossRef CAS PubMed
.
- B. J. Rollins, Blood, 1997, 90, 909–928 CAS
.
- A. Rouf and C. Tanyeli, Eur. J. Med. Chem., 2015, 97, 911–927 CrossRef CAS PubMed
.
- Y. Lu, et al., J. Med. Chem., 2009, 52, 1701–1711 CrossRef CAS PubMed
.
- M. L. Barreca, et al., J. Med. Chem., 2002, 45, 5410–5413 CrossRef CAS PubMed
.
- M. Cuenca-Estrella, A. Gomez-Lopez, E. Mellado, G. Garcia-Effron and J. L. Rodriguez-Tudela, Antimicrob. Agents Chemother., 2004, 48, 3107–3111 CrossRef CAS PubMed
.
- L. M. Fox and L. D. Saravolatz, Clin. Infect. Dis., 2005, 40, 1173–1180 CrossRef CAS PubMed
.
- R. M. Mohareb, M. Y. Zaki and N. S. Abbas, Steroids, 2015, 98, 80–91 CrossRef CAS PubMed
.
- B. S. Holla, K. V. Malini, B. S. Rao, B. K. Sarojini and N. S. Kumari, Eur. J. Med. Chem., 2003, 38(3), 313–318 CrossRef CAS PubMed
.
- T. M. Potewar, S. A. Ingale and K. V. Srinivasan, Tetrahedron, 2007, 63, 11066–11069 CrossRef CAS
.
- Z. A. Kaplancıklı, M. D. Altıntop, Ö. Atlı, B. Sever, M. Baysal, H. E. Temel, F. Demirci and A. Özdemir, Anti Canc. Agents Med. Chem., 2017, 17, 674–681 CrossRef
.
| This journal is © The Royal Society of Chemistry 2017 |