Open Access Article
Open Access ArticleRing-opening polymerization of rac-lactide catalyzed by crown ether complexes of sodium and potassium iminophenoxides†
Bing-Bing Wua,
Lu-Lu Tiana and
Zhong-Xia Wang *ab
*ab
aCAS Key Laboratory of Soft Matter Chemistry, Hefei National Laboratory for Physical Sciences at Microscale, Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, People's Republic of China. E-mail: zxwang@ustc.edu.cn; Tel: +86 551 63603043
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, People's Republic of China
First published on 3rd May 2017
Abstract
A series of iminophenoxide ligand precursors [2-(RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4OH] (HL1: R = C6H5; HL2: R = 2,6-iPr2C6H3) and [2-(RN
CH)C6H4OH] (HL1: R = C6H5; HL2: R = 2,6-iPr2C6H3) and [2-(RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)-4,6-tBu2C6H2OH] (HL3: R = C6H5; HL4: R = 2,6-iPr2C6H3) were synthesized. These compounds reacted with NaN(SiMe3)2/15-crown-5 or KN(SiMe3)2/18-crown-6 to afford corresponding crown ether complexes of sodium and potassium iminophenoxides (1, (15-C-5)NaL2; 2, (15-C-5)NaL3; 3, (15-C-5)NaL4; 4, (18-C-6)KL1; 5, (18-C-6)KL2; 6, (18-C-6)KL3; 7, (18-C-6)KL4). Catalysis of the complexes toward the ring-opening polymerization of rac-lactide was studied. Each of the complexes exhibited high catalytic activity at room temperature. Complexes 2, 3, 6 and 7 showed poor isotactic selectivity and relatively broad molecular weight distributions. Complexes 1 and 5 resulted in more stereoregular polymers with Pm values of 0.58 and 0.66, respectively. Complex 4 led to the best selectivity for isotacticity (Pm = 0.75) when the polymerization was performed in toluene at 0 °C.
CH)-4,6-tBu2C6H2OH] (HL3: R = C6H5; HL4: R = 2,6-iPr2C6H3) were synthesized. These compounds reacted with NaN(SiMe3)2/15-crown-5 or KN(SiMe3)2/18-crown-6 to afford corresponding crown ether complexes of sodium and potassium iminophenoxides (1, (15-C-5)NaL2; 2, (15-C-5)NaL3; 3, (15-C-5)NaL4; 4, (18-C-6)KL1; 5, (18-C-6)KL2; 6, (18-C-6)KL3; 7, (18-C-6)KL4). Catalysis of the complexes toward the ring-opening polymerization of rac-lactide was studied. Each of the complexes exhibited high catalytic activity at room temperature. Complexes 2, 3, 6 and 7 showed poor isotactic selectivity and relatively broad molecular weight distributions. Complexes 1 and 5 resulted in more stereoregular polymers with Pm values of 0.58 and 0.66, respectively. Complex 4 led to the best selectivity for isotacticity (Pm = 0.75) when the polymerization was performed in toluene at 0 °C.
Introduction
Polylactide (PLA), an important biodegradable and biocompatible polymer, has been used in a wide variety of applications such as drug delivery systems, resorbable sutures, and medical implants.1–11 The ring-opening polymerization (ROP) of lactides catalyzed by metal complexes is a major method for the synthesis of PLA with controlled molecular weights, low polydispersity indices (PDIs), and specific stereo-microstructures.12–14 A lot of metal complexes such as Zn,15–24 Mg,25–37 Al,38–45 Sn,46–57 and rare earth metal complexes58–74 have been proven to be effective for the ROP of lactides. In recent years alkali metal complexes also attracted great interest owing to their high catalytic activity and cheapness, wide availability and nontoxic nature of alkali metals.75–101 Several alkali metal complexes supported by iminophenoxide ligands have been reported to efficiently initiate the ROP of lactides. In 2012 Chen et al. reported the synthesis and catalysis of lithium and sodium iminophenoxide complexes toward the ROP of L-lactide.91 In 2015 Cano et al. synthesized a series of lithium, sodium, and potassium iminophenoxide complexes, and found that the lithium complexes resulted in heterorich-PLA (Pr = 0.75),102 while sodium and potassium complexes presented poor stereo-selectivity in the polymerization of rac-lactide.92 Chakraborty et al. reported similar complexes which catalyzed solvent free ROP of rac-lactide with high catalytic activity and led to heterorich-PLA.93 Recently Wu and co-workers reported that crown ether complexes of sodium and potassium mono-phenoxides exhibited high catalytic activity and high iso-selectivity for the ROP of rac-lactide.94–101 However, the phenoxide ligands are required to have very bulky ortho-substituents on the aromatic rings to achieve good selectivity.94–101 It is interesting to design simpler ligands to get high catalytic selectivity of complexes. A strategy is to use chelate ligands to restrict rotation of M–OAr bonds of alkali metal complexes. In a earlier work we demonstrated that crown ether complex of potassium quinolin-8-olate did initiate iso-selective ROP of rac-lactide. The Pm value103 reached 0.75 when the polymerization of 100 equiv. of rac-lactide was performed in toluene at 0 °C.104 We intended to examine more chelate ligands to improve catalytic selectivity of the chelate alkali metal complexes. Herein we report synthesis and catalytic study of crown ether complexes of sodium and potassium iminophenoxides with different substituents on the aromatic rings.Results and discussion
Iminophenols 2-(PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4OH (HL1), 2-(2,6-iPr2C6H3N
CH)C6H4OH (HL1), 2-(2,6-iPr2C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)–C6H4OH (HL2), 2-(PhN
CH)–C6H4OH (HL2), 2-(PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)-3,5-tBu2C6H2OH (HL3) and 2-(2,6-iPr2 C6H3N
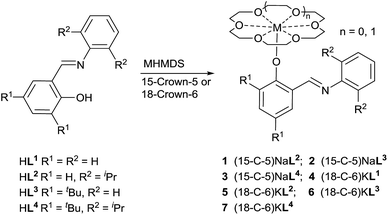
CH)-3,5-tBu2C6H2OH (HL3) and 2-(2,6-iPr2 C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)-3,5-tBu2C6H2OH (HL4) were synthesized according to reported procedures.92 Treatment of the iminophenols with NaN(SiMe3)2/15-crown-5 or KN(SiMe3)2/18-crown-6 in toluene at room temperature afforded corresponding crown ether complexes of sodium and potassium iminophenoxides 1–7 respectively (Scheme 1). However, treatment of HL1 with NaN(SiMe3)2 in toluene or THF in the presence of 15-crown-5 could not generate expected crown ether sodium complex.
CH)-3,5-tBu2C6H2OH (HL4) were synthesized according to reported procedures.92 Treatment of the iminophenols with NaN(SiMe3)2/15-crown-5 or KN(SiMe3)2/18-crown-6 in toluene at room temperature afforded corresponding crown ether complexes of sodium and potassium iminophenoxides 1–7 respectively (Scheme 1). However, treatment of HL1 with NaN(SiMe3)2 in toluene or THF in the presence of 15-crown-5 could not generate expected crown ether sodium complex.
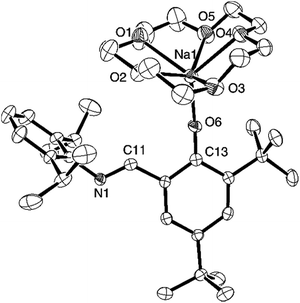
Complexes 1–7 are yellow solids, soluble in DMSO, THF, and slightly soluble in toluene. They were characterized by elemental analyses, 1H and 13C{1H} NMR spectroscopy. Complexes 3 and 4 were further characterized by single crystal X-ray diffraction techniques. The ORTEP drawing of complex 3 is presented in Fig. 1, along with selected bond lengths and angles. The structure shows that the phenoxy ligand coordinates to the central sodium cation in a monodentate mode, rather than a O,N-chelate mode. This might be due to steric repulsion between the N-arylimino group and the crown ether ligand which combines with Na+ through coordination of the five oxygen atoms. The C(13)–O(6)–Na(1) angle of 159.67(17)° reveals approximately linear arrangement of C(13), O(6) and Na(1) atoms. The O6–Na1 distance of 2.1393(18) Å is slightly shorter than corresponding one in sodium 2-(anthracen-9-yl)-4,6-di-tert-butylphenolate crown ether complex (2.1478(18) Å). The O(6)–C(13) distance of 1.277(2) Å is also slightly shorter than corresponding that in the above-mentioned complex (1.287(3) Å).98
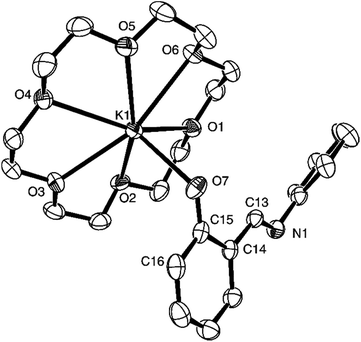
The ORTEP drawing of complex 4 is presented in Fig. 2, along with selected bond lengths and angles. In the structure the phenoxy ligand also coordinates to the central metal in a monodentate mode. However, the C(15)–O(7)–K(1) angle of 130.8(3)° shows that the connectivity of C15, O7 and K1 atoms is not linear. The C15–O7–K1 plane is almost perpendicular to the adjacent aromatic ring plane, the torsion angle of K1–O7–C15–C14 being 96°. The K1–C7 distance of 2.623(3) Å is within a normal range compared with the reported crown ether potassium phenoxide complexes.94–100 The O(7)–C(15) distance of 1.289(5) Å is comparable with those in crown ether pota-ssium complex bearing 2-(anthracen-9-yl) phenolate ligands.98
In the presence of benzyl alcohol, catalysis of complexes 1–7 in the ROP of rac-lactide was tested, and the results are summarized in Table 1. Each of the complexes showed high catalytic activity in toluene at room temperature (Table 1, entries 1–7). When using a molar ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for [LA]0/[M]0/[BnOH]0, complex 1 led to faster polymerization of rac-lactide than complexes 2 and 3 for the three similar sodium complexes (Table 1, entries 1–3). Potassium complexes 4–7 exhibited same activity order. Thus, the complexes without ortho-tert-butyl group on the phenoxo rings led to faster ROP of rac-lactide than the other ones with ortho-tert-butyl groups on the phenoxo rings. It seems that the stereo hindrance of ortho-tert-butyl groups on the phenoxo rings decreases the ROP rate (Table 1, entries 4–7). But we cannot rule out influence of electron effect of the ortho- and para-position tert-butyl groups. The ortho-position substituents on the N-aryl rings exhibited less effect on the catalytic activity of the complexes, which might be because the aromatic rings are far from the metal centers. In addition, the potassium complexes are less active than corresponding sodium complexes (5 vs. 1, 6 vs. 2, and 7 vs. 3), which is different from the previous report results.95,98 The molecular weights of the final polymers generated by those catalysis are lower than expected. A plausible reason could be the presence of transesterification side reaction in the process of polymerization.94–101 The relatively wide molecular weight distributions (1.18 to 1.39) and MALDI-TOF MS analysis (Fig. 3) of the polymers support this explanation. For example, the MS analysis of the polymer showed two main series of peaks. A series of peaks at 144 m + 108 + 23, which can be assigned to m(C6H8O4) + BnOH + Na+. Another series of peaks at 72 m + 108 + 23, which can be ascribed to m(C3H4O2) + BnOH + Na+. The series of peaks separated by m/z = 72 Da is indicative of transesterification occurring in the process of polymerization.
1 for [LA]0/[M]0/[BnOH]0, complex 1 led to faster polymerization of rac-lactide than complexes 2 and 3 for the three similar sodium complexes (Table 1, entries 1–3). Potassium complexes 4–7 exhibited same activity order. Thus, the complexes without ortho-tert-butyl group on the phenoxo rings led to faster ROP of rac-lactide than the other ones with ortho-tert-butyl groups on the phenoxo rings. It seems that the stereo hindrance of ortho-tert-butyl groups on the phenoxo rings decreases the ROP rate (Table 1, entries 4–7). But we cannot rule out influence of electron effect of the ortho- and para-position tert-butyl groups. The ortho-position substituents on the N-aryl rings exhibited less effect on the catalytic activity of the complexes, which might be because the aromatic rings are far from the metal centers. In addition, the potassium complexes are less active than corresponding sodium complexes (5 vs. 1, 6 vs. 2, and 7 vs. 3), which is different from the previous report results.95,98 The molecular weights of the final polymers generated by those catalysis are lower than expected. A plausible reason could be the presence of transesterification side reaction in the process of polymerization.94–101 The relatively wide molecular weight distributions (1.18 to 1.39) and MALDI-TOF MS analysis (Fig. 3) of the polymers support this explanation. For example, the MS analysis of the polymer showed two main series of peaks. A series of peaks at 144 m + 108 + 23, which can be assigned to m(C6H8O4) + BnOH + Na+. Another series of peaks at 72 m + 108 + 23, which can be ascribed to m(C3H4O2) + BnOH + Na+. The series of peaks separated by m/z = 72 Da is indicative of transesterification occurring in the process of polymerization.
| Entry | Cat. | [LA]0:[Cat.]0:[BnOH]0 | Time (min) | Conv.b (%) | Mn,calcc (g mol−1) | Mn,GPCd (g mol−1) | PDI | Pme |
|---|---|---|---|---|---|---|---|---|
| a [Cat.]0 = 5 mmol L−1, reactions were conducted in 4 cm3 solvent under 25 °C.b rac-LA conversion was determined by 1H NMR spectra.c Mn,cald = 144.13 × [LA]0/[BnOH]0 × conv. (%) + 108.14.d Obtained from GPC analysis and calibrated against the polystyrene standard, multiplied by 0.58.105e Determined by analysis of all of the tetrad signals in the methine region of the homonuclear-decoupled 1H NMR spectra.f Polymerization reaction was run at 0 °C.g Polymerization reaction was carried out in CH2Cl2.h Polymerization reaction was carried out in THF. | ||||||||
| 1 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 99 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.32 | 0.58 |
| 2 | 2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
9200 | 1.25 | 0.52 |
| 3 | 3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
8600 | 1.30 | 0.52 |
| 4 | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 98 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7400 | 1.18 | 0.69 |
| 5 | 5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 98 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.39 | 0.66 |
| 6 | 6 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | 98 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
8900 | 1.27 | 0.53 |
| 7 | 7 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
7800 | 1.28 | 0.52 |
| 8 | 1 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 99 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.36 | 0.60 |
| 9 | 1 | 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 99 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.39 | 0.59 |
| 10 | 1 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 99 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.36 | 0.62 |
| 11 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 99 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.37 | 0.63 |
| 12 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2 | 99 | 7200 | 8500 | 1.34 | 0.61 |
| 13f | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 99 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
9500 | 1.16 | 0.71 |
| 14 | 4 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 97 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.11 | 0.70 |
| 15 | 4 | 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 99 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.28 | 0.69 |
| 16 | 4 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 96 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.25 | 0.69 |
| 17 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 89 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.18 | 0.66 |
| 18 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
3 | 99 | 7200 | 8100 | 1.37 | 0.70 |
| 19g | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 19 | 2800 | 400 | 1.21 | — |
| 20h | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 15 | 2300 | 400 | 1.19 | — |
| 21f | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5600 | 1.06 | 0.75 |
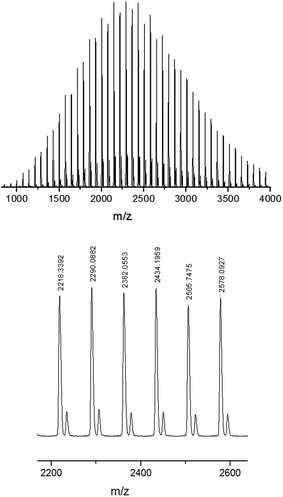 | ||
Fig. 3 The MALDI-TOF MS analysis of the polymer prepared by using 4/BnOH as catalyst in toluene at room temperature, ratio of [LA]0/[M]0/[BnOH]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The sodium and potassium complexes 1 and 4 were selected for a more detailed study on their catalysis. At first, higher monomer loadings were tested using 1/BnOH and 4/BnOH, respectively (Table 1, entries 8–11 and 14–17). 1/BnOH showed higher activity than 4/BnOH. For example, 1/BnOH drove 500 equiv. of rac-lactide to PLA in 15 min in 99% conversion, whereas 4/BnOH drove 89% conversion of 500 equiv. of rac-lactide to spend 30 min. Each of 1- and 4-catalyzed ROP gave a linear relationship of the molecular weights of polymers to the ratio of monomer to catalyst (Fig. 4 and 5), implying that the polymerizations are controlled. Complexes 1 and 4 were demonstrated to catalyze the immortal ROP of 500 equiv. of rac-LA in the presence of 10 equiv. of BnOH, resulting in polymers with controlled molecular weights (Table 1, entries 12 and 18). We also examined the catalytic reaction by 4/BnOH in CH2Cl2 and THF, respectively (Table 1, entries 19 and 20). Both CH2Cl2 and THF were less effective than toluene. In these solvents the catalyst exhibited lower activity than in toluene and the molecular weight of the polymers were markedly lower than the theoretical values.
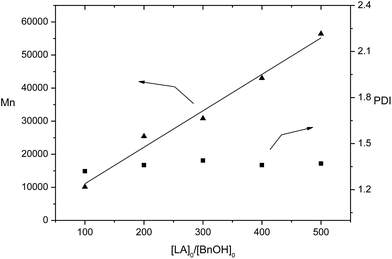 | ||
| Fig. 4 Polymerization of rac-LA catalyzed by 1/BnOH in toluene at room temperature. The relationships between Mn(▲), PDI (■) of the polymer and the initial mole ratios [LA]0/[BnOH]0 is shown (Table 1, entries 1 and 8–11). | ||
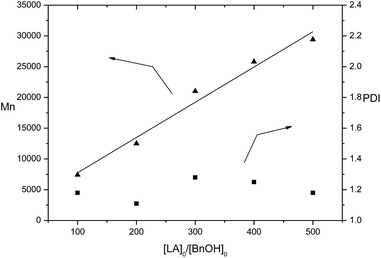 | ||
| Fig. 5 Polymerization of rac-LA catalyzed by 4/BnOH in toluene at room temperature. The relationships between Mn(▲), PDI (■) of the polymer and the initial mole ratios [LA]0/[BnOH]0 is shown (Table 1, entries 4 and 14–17). | ||
The reactions in toluene exhibited isoselectivity (e.g. Fig. 6). Potassium complex 4/BnOH showed the highest selectivity when using a ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for [LA]0/[4]0/[BnOH]0, producing isotactic PLA with a Pm value of 0.69 at room temperature. Among the three sodium complexes, 1 exhibited better catalytic selectivity. These experimental facts demonstrated that the complexes with less sterically hindered ligands had better catalytic selectivity. Based on this experimental result, we speculate that the phenoxide ligands might coordinate to alkali metal center in a O,N-chelate mode during the process of catalysis, which improved the catalytic selectivity of the complexes through restricting rotation of the M–Oaryl single bond. A less sterically hindered phenoxo group and N-aryl group would benefit formation of O,N-chelate complexes.
1 for [LA]0/[4]0/[BnOH]0, producing isotactic PLA with a Pm value of 0.69 at room temperature. Among the three sodium complexes, 1 exhibited better catalytic selectivity. These experimental facts demonstrated that the complexes with less sterically hindered ligands had better catalytic selectivity. Based on this experimental result, we speculate that the phenoxide ligands might coordinate to alkali metal center in a O,N-chelate mode during the process of catalysis, which improved the catalytic selectivity of the complexes through restricting rotation of the M–Oaryl single bond. A less sterically hindered phenoxo group and N-aryl group would benefit formation of O,N-chelate complexes.
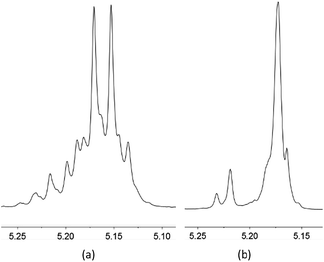 | ||
| Fig. 6 Methine region of the (a) normal 1H NMR spectrum and (b) homonuclear decoupled 1H NMR spectrum of the PLA prepared by complex 4/BnOH catalysis (Table 1, entry 21). | ||
We also noticed that the Pm values of the polymers catalyzed by 1/BnOH slightly increased with the increase of the ratio of rac-LA to catalyst (Table 1, entries 1 and 8–11). However, the Pm values of the polymers catalyzed by 4/BnOH were approximately unchanged when the ratio of monomer to catalyst varied from 100 to 400 and slightly decreased when the ratio of monomer to catalyst was 500 (Table 1, entries 4 and 14–17). Reduction of polymerization temperature led to increase of selectivity whether the catalyst was 1 or 4. The Pm value of the PLA was 0.71 when 100 equiv. of rac-LA was catalytically polymerized by 1/BnOH at 0 °C and the Pm value of the PLA reached 0.75 when 100 equiv. of rac-LA was catalytically polymerized by 4/BnOH at 0 °C (Table 1, entries 13 and 21). The end group analysis of the PLA proved that the polymer chain was capped with one benzyl ester end and one hydroxyl end (Fig. 7), which are consistent with an insertion mechanism of a benzyloxy group into the lactide as supposed for most alkali metal phenoxides.94–100
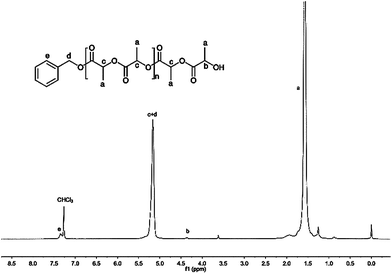 | ||
| Fig. 7 The 1H NMR spectrum of PLA initiated by 4/BnOH (Table 1, entry 4) (NMR: 400 MHz, solvent: CDCl3, reference: TMS). | ||
Conclusions
In summary, we have synthesized and characterized a series of sodium and potassium iminophenoxides bearing 15-crown-5 or 18-crown-6 as the auxiliary ligand. In the presence of benzyl alcohol, all the complexes can efficiently catalyze the ROP of rac-lactide. The catalysts with bulky substituents on the aromatic rings of phenoxo and N-aryl groups are unfavored for the selectivity of the polymers. The potassium complex without any substituents except imino group on the aromatic rings, [(18-crown-6)KOC6H4{2-(PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)}] (4), exhibited the best selectivity of isotacticity (Pm = 0.75) when the ROP was carried out at 0 °C. During the catalytic process, the iminophenoxide ligands might coordinate to the alkali metal center in a O,N-chelate mode which improved the catalytic selectivity of the complexes through restricting rotation of the M–Oaryl single bond.
CH)}] (4), exhibited the best selectivity of isotacticity (Pm = 0.75) when the ROP was carried out at 0 °C. During the catalytic process, the iminophenoxide ligands might coordinate to the alkali metal center in a O,N-chelate mode which improved the catalytic selectivity of the complexes through restricting rotation of the M–Oaryl single bond.
Experimental
All air or moisture sensitive manipulations were performed under dry N2 using standard Schlenk techniques. Solvents were distilled under nitrogen over sodium (toluene), sodium/benzophenone (n-hexane and tetrahydrofuran), or CaH2 (dichloromethane) and degassed prior to use. NaN(SiMe3)2, KN(SiMe3)2 and other reagents were purchased from local chemical companies and used as received. BnOH was distilled from CaO. rac-Lactide was purchased from Daigang Biomaterial Co. and recrystallized three times from toluene prior to use. DMSO-d6, purchased from EMD Millipore Corporation, was degassed, and stored over 4 Å molecular sieves. CDCl3 was purchased from Cambridge Isotope Laboratories and used as received. NMR spectra were recorded on a Bruker AVANCE III 400 spectrometer at ambient temperature. The chemical shifts of 1H and 13C{1H} NMR spectra were referenced to internal solvent resonances. Elemental analyses were performed on an Elementar Vario EL III analyzer. Gel permeation chromatography (GPC) was recorded on a Waters 150C instrument equipped with UltraStyragel columns (103, 104, and 105 Å) and a 410 refractive index detector, using monodispersed polystyrene as the calibration standard. THF (HPLC grade) was used as an eluent at a flow rate of 1 cm3 min−1.Synthesis of complex 1
To a stirred solution of HL2 (0.28 g, 1.00 mmol) and 15-crown-5 (0.22 g, 1.00 mmol) in toluene (10 cm3) was slowly added NaN(SiMe3)2 (0.55 cm3, 2 M solution in THF, 1.1 mmol) at room temperature. The mixture was stirred for 10 h to generate a yellow solution. The resulting solution was concentrated in vacuo. n-Hexane (20 cm3) was added to the solution to afford complex 1 as a yellow solid (0.30 g, 57%). 1H NMR (400 MHz, DMSO-d6): δ 8.44 (s, 1H), 7.59 (dd, J = 7.7, 2.0 Hz, 1H), 7.04 (d, J = 7.6 Hz, 2H), 6.98–6.91 (m, 1H), 6.89–6.81 (m, 1H), 6.17 (d, J = 8.4 Hz, 1H), 5.93 (t, J = 7.2 Hz, 1H), 3.56 (s, 20H), 3.02–2.91 (m, 2H), 1.09 (d, J = 6.9 Hz, 12H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 174.06, 161.93, 152.06, 137.66, 132.25, 126.78, 123.05, 122.87, 122.28, 122.18, 106.98, 69.34, 27.16, 23.39. Anal. calcd for C29H42NNaO6: C 66.52, H 8.08, N 2.67; found: C 66.23, H 8.22, N 2.70.Synthesis of complex 2
To a stirred solution of HL3 (0.30 g, 1.00 mmol) and 15-crown-5 (0.22 g, 1.00 mmol) in toluene (10 cm3) was slowly added NaN(SiMe3)2 (0.55 cm3, 2 M solution in THF, 1.1 mmol) at room temperature. The mixture was stirred for 10 h and yellow precipitates were formed. The precipitates were collected by filtration, washed with toluene, and dried in vacuo to give yellow solids (0.25 g, 45%). 1H NMR (400 MHz, DMSO-d6): δ 8.79 (s, 1H), 7.38 (d, J = 2.9 Hz, 1H), 7.31–7.24 (m, 2H), 7.07–6.97 (m, 3H), 6.95 (d, J = 2.9 Hz, 1H), 3.55 (s, 20H), 1.33 (s, 9H), 1.19 (s, 9H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 173.23, 162.37, 155.59, 139.89, 128.71, 126.24, 126.04, 122.50, 120.82, 120.28, 69.46, 34.90, 33.37, 31.79, 29.52. Anal. calcd for C31H46NNaO6: C 67.49, H 8.40, N 2.54. Found: C 67.02, H 8.57, N 2.66.Synthesis of complex 3
To a stirred solution of HL4 (0.39 g, 1.00 mmol) and 15-crown-5 (0.22 g, 1.00 mmol) in toluene (10 cm3) was slowly added NaN(SiMe3)2 (0.55 cm3, 2 M solution in THF, 1.1 mmol) at room temperature. The mixture was stirred for 10 h and yellow precipitates were formed. The precipitates were collected by filtration, washed with toluene, and dried in vacuo to give yellow solids (0.32 g, 50%). 1H NMR (400 MHz, DMSO-d6): δ 8.46 (s, 1H), 7.50 (d, J = 2.7 Hz, 1H), 7.02 (d, J = 7.6 Hz, 2H), 6.96 (d, J = 2.8 Hz, 1H), 6.90 (t, J = 7.6 Hz, 1H), 3.54 (s, 20H), 3.05–2.93 (m, 2H), 1.33 (s, 9H), 1.21 (s, 9H), 1.09 (d, J = 6.9 Hz, 12H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 172.41, 163.01, 152.59, 139.42, 137.74, 125.98, 125.67, 122.13, 121.69, 120.92, 119.87, 69.20, 34.87, 33.33, 31.86, 29.64, 27.09, 23.40. Anal. calcd for C37H58NNaO6·0.4C7H8: C 71.06, H 9.17, N 2.08. Found: C 71.06, H 9.19, N 2.15.Single crystals of complex 3 suitable for X-ray diffraction analysis were obtained from a saturatted toluene solution.
Synthesis of complex 4
To a stirred solution of HL1 (0.19 g, 1.00 mmol) and 18-crown-6 (0.27 g, 1.00 mmol) in toluene (10 cm3) was slowly added KN(SiMe3)2 (1.1 cm3, 1 M solution in THF, 1.1 mmol) at room temperature. The resultant mixture was stirred at room temperature for 10 h and yellow precipitates were formed. The precipitates were collected by filtration, washed with toluene, and dried in vacuo to give yellow solids (0.21 g, 42%). 1H NMR (400 MHz, DMSO-d6): δ 8.78 (s, 1H), 7.51 (dd, J = 7.8, 2.1 Hz, 1H), 7.29 (t, J = 7.8 Hz, 2H), 7.09–6.96 (m, 3H), 6.83–6.75 (m, 1H), 6.09 (dd, J = 8.6, 0.7 Hz, 1H), 5.82 (t, J = 7.2 Hz, 1H), 3.53 (s, 24H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 175.39, 160.70, 154.95, 132.55, 128.82, 126.85, 123.57, 123.08, 122.89, 120.76, 106.30, 69.44. Anal. calcd for C25H34KNO7·0.15C7H8: C 60.94, H 6.91, N 2.73; found: C 61.15, H 6.68, N 2.96.Single crystals of complex 4 suitable for X-ray diffraction analysis were obtained from a THF/toluene solution.
Synthesis of complex 5
To a stirred solution of HL2 (0.28 g, 1.00 mmol) and 18-crown-6 (0.27 g, 1.00 mmol) in toluene (10 cm3) was slowly added KN(SiMe3)2 (1.1 cm3, 1 M solution in THF, 1.1 mmol) at room temperature. The resultant mixture was stirred at room temperature for 10 h to generate a yellow solution. The solution was concentrated in vacuo. Then n-hexane (20 cm3) was added to the concentrated solution to afford complex 5 as a yellow solid (0.28 g, 48%). 1H NMR (400 MHz, DMSO-d6): δ 8.40 (s, 1H), 7.56 (dd, J = 7.6, 1.9 Hz, 1H), 7.02 (d, J = 7.6 Hz, 2H), 6.96–6.87 (m, 1H), 6.83–6.75 (m, 1H), 6.05 (d, J = 8.6 Hz, 1H), 5.82 (t, J = 7.1 Hz, 1H), 3.54 (s, 24H), 3.04–2.89 (m, 2H), 1.07 (d, J = 6.9 Hz, 12H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 174.88, 161.91, 152.26, 137.68, 132.20, 126.44, 123.30, 122.80, 122.23, 122.01, 105.92, 69.44, 27.13, 23.37. Anal. calcd for C31H46KNO7·0.15C6H14: C 64.21, H 8.12, N 2.35; found: C 63.83, H 8.58, N 2.67.Synthesis of complex 6
To a stirred solution of HL3 (0.30 g, 1.00 mmol) and 18-crown-6 (0.27 g, 1.00 mmol) in toluene (10 cm3) was slowly added KN(SiMe3)2 (1.1 cm3, 1 M solution in THF, 1.1 mmol) at room temperature. The resultant mixture was stirred at room temperature for 10 h and yellow precipitates were formed. The precipitates were collected by filtration, washed with toluene, and dried in vacuo to give yellow solids (0.39 g, 64%). 1H NMR (400 MHz, DMSO-d6): δ 8.83 (s, 1H), 7.40 (d, J = 2.8 Hz, 1H), 7.27 (t, J = 7.7 Hz, 2H), 7.02–6.96 (m, 3H), 6.95 (d, J = 2.9 Hz, 1H), 3.52 (s, 24H), 1.33 (s, 9H), 1.20 (s, 9H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 173.51, 162.32, 155.71, 139.94, 128.69, 126.00, 125.93, 122.37, 120.76, 119.88, 69.42, 34.89, 33.36, 31.79, 29.48. Anal. calcd for C33H50KNO7: C 64.78, H 8.24, N 2.29; found: C 64.60, H 8.39, N 2.42.Synthesis of complex 7
To a stirred solution of HL4 (0.39 g, 1.00 mmol) and 18-crown-6 (0.27 g, 1.00 mmol) in toluene (10 cm3) was slowly added KN(SiMe3)2 (1.1 cm3, 1 M solution in THF, 1.1 mmol) at room temperature. The resultant mixture was stirred at room temperature for 10 h and yellow precipitates were formed. The precipitates were collected by filtration, washed with toluene, and dried in vacuo to give yellow solids (0.32 g, 46%). 1H NMR (400 MHz, DMSO-d6): δ 8.48 (s, 1H), 7.51 (s, 1H), 7.02 (d, J = 7.6 Hz, 2H), 6.95 (s, 1H), 6.90 (t, J = 7.6 Hz, 1H), 3.52 (s, 24H), 3.07–2.94 (m, 2H), 1.34 (s, 9H), 1.21 (s, 9H), 1.10 (d, J = 6.8 Hz, 12H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 172.81, 162.99, 152.70, 139.49, 137.72, 125.59, 125.46, 122.10, 121.59, 120.77, 119.63, 69.41, 34.87, 33.30, 31.86, 29.57, 27.09, 23.37. Anal. calcd for C39H62KNO7: C 67.30, H 8.98, N 2.01; found: C 67.30, H 8.87, N 2.06.X-ray crystallography
Single crystals of complexes 3 and 4 were respectively mounted in Lindemann capillaries under nitrogen. Diffraction data were collected at 298(2) K on a Bruker Smart CCD area detector with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The structures were solved by direct methods using SHELXS-97 (ref. 106) and refined against F2 by full-matrix least squares using SHELXL-97.107 Hydrogen atoms were placed in calculated positions. Crystal data and experimental details of the structure determination are listed in Table 2.| Complex | 3 | 4 |
|---|---|---|
| a R1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo4)]1/2. | ||
| Empirical formula | C37H58NNaO6 | C25H34KNO7 |
| fw | 635.83 | 499.63 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P2/n | C2/c |
| a (Å) | 15.9289(13) | 24.817(2) |
| b (Å) | 11.4741(9) | 17.2980(15) |
| c (Å) | 21.5944(18) | 17.5045(16) |
| β (deg) | 102.398(2) | 133.796(3) |
| V (Å3) | 3854.8(5) | 5424.0(8) |
| Z | 4 | 8 |
| Dcalcd (g cm−3) | 1.096 | 1.224 |
| F(000) | 1384 | 2128 |
| μ (mm−1) | 0.082 | 0.237 |
| θ Range for data collecn (deg) | 2.21 to 25.02 | 2.33 to 25.02 |
| No. of reflns collected | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317 317 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
| No. of indep reflns (Rint) | 6798 (0.0635) | 4778 (0.1302) |
| Restraints/params | 0/416 | 0/307 |
| Goodness of fit on F2 | 1.052 | 1.011 |
| Final R indicesa [I > 2σ(I)] | R1 = 0.0492; | R1 = 0.0715, |
| wR2 = 0.0526 | wR2 = 0.1323 | |
| R indices (all data) | R1 = 0.1527, | R1 = 0.1586, |
| wR2 = 0.0597 | wR2 = 0.1517 | |
| Largest diff peak and hole [e Å−3] | 0.189 and −0.187 | 0.618 and −0.225 |
Polymerization of rac-LA
A typical polymerization procedure was exemplified using 1/BnOH as the catalyst. Complex 1 (10.94 mg, 0.02 mmol) and BnOH (0.20 cm3, 0.1 M in toluene, 0.02 mmol) were added in sequence to toluene (1.8 cm3). The resultant mixture was stirred at room temperature for 10 min and then added to a stirred mixture of rac-LA (0.2883 g, 2.00 mmol) and toluene (2.0 cm3) at the same temperature. The polymerization reaction was terminated after 1 min by adding a drop of water. White precipitates were filtered under reduced pressure and washed with hexane. Drying the wet cake under vacuum gave the polymer as white solid. For GPC analysis, the sample was dissolved in THF, passed through a short neutral aluminum oxide column, precipitated in methanol, and dried under vacuum.Acknowledgements
We thank the Foundation for Talents of Anhui Province (Grant No. 2008Z011) for financial support. We also thank Professor D.-Q. Wang for the crystal structure determinations.Notes and references
- H. Seyednejad, A. H. Ghassemi, C. F. van Nostrum, T. Vermonden and W. E. Hennink, J. Controlled Release, 2011, 168, 152 Search PubMed.
- A. Arbaoui and C. Redshaw, Polym. Chem., 2010, 1, 801 RSC.
- N. Ajellal, J.-F. Carpentier, C. Guillaume, S. M. Guillaume, M. Helou, V. Poirier, Y. Sarazin and A. Trifonov, Dalton Trans., 2010, 39, 8363 RSC.
- M. H. Chisholm, Pure Appl. Chem., 2010, 82, 1647 CrossRef CAS.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484 RSC.
- R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11 CrossRef CAS.
- V. R. Sinha, K. Bansal, R. Kaushik, R. Kumria and A. Trehan, Int. J. Pharm., 2004, 278, 1 CrossRef CAS PubMed.
- D. Mecerreyes, R. Jerôme and P. Dubois, Adv. Polym. Sci., 1999, 147, 1 CrossRef CAS.
- B. J. O'Keefe, M. A. Hillmyer and W. B. Tolman, Dalton Trans., 2001, 2215 RSC.
- K. M. Stridsberg, M. Ryner and A.-C. Albertsson, Adv. Polym. Sci., 2002, 157, 41 CrossRef CAS.
- E. Chiellini and R. Solaro, Adv. Mater., 1996, 8, 305 CrossRef CAS.
- M. J.-L. Tschan, E. Brulé, P. Haquette and C. M. Thomas, Polym. Chem., 2012, 3, 836 RSC.
- J. Wu, T.-L. Yu, C.-T. Chen and C.-C. Lin, Coord. Chem. Rev., 2006, 250, 602 CrossRef CAS.
- C. A. Wheaton, P. G. Hayes and B. J. Ireland, Dalton Trans., 2009, 4832 RSC.
- H. Wang, Y. Yang and H. Ma, Inorg. Chem., 2016, 55, 7356 CrossRef CAS PubMed.
- M. J. Walton, S. J. Lancaster, J. A. Wright, M. R. J. Elsegood and C. Redshaw, Dalton Trans., 2014, 43, 18001 RSC.
- M. Honrado, A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sáncheza and A. M. Rodrígueza, Dalton Trans., 2014, 43, 17090 RSC.
- W.-A. Ma and Z.-X. Wang, Organometallics, 2011, 30, 4364 CrossRef CAS.
- C. A. Wheaton and P. G. Hayes, Chem. Commun., 2010, 46, 8404 RSC.
- P. L. Arnold, I. J. Casely, Z. R. Turner, R. Bellabarba and R. B. Tooze, Dalton Trans., 2009, 7236 RSC.
- Y.-H. Tsai, C.-H. Lin, C.-C. Lin and B.-T. Ko, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4927 CrossRef CAS.
- C. K. Williams, L. E. Breyfogle, S. K. Choi, W. Nam, V. G. Young Jr, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2003, 125, 11350 CrossRef CAS PubMed.
- M. Cheng, A. B. Attygalle, E. B. Lobkowsky and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 11583 CrossRef CAS.
- B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229 CrossRef CAS PubMed.
- H. B. Wang, J. Guo, Y. Yang and H. Ma, Dalton Trans., 2016, 45, 10942 RSC.
- S. Ghosh, P. K. S. Antharjanam and D. Chakraborty, Polymer, 2015, 70, 38 CrossRef CAS.
- S. Ghosh, D. Chakraborty and V. Ramkumar, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1474 CrossRef CAS.
- M. Honrado, A. Otero, J. Fernandez-Baeza, L. F. Sanchez-Barba, A. Garces, A. Lara-Sanchez, J. Martinez-Ferrer, S. Sobrino and A. M. Rodriguez, Organometallics, 2015, 34, 3196 CrossRef CAS.
- Y. Sarazin, B. Liu, T. Roisnel, L. Maron and J.-F. Carpentier, J. Am. Chem. Soc., 2011, 133, 9069 CrossRef CAS PubMed.
- M. H. Chisholm, K. Choojun, J. C. Gallucci and P. M. Wambua, Chem. Sci., 2012, 3, 3445 RSC.
- C.-Y. Sung, C.-Y. Li, J.-K. Su, T.-Y. Chen, C.-H. Lin and B.-T. Ko, Dalton Trans., 2012, 41, 953 RSC.
- S. Song, H. Ma and Y. Yang, Dalton Trans., 2013, 42, 14200 RSC.
- R. A. Collins, J. Unruangsri and P. Mountford, Dalton Trans., 2013, 42, 759 RSC.
- Y. Huang, W. Wang, C.-C. Lin, M. P. Blake, L. Clark, A. D. Schwarz and P. Mountford, Dalton Trans., 2013, 42, 9313 RSC.
- Y. Gao, Z.-G. Dai, J.-J. Zhang, X.-X. Ma, N. Tang and J.-C. Wu, Inorg. Chem., 2014, 53, 716 CrossRef CAS PubMed.
- B. Gao, D.-H. Zhao, X. Li, Y. Cui, R.-L. Duan and X. Pang, RSC Adv., 2015, 5, 440 RSC.
- T. J. J. Whitehorne, B. Vabre and F. Schaper, Dalton Trans., 2014, 43, 6339 RSC.
- P. McKeown, M. G. Davidson, G. Kociok-Köhnb and M. D. Jones, Chem. Commun., 2016, 52, 10431 RSC.
- Z. Sun, R. Duan, J. Yang, H. Zhang, S. Li, X. Pang, W. Chen and X. Chen, RSC Adv., 2016, 6, 17531 RSC.
- A. Pilone, N. D. Maio, K. Press, V. Venditto, D. Pappalardo, M. Mazzeo, C. Pellecchia, M. Kol and M. Lamberti, Dalton Trans., 2015, 44, 2157 RSC.
- Y.-L. Hsieh, N. Huang, G.-H. Lee and C.-H. Peng, Polymer, 2015, 72, 281 CrossRef CAS.
- H. Z. Du, A. H. Velders, P. J. Dijkstra, Z. Y. Zhong, X. S. Chen and J. Feijen, Macromolecules, 2009, 42, 1058 CrossRef CAS.
- H.-L. Chen, S. Dutta, P.-Y. Huang and C.-C. Lin, Organometallics, 2012, 31, 2016 CrossRef CAS.
- B. Gao, X. Li, R. L. Duan, Q. Duan, Y. H. Li, X. Pang, H. J. Zhuang and X.-S. Chen, RSC Adv., 2015, 5, 29412 RSC.
- A. Pilone, K. Press, I. Goldberg, M. Kol, M. Mazzeo and M. Lamberti, J. Am. Chem. Soc., 2014, 136, 2940 CrossRef CAS PubMed.
- A. P. Dove, V. C. Gibson, E. L. Marshall, A. J. P. White and D. J. Williams, Chem. Commun., 2001, 283 RSC.
- N. Nimitsiriwat, V. C. Gibson, E. L. Marshall and M. R. J. Elsegood, Inorg. Chem., 2008, 47, 5417 CrossRef CAS PubMed.
- H. Y. Chen, H. J. Fang, Y. J. Chen, S. C. N. Hsu, Y. C. Lai, H. W. Ou, W. T. Peng, Y. J. Chang, Y. C. Tsou, T. Y. Wu, H. Chung, Y. Chen, T. C. Huang and B. S. Wu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3286 CrossRef CAS.
- P. Degee, P. Dubois, S. Jacobsen, H. G. Fritz and R. Jerome, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2413 CrossRef CAS.
- M. Moller, R. Kange and J. L. Hedrick, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2067 CrossRef CAS.
- S. J. Moravek, J. M. Messman and R. F. Storey, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 797 CrossRef CAS.
- X. C. Zhang, D. A. Macdonald, M. F. A. Goosen and K. B. McAuley, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2965 CrossRef CAS.
- A. P. Dove, V. C. Gibson, E. L. Marshall, H. S. Rzepa, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2006, 128, 9834 CrossRef CAS PubMed.
- N. Nimitsiriwat, E. L. Marshall, V. C. Gibson, M. R. J. Elsegood and S. H. Dale, J. Am. Chem. Soc., 2004, 126, 13598 CrossRef CAS PubMed.
- K. B. Aubrecht, M. A. Hillmyer and W. B. Tolman, Macromolecules, 2002, 35, 644 CrossRef CAS.
- M. Lahcini, P. M. Castro, M. Kalmi, M. Leskela and T. Repo, Organometallics, 2004, 23, 4547 CrossRef CAS.
- V. Poirier, T. Roisnel, S. Sinbandhit, M. Bochmann, J. F. Carpentier and Y. Sarazin, Chem.–Eur. J., 2012, 18, 2998 CrossRef CAS PubMed.
- M. Zhang, X. Ni and Z. Shen, Organometallics, 2014, 33, 6861 CrossRef CAS.
- L. Clark, M. G. Cushion, H. E. Dyer, A. D. Schwarz, R. Duchateau and P. Mountford, Chem. Commun., 2010, 46, 273 RSC.
- L. Clark, G. B. Deacon, C. M. Forsyth, P. C. Junk, P. Mountford, J. P. Townley and J. Wang, Dalton Trans., 2013, 42, 9294 RSC.
- H. E. Dyer, S. Huijser, N. Susperregui, F. Bonnet, A. D. Schwarz, R. Duchateau, L. Maron and P. Mountford, Organometallics, 2010, 29, 3602 CrossRef CAS.
- S. Yang, K. Nie, Y. Zhang, M.-Q. Xue, Y.-M. Yao and Q. Shen, Inorg. Chem., 2014, 53, 105 CrossRef CAS PubMed.
- J.-S. Qiu, M. Lu, Y.-M. Yao, Y. Zhang, Y.-R. Wang and Q. Shen, Dalton Trans., 2013, 42, 10179 RSC.
- X.-D. Shen, M.-Q. Xue, R. Jiao, Y. Ma, Y. Zhang and Q. Shen, Organometallics, 2012, 31, 6222 CrossRef CAS.
- W.-J. Zhang, S.-F. Liu, W.-H. Yang, X. Hao, R. Glaser and W.-H. Sun, Organometallics, 2012, 31, 8178 CrossRef CAS.
- W.-J. Zhang, S.-F. Liu, W.-H. Sun, X. Hao and C. Redshaw, New J. Chem., 2012, 36, 2392 RSC.
- M. Bouyahyi, N. Ajellal, E. Kirillov, C. M. Thomas and J. F. Carpentier, Chem.–Eur. J., 2011, 17, 1872 CrossRef CAS PubMed.
- E. Grunova, E. Kirillov, T. Roisnel and J. F. Carpentier, Dalton Trans., 2010, 39, 6739 RSC.
- J. C. Buffet, A. Kapelski and J. Okuda, Macromolecules, 2010, 43, 10201 CrossRef CAS.
- T. Cao, A. Buchard, X. F. Le Goff and C. K. Williams, Inorg. Chem., 2012, 51, 2157 CrossRef CAS PubMed.
- C. Bakewell, T. Cao, N. Long, X. F. Le Goff, A. Auffrant and C. K. Williams, J. Am. Chem. Soc., 2012, 134, 20577 CrossRef CAS PubMed.
- S. Sun, K. Nie, Y. F. Tan, B. Zhao, Y. Zhang, Q. Shen and Y.-M. Yao, Dalton Trans., 2013, 42, 2870 RSC.
- Y. Cui, W. Gu, Y. Wang, B. Zhao, Y. Yao and Q. Shen, Catal. Sci. Technol., 2015, 5, 3302 CAS.
- Y.-L. Duan, J.-X. He, W. Wang, J.-J. Zhou, Y. Huang and Y. Yang, Dalton Trans., 2016, 45, 10807 RSC.
- A. K. Sutar, T. Maharana, S. Dutta, C.-T. Chen and C.-C. Lin, Chem. Soc. Rev., 2010, 39, 1724 RSC.
- E. Kober, R. Petrus, P. Kociecka, Z. Janas and P. Sobota, Polyhedron, 2015, 85, 814 CrossRef CAS.
- Y. Huang, Y.-H. Tsai, W.-C. Hung, C.-S. Lin, W. Wang, J.-H. Huang, S. Dutta and C.-C. Lin, Inorg. Chem., 2010, 49, 9416 CrossRef CAS PubMed.
- L. Wang, X. Pan, L. Yao, N. Tang and J. Wu, Eur. J. Inorg. Chem., 2011, 632 CrossRef.
- B. Calvo, M. G. Davidson and D. García-Vivó, Inorg. Chem., 2011, 50, 3589 CrossRef CAS PubMed.
- J. Zhang, C. Jian, Y. Gao, L. Wang, N. Tang and J. Wu, Inorg. Chem., 2012, 51, 13380 CrossRef CAS PubMed.
- H.-Y. Chen, L. Mialon, K. A. Abboud and S. A. Miller, Organometallics, 2012, 31, 5252 CrossRef CAS.
- R. K. Dean, A. M. Reckling, H. Chen, L. N. Dawe, C. M. Schneider and C. M. Kozak, Dalton Trans., 2013, 42, 3504 RSC.
- S.-C. Roşca, D.-A. Roşca, V. Dorcet, C. M. Kozak, F. M. Kerton, J.-F. Carpentier and Y. Sarazin, Dalton Trans., 2013, 42, 9361 RSC.
- C. Thomas, A. Milet, F. Peruch and B. Bibal, Polym. Chem., 2013, 4, 3491 RSC.
- D. Alhashmialameer, N. Ikpo, J. Collins, L. N. Dawe, K. Hattenhauera and F. M. Kerton, Dalton Trans., 2015, 44, 20216 RSC.
- C. Gallegos, V. Tabernero, M. E. G. Mosquera, T. Cuenca and J. Cano, Eur. J. Inorg. Chem., 2015, 5124 CrossRef CAS.
- F. M. García-Valle, R. Estivill, C. Gallegos, T. Cuenca, M. E. G. Mosquera, V. Tabernero and J. Cano, Organometallics, 2015, 34, 477 CrossRef.
- H.-W. Ou, K.-H. Lo, W.-T. Du, W.-Y. Lu, W.-J. Chuang, B.-H. Huang, H.-Y. Chen and C.-C. Lin, Inorg. Chem., 2016, 55, 1423 CrossRef CAS PubMed.
- Q. Zhang, W. Zhang, S. Wang, G. A. Solan, T. Liang, N. M. Rajendrana and W.-H. Sun, Inorg. Chem. Front., 2016, 3, 1178 RSC.
- W.-J. Chuang, Y.-T. Huang, Y.-H. Chen, Y.-S. Lin, W.-Y. Lu, Y.-C. Lai, M. Y. Chiang, S. C. N. Hsu and H.-Y. Chen, RSC Adv., 2016, 6, 33014 RSC.
- W.-Y. Lu, M.-W. Hsiao, S. C. N. Hsu, W.-T. Peng, Y.-J. Chang, Y.-C. Tsou, T.-Y. Wu, Y.-C. Lai, Y. Chen and H.-Y. Chen, Dalton Trans., 2012, 41, 3659 RSC.
- F. M. García-Valle, R. Estivill, C. Gallegos, T. Cuenca, M. E. G. Mosquera, V. Tabernero and J. Cano, Organometallics, 2015, 34, 477 CrossRef.
- S. Ghosh, D. Chakraborty and B. Varghese, Eur. Polym. J., 2015, 62, 51 CrossRef CAS.
- J.-J. Zhang, J. Xiong, Y.-Y. Sun, N. Tang and J.-C. Wu, Macromolecules, 2014, 47, 7789 CrossRef CAS.
- J. Xiong, J.-J. Zhang, Y.-Y. Sun, Z.-R. Dai, X.-B. Pan and J.-C. Wu, Inorg. Chem., 2015, 54, 1737 CrossRef CAS PubMed.
- Z.-R. Dai, Y.-Y. Sun, J. Xiong, X.-B. Pan and J.-C. Wu, ACS Macro Lett., 2015, 4, 556 CrossRef CAS.
- Z. R. Dai, Y.-Y. Sun, J. Xiong, X.-B. Pan, N. Tang and J.-C. Wu, Catal. Sci. Technol., 2016, 6, 515 CAS.
- Y.-Y. Sun, J. Xiong, Z.-R. Dai, X.-B. Pan, N. Tang and J.-C. Wu, Inorg. Chem., 2016, 55, 136 CrossRef CAS PubMed.
- Y.-G. Li, H.-W. Zhao, X.-Y. Mao, X.-B. Pan and J.-C. Wu, Dalton Trans., 2016, 45, 9636 RSC.
- Y. Cui, C. Chen, Y. Sun, J. Wu and X. Pan, Inorg. Chem. Front., 2017, 4, 261 RSC.
- C. Chen, Y. Cui, X. Mao, X. Pan and J. Wu, Macromolecules, 2017, 50, 83 CrossRef CAS.
- Pr is the probability of racemic linkages between monomer units. [mmm] = [2(1 − Pr)2 + Pr(1 − Pr)]/2; [mrm] = [Pr2 + Pr(1 − Pr)]/2; [mmr] = [rmm] = Pr(1 − Pr)/2; [rmr] = Pr2/2.
- Pm is the probability of meso linkages between monomer units. [mmm] = Pm2 + (1 − Pm)Pm/2, [mmr] = [rmm] = (1 − Pm)Pm/2, [rmr] = (1 − Pm)2/2, [mrm] = [(1 − Pm)2 + Pm(1 − Pm)]/2.
- B.-B. Wu and Z.-X. Wang, RSC Adv., 2017, 7, 11657 RSC.
- M. Save, M. Schappacher and A. Soum, Macromol. Chem. Phys., 2002, 203, 889 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXL97: Programs for Structure Refinement, Universität Göttingen, Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † CCDC 1539732 and 1539733 contain the supplementary crystallographic data for this paper. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra03394j |
| This journal is © The Royal Society of Chemistry 2017 |