Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of mesoporous silica by CO2/N2 switchable templates using a convenient compound†
Jianzhong Jiang *a,
Jinchao Yin‡
a,
Li Dai‡a,
Rukuan Liub and
Zhihong Xiaob
*a,
Jinchao Yin‡
a,
Li Dai‡a,
Rukuan Liub and
Zhihong Xiaob
aThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu 214122, P. R. China. E-mail: jzjiang@jiangnan.edu.cn
bInstitute of Bioenergy, Hunan Academy of Forestry, Changsha 410004, China
First published on 10th May 2017
Abstract
A facile synthesis method for the preparation of mesoporous silica with CO2/N2 switchable templates using a commercially available compound was described. Dodecyl dimethyl ammonium bicarbonate, which can be in situ formed by N,N-dimethyldodecylamine and CO2, was used as a soft template, and 98% of the templates could be easily removed.
Mesoporous silica nanoparticles (MSNs) have attracted extensive attention in recent years and have been applied in various fields such as catalysis, adsorption and storage, controlled drug release, targeted drug delivery, and simultaneous diagnosis, owing to their low density, large void space, large specific surface area, and good biocompatibility.1
Soft templates are most commonly used in the preparation of various mesoporous silica materials. MSNs with spherical pores can always be obtained after the removal of the templates. The use of different removal methods certainly influence the characteristics of mesoporous materials. The most common method to remove the templates is calcination. The calcination process causes structure shrinkage or collapse, no recovery of the surfactants, and sacrifice of the surface hydroxyl groups.2 Pinnavaia and co-workers have extensively studied alkylamines as silica templates;3 however, solvent extraction was needed to remove the templates. Although solvent extraction allows the recovery and reuse of expensive templates,4 a great amount of solvent is needed. Supercritical carbon dioxide extraction,5 ultrasonic extraction,6 microwave-assisted methods,7 and oxidative treatment, such as UV/H2O2 treatment8 and oxidation using perchlorates,9 have also been employed to remove the templates. Recently, Zhao et al. reported UV irradiation and solvent extraction to remove an azobenzene-derived surfactant template that was used to prepare MSNs, and 80% of the surfactant could be recycled.10 However, new strategies are still needed for the facile and non-destructive preparation of MSNs.
Jessop et al. have developed CO2-switchable surfactants.11 Compared to other triggers, such as light or pH, CO2 is an inexpensive, environmentally benign, and effective trigger for switchable surfactants. Hydrophobic long-chain alkyl amidines could be protonated and become water-soluble surfactants upon the addition of CO2. Amidinium ion could then be deprotonated by removing CO2 using N2. However, most of the switchable surfactants need complex synthesis methods and are commercially unavailable.
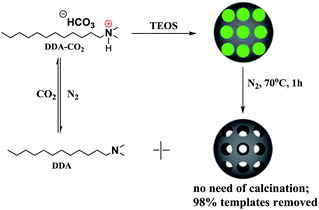
Herein, we described a CO2/N2 switchable template for the synthesis of MSNs using a commercially available compound, N,N-dimethyldodecylamine (abbreviated as DDA), which can be in situ converted to amphiphilic dodecyl dimethyl ammonium bicarbonate (abbreviated as DDA-CO2) by bubbling CO2. The templates collapsed after the preparation of the MSNs and could be easily removed from the mesopores by bubbling N2 at 70 °C and washing with water/acetone. Most of the template (98%) could be removed without calcination (Scheme 1).
In an initial experiment, DDA was used as a template for the preparation of MSNs. As shown in Fig. 1a, there was no peak in the X-ray diffraction (XRD) curve, which indicated that DDA was not a suitable template for MSNs. Pinnavaia et al. have reported that primary amines can be used as a silica template. However, contrary to primary amines, N,N-dimethyldodecylamine is too hydrophobic to form micelles in water.
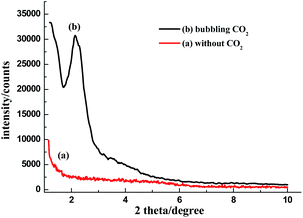 | ||
| Fig. 1 The X-ray diffraction (XRD) curves obtained for the MSNs fabricated using N,N-dimethyldodecylamine aqueous solution: (a) without bubbling CO2 and (b) after bubbling CO2 (0.1 MPa). | ||
DDA can be protonated to amphiphilic ammonium bicarbonate (DDA-CO2, surface-active form) by bubbling CO2, and the opaque aqueous solution of DDA quickly turns transparent. Bubbling the latter solution with N2 or air converts the surface-active form to its uncharged surface-inactive form (Scheme 1). The interconversion can be monitored by measuring the conductivity of the aqueous solution (Fig. S1†). Therefore, the in situ formed amphiphilic DDA-CO2 was used as a soft template for the preparation of MSNs.
As shown in Scheme 1, tetraethyl orthosilicate (TEOS) and Na2EDTA were then added into the DDA aqueous solution in a bottle under stirring. The bottle was connected to a CO2 cylinder (0.1 MPa). The reaction was carried out under static conditions at a certain temperature for 6 days. N2 was then bubbled into the suspension at 70 °C for an hour when the reaction was completed. The product was ultrasonically washed 6 times using distilled water and acetone (5 minutes per washing step) at room temperature.
Silica was investigated using XRD. There was one strong and narrow peak at 2 degree in the XRD curve, as shown in Fig. 1b, which indicated the disordered mesopores in the silica product. The result is similar to that previously reported in the literature.2a,12 At neutral pH ∼7, only disordered amorphous silica was obtained.
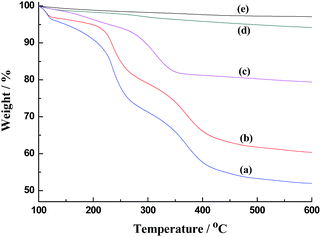
Thermogravimetric analysis (TGA) was performed to determine the template residue in the mesopores (Fig. 2). The weight loss can be divided into the following stages: the first stage is associated with the removal of physically adsorbed water (temperature below 150 °C), the second stage is associated with the decomposition of organic compounds (150 to 350 °C), and the third stage is associated with the dehydroxylation of the silicon hydroxyl groups (temperature higher than 350 °C).13 It was calculated that the template residue in the as-made silica without any treatment was 30% (Fig. 2a). Although the template residue was decreased to 22% after washing with acetone and water, the washing process did not efficiently remove the template from the mesopores (Fig. 2b). The template residue in silica was only 2% after bubbling N2 and washing (Fig. 2d), which was close to the residue value obtained after calcination (∼1%, Fig. 2e).
The IR transmission spectra show a slight peak at 2900 cm−1 corresponding to the stretching vibration band of –CH3 and –CH2, which indicates the efficient removal of the organic templates by calcination and bubbling N2 (Fig. S2†). The relative intensity of the Si–OH bending bands around 960 cm−1 was nearly the same as that observed for the as-made and bubbling N2 samples, whereas a much weaker peak intensity was observed for the calcined sample. This result indicated that calcination would inevitably reduce the level of silanol groups.
Compare with the time-consuming process of calcination or solvent extraction, the entire process of bubbling N2 and washing only required 1.5 h. There are three reasons that DDA-CO2 template could be efficiently removed just by switching off the template upon bubbling N2. The first reason is that DDA-CO2 can be easily deprotonated into its surface-inactive compound (DDA). The hydrophilic DDA cannot form micelles as amphiphilic DDA-CO2. Therefore, the micelles collapse after the DDA-CO2 is deprotonated by bubbling N2.11b The second reason is that the negative charges on the wall of the mesopores in a neutral solution were much lower than those under basic conditions. The electrostatic attraction between the micelles and the wall of the mesopores is weaker under neutral conditions. The third reason is the addition of Na2EDTA to the surfactant solution. Wong and Xiao have reported that EDTA2− can co-assemble with the micelles of the cationic surfactant via electrostatic interactions to afford a low incorporation of the surfactant on the mesoporous,14 which facilitates the removal of the template. The TGA results show that the addition of Na2EDTA decreases the template residue from 15% to 2% (Fig. 2c and d).
The recyclability of the template was also studied. The solution after bubbling N2 and washing was obtained, evaporated, and then extracted with diethyl ether. The diethyl ether layer was evaporated and DDA was then obtained. Most of the DDA could be obtained (≈97%) and could be reused at least 10 times although there was a small weight loss during the entire process (Fig. S3†).
Scanning electron microscopy (SEM) (Fig. 3a) and transmission electron microscopy (TEM) (Fig. 3b) were also performed to observe the morphology and structure of the silica product. It was seen that the silica product comprised aggregated particles with sizes of 150 nm and had a clear mesoporous structure. Moreover, the N2 adsorption isotherm shown in Fig. 3c was a type IV isotherm according to IUPAC nomenclature.15 The steep increase at a relative pressure of 0.9 < p/p0 < 1.0 indicated that the pores were formed by stacked particles. The pore size distribution curve indicated a uniform pore size of 2.3 nm. The Brunauer–Emmett–Teller (BET) surface area SBET was 565.8 m2 g−1 and pore volume at P/P0 = 0.99 was 0.4 cm3 g−1.
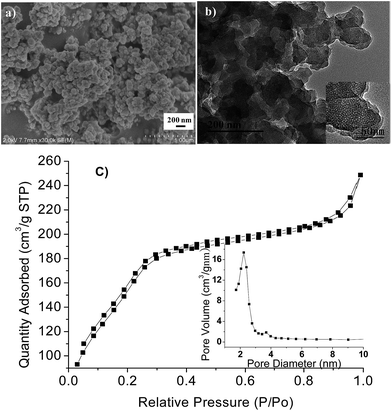 | ||
| Fig. 3 (a) SEM image of the MSNs after removal of the template by bubbling N2, (b) TEM image, and (c) the N2 adsorption isotherm and pore size distribution. | ||
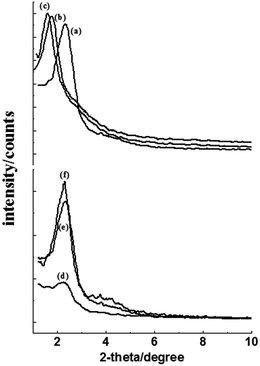
As the hydrocarbon chain length is an important factor that can affect the surface activity of a surfactant and the size of the micelles, tertiary amines with a carbon number of 12, 14, and 16 in the alkyl chain were used under the same conditions (Fig. 4a–c). The particle size decreased upon increasing the alkyl chain (Fig. S4†). In general, the longer the cation alkyl chain, the stronger the cation amphiphilicity. The tertiary amines with a longer alkyl chain have a higher surface activity, which can facilitate the surfactant assembly with the silica monomer, resulting in smaller particles.16,17 The 2-theta of peak in the XRD pattern decreased as the alkyl chain increased (Fig. 4a–c), which indicated larger cell spacing.
As shown in Fig. 4d–f, the intensity at higher temperature was stronger, which indicated better long-range order. In addition, the morphology at higher temperature was more uniform (Fig. S5†). DDA-CO2 with a much lower surface activity at lower temperature could not effectively assemble with the silica monomer; thus, it was harder to form the uniform mesoporous particles.1b
In conclusion, a new method to synthesize mesoporous silica using an inexpensive N2/CO2 switchable surfactant as the template was introduced, in which the template could be easily removed via bubbling N2.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21473080, 20901032) and the Fundamental Research Funds for the Central Universities (JUSRP51405A).Notes and references
- (a) Y. H. Deng, J. Wei, Z. K. Sun and D. Y. Zhao, Chem. Soc. Rev., 2013, 40, 4254 Search PubMed; (b) H. P. Lin and C. Y. Mou, Acc. Chem. Res., 2002, 35, 927 CrossRef CAS PubMed.
- (a) D. Zhao, J. Feng, Q. Huo, N. Melosh, G. Fredrickson, B. Chmelka and G. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed; (b) F. Berube and S. Kaliaguine, Microporous Mesoporous Mater., 2008, 115, 469 CrossRef CAS; (c) R. Grieken, G. Calleja, G. Stucky, J. Melero, R. Garcia and J. Iglesias, Langmuir, 2003, 19, 3966 CrossRef.
- (a) Y. Mori and T. J. Pinnavaia, Chem. Mater., 2001, 13, 2173 CrossRef CAS; (b) R. W. Hicks and T. J. Pinnavaia, Chem. Mater., 2003, 15, 78 CrossRef CAS; (c) I. Park, Z. Wang and T. J. Pinnavaia, Chem. Mater., 2005, 17, 383 CrossRef CAS.
- (a) H. Ji, Y. Fan, W. Jin, C. Chen and N. Xu, J. Non-Cryst. Solids, 2008, 354, 2010 CrossRef CAS; (b) C. Knofel, M. Lutecki, C. Martin, M. Mentens, V. Hornebecq and P. Llewellyn, Microporous Mesoporous Mater., 2010, 128, 26 CrossRef.
- (a) S. Kawi and M. Lai, AIChE J., 2002, 48, 1572 CrossRef CAS; (b) Z. Huang, L. Xu, J. Li, S. Kawi and A. Goh, Sep. Purif. Technol., 2011, 77, 112 CrossRef CAS.
- J. Jin, L. Cao, G. X. Su, C. Y. Xu, Z. Zhang, X. Gao, H. Liu and H. T. Liu, Ultrason. Sonochem., 2014, 21, 1688 CrossRef CAS PubMed.
- B. Tian, X. Liu, C. Yu, F. Gao, Q. Luo, S. Xie, B. Tu and D. Zhao, Chem. Commun., 2002, 11, 1186 RSC.
- L. Xiao, J. Li, H. Jin and R. Xu, Microporous Mesoporous Mater., 2006, 96, 413 CrossRef CAS.
- H. Cai and D. Zhao, Microporous Mesoporous Mater., 2009, 118, 513 CrossRef CAS.
- J. Wei, Y. Liu, J. Chen, Y. Li, Q. Yue, G. Pan, Y. Yu, Y. Deng and D. Zhao, Adv. Mater., 2014, 26, 1782 CrossRef CAS PubMed.
- (a) Y. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958 CrossRef CAS PubMed; (b) P. G. Jessop, S. M. Mercera and D. J. Heldebrant, Energy Environ. Sci., 2012, 5, 7240 RSC.
- (a) E. Prouzet and T. J. Pinnavaia, Angew. Chem., Int. Ed. Engl., 1997, 36, 516 CrossRef CAS; (b) C. G. Goltner and M. Antonietti, Adv. Mater., 1997, 9, 431 CrossRef CAS; (c) M. Antonietti and C. Goltner, Angew. Chem., Int. Ed. Engl., 1997, 36, 910 CrossRef.
- (a) S. Udayakumara, A. Pandurangan and P. K. Sinha, Appl. Catal., A, 2004, 272, 267 CrossRef; (b) L. K. C. de Souza, J. J. R. Pardauil and J. R. Zamian, Powder Technol., 2012, 229, 1 CrossRef CAS.
- (a) R. K. Rana, V. V. Murthy, J. Yu and M. S. Wong, Adv. Mater., 2005, 17, 1145 CrossRef CAS; (b) Q. Xiao, Y. Zhong, W. Zhu, T. Chen and L. Wang, Microporous Mesoporous Mater., 2008, 116, 339 CrossRef CAS.
- L. B. McCusker, F. Liebau and G. Engelhardt, Pure Appl. Chem., 2001, 73, 381 CrossRef CAS.
- K. Yano and Y. Fukushima, J. Mater. Chem., 2004, 14, 1579 RSC.
- B. G. Trewyn, C. M. Whitman and V. S.-Y. Lin, Nano Lett., 2004, 4, 2139 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03134c |
| ‡ The two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |