Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
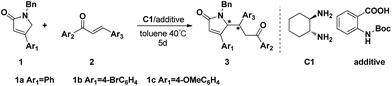
Asymmetric Michael addition reactions of pyrrolones with chalcones catalyzed by vicinal primary-diamine salts†
Xiaolei Du‡
,
Dawei Yin‡,
Zemei Ge,
Xin Wang* and
Runtao Li *
*
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Science, Peking University, Beijing 100191, China. E-mail: xinwang@bjmu.edu.cn; lirt@bjmu.edu.cn
First published on 5th May 2017
Abstract
The efficient asymmetric Michael addition reactions of pyrrolones with chalcones catalyzed by a simple and commercially available chiral 1,2-diaminocyclohexane-2-(N-Boc-amino)benzoic acid have been developed to provide the corresponding Michael adducts in good yields (up to 90%) and high enantioselectivities (up to 95% ee).
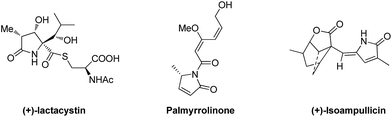
Pyrrolones are privileged heterocyclic scaffolds found in a number of natural and synthetic molecules (Fig. 1),1 which are reported to possess important pharmacological activities, especially antibacterial and antifungal,2 anti-tubercular,3 anticonvulsant activity,4 immunosuppressive activity,5 anticancer activity,6 analgesic and anti-inflammatory activity.7 Additionally, optical pyrrolones can act as synthetic precursors of some natural products.8 In particular, chiral 5-substituted pyrrolones and their derivatives display marvelous biological properties,9 which undoubtably increase their importance both in chemical synthesis and synthetic methodologies. Therefore, the exploration of asymmetric reactions from readily available starting material pyrrolones to their 5-substituted derivatives has recently appeared extremely attractive.
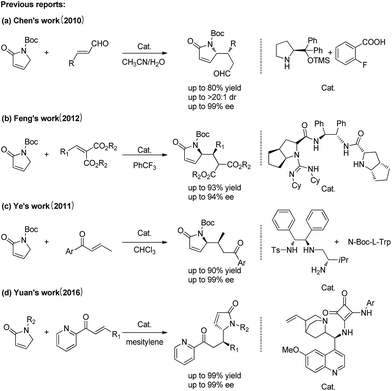
In general, these asymmetric reactions include asymmetric Michael addition reaction, asymmetric Aldol condensation reaction and asymmetric Mannich reaction.10 Recently, some secondary and tertiary amines, such as proline and its derivatives, thioureas, quinines and cinchona alkaloids were reported to catalyze above asymmetric reactions.11 Great improvement has been made in asymmetric Michael addition reaction (Fig. 2). For example, Chen and co-workers achieved satisfied results in the enantio- and diastereoselective Michael reaction of N-Boc pyrrolone with α,β-unsaturated aldehydes catalyzed by proline,12 Feng's group developed a novel guanidine combining with secondary amine as bifunctional catalysts for the asymmetric Michael reaction of N-Boc pyrrolone with malonates.13 However, to the best of our knowledge, chiral primary amine has rarely been used to the 5-deprotonation of pyrrolone pathway,14 and the poor reactive chalcones have never been reported to proceed asymmetric Michael reaction with pyrrolones. So it still represents a challenging task regarding the reactivity and stereoselectivity of the two relatively inert reactants.
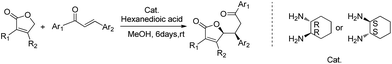
In our previous report, we have successfully realized the asymmetric Michael addition reactions of furanones with chalcones using simple chiral primary-diamine salts (Scheme 1).15 As an extension of our work, herein, we wish to disclose an efficient asymmetric Michael addition reaction of pyrrolones with chalcones catalyzed by chiral primary-diamine salts (Table 1).
| Entry | Cat. | Solvent | Additive | T (°C) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|---|
| a All reactions were carried out using 1.0 equiv. of 1a (0.15 mmol), 1.5 equiv. of 2a (0.225 mmol), and 20 mol% of catalyst (0.03 mmol), 40 mol% of additive (0.06 mmol).b Isolated yield.c Determined by NMR.d Determined by chiral HPLC analysis. | |||||||
| 1 | C1 | MeOH | A1 | r.t. | 25 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 |
| 2 | C2 | MeOH | A1 | r.t. | Trace | — | — |
| 3 | C3 | MeOH | A1 | r.t. | 0 | — | — |
| 4 | C4 | MeOH | A1 | r.t. | Trace | — | — |
| 5 | C1 | MeOH | A2 | r.t. | 0 | — | — |
| 6 | C1 | MeOH | A3 | r.t. | 20 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 7 | C1 | MeOH | A4 | r.t. | 25 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 8 | C1 | MeOH | A5 | r.t. | 27 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 9 | C1 | MeOH | A5 | 40 | 45 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55 |
| 11 | C1 | EtOH | A5 | 40 | 30 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 12 | C1 | PhMe | A5 | 40 | 48 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 13 | C1 | PhMe | A6 | 40 | 50 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 14 | C1 | PhMe | A7 | 40 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
| 15 | C1 | PhMe | A8 | 40 | 65 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
95 |
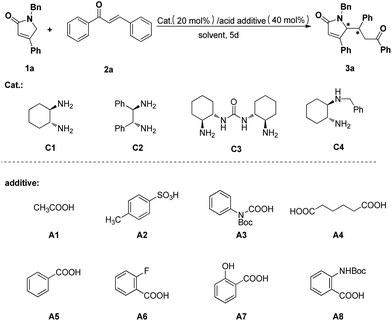
Our initial investigation began with the reaction of 4-phenyl N-benzyl pyrrolone (1a) and chalcone (2a) using chiral (1R, 2R)-cyclohexane-1,2-diamine (C1, 20 mol%) as catalyst and acetic acid (A1, 40 mol%) as additive in methanol at room temperature, and the desired product 3a was obtained in 25% yield with 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 89% ee (Table 1, entry 1). Encouraged by this result, we began the further optimization as follows. Firstly, different chiral primary amine catalysts were screened (Table 1, entries 1–4) and C1 still was the best one. Then, the effect of the additive on the reaction was tested (Table 1, entries 5–8). It can been seen that all selected additives except A2 worked well and A5 is better by comparison (Table 1, entry 8). By raising the reaction temperature from r.t. to 40 °C, the yield of 3a was improved to 45%, unfortunately, its stereoselectivity was significantly decreased (Table 1, entry 9). Furtherly, solvent screening revealed that compound 3a could be obtained in 48% yield with 90% ee in toluene at 40 °C (Table 1, entry 12). In order to further optimize the yield and stereoselectivity, the derivatives of benzoic acid (A6–A8) were examined (Table 1, entries 13–15). The results revealed that, using C1 as catalyst and A8 as additive, the reaction between substrates 1a and 2a in toluene at 40 °C gave the desired product 3a in 65% yield, 3
1 dr and 89% ee (Table 1, entry 1). Encouraged by this result, we began the further optimization as follows. Firstly, different chiral primary amine catalysts were screened (Table 1, entries 1–4) and C1 still was the best one. Then, the effect of the additive on the reaction was tested (Table 1, entries 5–8). It can been seen that all selected additives except A2 worked well and A5 is better by comparison (Table 1, entry 8). By raising the reaction temperature from r.t. to 40 °C, the yield of 3a was improved to 45%, unfortunately, its stereoselectivity was significantly decreased (Table 1, entry 9). Furtherly, solvent screening revealed that compound 3a could be obtained in 48% yield with 90% ee in toluene at 40 °C (Table 1, entry 12). In order to further optimize the yield and stereoselectivity, the derivatives of benzoic acid (A6–A8) were examined (Table 1, entries 13–15). The results revealed that, using C1 as catalyst and A8 as additive, the reaction between substrates 1a and 2a in toluene at 40 °C gave the desired product 3a in 65% yield, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr and 95% ee (Table 2, entry 15).
2 dr and 95% ee (Table 2, entry 15).
| Entry | 1 | Ar2 | Ar3 | 3/yieldb (%) | dr syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) antic antic |
ee (%) (syn)d |
|---|---|---|---|---|---|---|
| a All reactions were carried out using 1.0 equiv. of 1a (0.15 mmol), 1.5 equiv. of 2a (0.225 mmol), and 20 mol% of catalyst (0.03 mmol), 40 mol% of additive (0.06 mmol).b Isolated yield.c Determined by NMR.d Determined by chiral HPLC analysis. | ||||||
| 1 | 1a | Ph | Ph | 3a/65 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
95 |
| 2 | 1b | Ph | Ph | 3b/40 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 3 | 1c | Ph | Ph | 3c/60 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 4 | 1a | Ph | 3-MeOC6H4 | 3d/70 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90 |
| 5 | 1a | Ph | 4-ClC6H4 | 3e/62 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 |
| 6 | 1a | Ph | 3-NO2C6H4 | 3f/75 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 7 | 1c | Ph | 3-MeOC6H4 | 3g/64 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 8 | 1c | Ph | 3-MeC6H4 | 3h/55 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85 |
| 9 | 1c | Ph | 4-MeC6H4 | 3i/60 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 10 | 1c | Ph | 3-ClC6H4 | 3j/82 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 11 | 1c | Ph | 4-ClC6H4 | 3k/64 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 12 | 1c | Ph | 4-FC6H4 | 3l/55 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 13 | 1c | 4-MeC6H4 | Ph | 3m/69 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
84 |
| 14 | 1c | 4-MeC6H4 | 3-MeC6H4 | 3n/66 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 15 | 1c | 4-MeC6H4 | 3-BrC6H4 | 3o/64 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 |
| 16 | 1c | 4-MeC6H4 | 4-FC6H4 | 3p/90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
With the optimized conditions in hand, the application scope of the catalytic system was then explored. As shown in Table 2, different 4-aromatic ring substituted N-benzyl pyrrolones react well with variety of chalcones giving the corresponding products 3 in moderate to good yields and high enantioselectivities. For N-benzyl pyrrolones (Table 2, entries 1–3), the electron nature of the substituents on the aromatic ring at the 4-position of N-benzyl pyrrolones (1) did not have an obvious effect on either diastereoselectivity or enantioselectivity when ignoring the fact that 4-bromo substituent decreased the yield (Table 2, entry 2). As regards chalcones, whatever their aromatic rings Ar2 or Ar3 contained electron-rich or electron-deficient substituents, the reaction remained stable yields and high enantioselectivities.
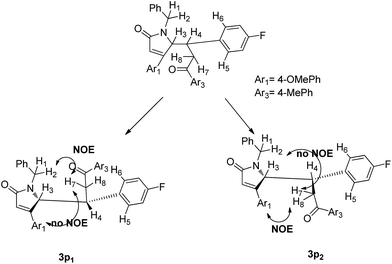
NOESY experiments performed on compound 3p,16 revealed strong correlations between hydrogen 2 and 5, 6, 7, 8 on 3p1, and no correlations between the hydrogens on Ar1 and hydrogen 7. As for 3p2, on the contrary, there were strong correlations between the hydrogens on Ar1, hydrogen 7, but no correlations between hydrogen 2 and 7, 8. Thus, the NOESY experiments allowed us to confirm the relative configuration of product 3p (Fig. 3). (see ESI†). Unfortunately, we were unable to grow quality crystals to determine compound 3p's absolute configuration.
Conclusions
In conclusion, we have developed an efficient asymmetric Michael addition reaction of 4-aromatic ring substituted N-benzyl pyrrolones with chalcones utilizing the simple and commercially available chiral 1,2-diaminocyclohexane-2-(N-Boc-amino)benzoic acid as the cooperative catalysts. The corresponding Michael addition products were obtained in moderate to good yields (up to 90%) and excellent enantioselectivity (up to 95% ee). Further studies and applications of vicinal primary diamine as catalyst in asymmetric reactions are currently underway in our laboratory.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (no. 81673287).Notes and references
- (a) K. C. Nicolaou, S. M. Dalby and U. Majumder, J. Am. Chem. Soc., 2008, 130, 14942–14943 CrossRef CAS PubMed; (b) P. Magnus, T. Katoh, I. R. Matthews and J. C. Huffman, J. Am. Chem. Soc., 1989, 111, 6707–6711 CrossRef CAS; (c) S. E. Denmark, Y. C. Moon and C. B. W. Senanayake, J. Am. Chem. Soc., 1990, 112, 311–315 CrossRef CAS.
- A. Husain, M. S. Y. Khan, S. M. Hasan and M. M. Alam, Eur. J. Med. Chem., 2005, 40, 1394–1404 CrossRef CAS PubMed.
- (a) A. Husain, S. M. Hasan, S. Lal and M. M. Alam, Indian J. Pharm. Sci., 2006, 68, 536–538 CrossRef CAS; (b) A. Ahmad, A. Husain, S. A. Khan, M. Mujeeb and A. Bhandari, J. Saudi Chem. Soc., 2015, 19, 340–346 CrossRef.
- C. Grunwald, C. Rundfeldt, H. J. Lankau, T. Arnold, N. Hofgen, R. Dost, U. Egerland, H. J. Hofmann and K. Unverferth, J. Med. Chem., 2006, 49, 1855–1866 CrossRef CAS PubMed.
- R. D. Alessio, A. Bargiotti, O. Carlini, F. Colotta, M. Ferrari, P. Gnocchi, A. Isetta, N. Mongelli, P. Motta, A. Rossi, M. Rossi, M. Tibolla and E. Vanotti, J. Med. Chem., 2000, 43, 2557–2565 CrossRef PubMed.
- M. M. Alam, A. Husain, S. M. Hasan and T. Anwer, Eur. J. Med. Chem., 2009, 44, 2636–2642 CrossRef CAS PubMed.
- S. Olla, F. Manetti, E. Crespan, G. Maga, A. Angelucci, S. Schenone, M. Bologna and M. Botta, Bioorg. Med. Chem. Lett., 2009, 19, 1512–1516 CrossRef CAS PubMed.
- (a) G. R. Pettit, S. Freeman, M. J. Simpson, M. A. Thompson, M. R. Boyd, M. D. Williams, G. R. Pettit and D. L. Doubek, Anti-Cancer Drug Des., 1995, 10, 243–249 CAS; (b) K. Sakata, K. Aoki, C. F. Chang, A. Sakurai, S. Tamura and S. Murakoshi, Agric. Biol. Chem., 1978, 42, 457–459 CAS; (c) M. Tereda, M. Sano, A. I. Ishii, H. Kino, S. Fukushima and T. J. Noro, J. Pharm. Soc. Jpn., 1982, 79, 93–98 Search PubMed; (d) H. Shinozaki and M. Ishida, Brain Res., 1985, 334, 33–40 CrossRef CAS PubMed; (e) D. Li, Y. J. Wang, L. Q. Wang, J. Wang, P. X. Wang, K. Z. Wang, L. Liu, D. S. Liu, X. X. Jiang and D. X. Yang, Chem. Commun., 2016, 52, 9640–9643 RSC.
- (a) L. Lin, J. Zhang, X. Ma, X. Fu and R. Wang, Org. Lett., 2011, 13, 6410–6413 CrossRef CAS PubMed; (b) J. Zhang, X. Liu, X. Ma and R. Wang, Chem. Commun., 2013, 49, 9329–9331 RSC; (c) C. Curti, B. Ranieri, L. Battistini, G. Rassu, V. Zambrano, G. Pelosi, G. Casiraghi and F. Zanardi, Adv. Synth. Catal., 2010, 352, 2011–2022 CrossRef CAS; (d) N. E. Shepherd, H. Tanabe, Y. Xu, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2010, 132, 3666–3667 CrossRef CAS PubMed.
- (a) A. R. Choudhury and S. Mukherjee, Org. Biomol. Chem., 2012, 10, 7313–7320 RSC; (b) Y. Chen, U. Das, M. Liu and W. Lin, J. Org. Chem., 2015, 80, 1985–1992 CrossRef CAS PubMed; (c) J. L. Zhang, X. H. Liu, X. J. Ma and R. Wang, Chem. Commun., 2013, 49, 3300–3302 RSC; (d) J. L. Zhang, X. H. Liu, X. J. Ma and R. Wang, Chem. Commun., 2013, 49, 9329–9331 RSC; (e) Y. Zhang, Y. L. Shao, H. S. Xu and W. Wang, J. Org. Chem., 2011, 76, 1472–1474 CrossRef CAS PubMed; (f) T. Y. Liu, H. L. Cui, J. Long, B. J. Li, Y. Wu, L. S. Ding and Y. C. Chen, J. Am. Chem. Soc., 2007, 129, 1878–1879 CrossRef CAS PubMed; (g) N. E. Shepherd, H. Tanabe, Y. J. Xu, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2010, 132, 3666–3667 CrossRef CAS PubMed; (h) J. T. Li, S. Qiu, X. Y. Ye, B. Zhu, H. J. Liu and Z. Y. Jiang, J. Org. Chem., 2016, 81, 11916–11923 CrossRef CAS PubMed; (i) H. Tanabe, Y. J. Xu, B. Sun, S. Matsunaga and M. Shibasaki, Heterocycles, 2012, 86, 611–622 CrossRef CAS; (j) S. G. Zlotin and S. V. Kochetkov, Russ. Chem. Rev., 2015, 84, 1077–1099 CrossRef CAS; (k) J. C. Kizirian, Chem. Rev., 2008, 108, 140–205 CrossRef CAS PubMed; (l) Y. H. Lam, M. N. Grayson, M. C. Holland, A. Simon and K. N. Houk, Acc. Chem. Res., 2016, 49, 750–762 CrossRef CAS PubMed.
- (a) W. Wu, X. Li, H. C. Huang, X. Q. Yuan, J. Z. Lu, K. L. Zhu and J. X. Ye, Angew. Chem., Int. Ed., 2013, 52, 1743–1747 CrossRef CAS PubMed; (b) J. W. Xie, L. Yue, D. Xue, X. L. Ma, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Chem. Commun., 2006, 48, 1563–1565 RSC; (c) T. B. Poulsen, C. Alemparte and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 11614–11615 CrossRef CAS PubMed; (d) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2001, 40, 3726–3748 CrossRef CAS; (e) J. L. Zhang, X. H. Liu, X. J. Ma and R. Wang, Chem. Commun., 2013, 49, 3300–3302 RSC.
- X. Feng, H. Cui, S. Xu, L. Wu and Y. Chen, Chem.–Eur. J., 2010, 16, 10309–10312 CrossRef CAS PubMed.
- Y. Yang, S. Dong, X. Liu, L. Lin and X. Feng, Chem. Commun., 2012, 48, 5040–5042 RSC.
- H. Huang, Z. Jin, K. Zhu, X. Liang and J. Ye, Angew. Chem., Int. Ed., 2011, 50, 3232–3235 CrossRef CAS PubMed.
- J. F. Wang, C. Qi, Z. M. Ge, T. M. Cheng and R. T. Li, Chem. Commun., 2010, 46, 2124–2126 RSC.
- G. Chaubet, T. Coursindel, X. Morelli, S. Betzi, P. Roche, Y. Guari, A. Lebrun, L. Toupet, Y. Collette, I. Parrot and J. Martinez, Org. Biomol. Chem., 2013, 11, 4719–4726 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03069j |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2017 |