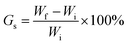Open Access Article
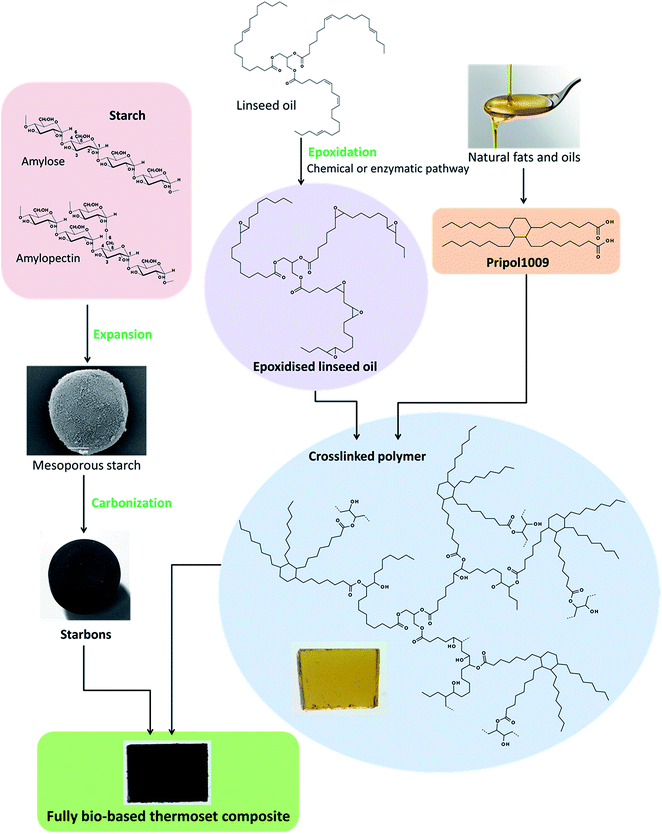
Open Access ArticleBio-based carbonaceous composite materials from epoxidised linseed oil, bio-derived curing agent and starch with controllable functionality†
N. Supanchaiyamat *a,
P. S. Shuttleworth
*a,
P. S. Shuttleworth b,
C. Sikhomc,
S. Chaengkhama,
H.-B. Yuede,
J. P. Fernández-Blázquezd,
V. L. Budarinc and
A. J. Hunt
b,
C. Sikhomc,
S. Chaengkhama,
H.-B. Yuede,
J. P. Fernández-Blázquezd,
V. L. Budarinc and
A. J. Hunt c
c
aMaterials Chemistry Research Center, Department of Chemistry, Faculty of Science, Khon Kaen Univesity, Khon Kaen, 40002, Thailand. E-mail: nontsu@kku.ac.th
bDepartamento de Física de Polímeros, Elastómeros y Aplicaciones Energéticas, Instituto de Ciencia y Tecnología de Polímeros, CSIC, 28006 Madrid, Spain
cDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK
dIMDEA Materials Institute, c/Eric Kandel 2, Getafe 28906, Madrid, Spain
eSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, China
First published on 4th May 2017
Abstract
Development of biomass-derived materials using sustainable practices has been one of the major scientific aims over the last few decades. A new class of bio-derived nanocomposite derived from epoxidised linseed oil, a bio-derived crosslinker and a starch based carbonaceous mesoporous material (Starbon®) has been developed. The use of Starbons® technology enables the incorporation of carbonaceous materials with tuneable surface functionality (from hydrophilic to hydrophobic). The resulting composite demonstrated good thermal stability up to 300 °C, good low temperature modulus, flexibility and uniformity, as demonstrated by TGA, DMA and SEM studies, respectively. Furthermore, the thermoset composites' swelling behaviour in solvents with a high polar index through to non-polar ones was investigated, revealing initially that non polar solvents have a greater impact on swelling than polar solvents and that in all cases the addition of filler reduces the extent of swelling. The inclusion of this carbonaceous material with hierarchical pore structure and high BET surface area may further aid the use of such composites in membrane separation applications.
Introduction
Petroleum resources have been used as the primary source of chemicals and materials from the early 20th century, however, critical issues relating to their finite availability and environmental concerns have resulted in the search for sustainable alternatives.1 Biomass has received significant attention as a feedstock for the production of chemicals and materials due to its inherent renewability and positive environmental credentials.2–4 Plant oils primarily derived of triglycerides and fatty acids are one such potential resource derived from biomass. Epoxidised plants oils have been intensively investigated for the production of novel materials such as alkyd resins, blends and composites.5–8 Thermoset materials are highly-crosslinked polymers that are cured by heat, pressure, light radiation or a combination of these energy sources.9 In many instances epoxidised plant oils are used as precursors for thermoset matertials.10 Bio-derived diacids have been utilised to crosslink epoxidised linseed oil, resulting in highly flexible transparent films, with significant water resistance.11In addition, fibres and/or particles have been extensively used as reinforcement agents for plant oil based resins, thus enhancing their mechanical properties.12–14 Carbonaceous fillers have also been used to improve thermoset plastic properties e.g. mechanical and thermal behaviours, and thus expand further their range of viable applications.15–17 However, carbonaceous fillers typically have limited surface functionality, are extensively microporous and often their manufacture is energy intensive, and in some cases these materials are costly.18 The polysaccharide-derived carbonaceous materials registered as Starbon®, with a highly developed mesoporous structure and temperature tuneable surface functionality (from hydrophilic to hydrophobic) are one potential alternative.19,20 These materials can be prepared through a simple expansion and carbonisation methodology from the parent polysaccharide.21,22 This flexibility, along with hierarchical pore structure and high BET surface area make Starbon® successful in a wide range of applications including catalysis, chromatography, adsorption of organic compounds (i.e. dyes), precious metals (i.e. gold, and palladium) and CO2 capture.23–28 Furthermore, Starbon® materials have significant aromatic/graphitic character at relatively low temperatures of preparation and therefore less energy is consumed during preparation.
Herein, Starbon® materials carbonised at different temperatures were combined with epoxidised linseed oil and a bio-derived dicarboxylic acid crosslinker to produce thermoset composites. The use of these renewable materials strongly adheres with the principles of Green chemistry, as does the composites synthesis that requires no addition of solvents (Fig. 1). Curing of the resin (control) and composites containing Starbon® with varying functionality (hydrophilic to hydrophobic) was confirmed by both FT-IR and DSC, whilst their morphology, thermal and dynamic mechanical properties were characterised using SEM, TGA and DMA respectively. Furthermore, swelling behaviour of the composites in solvents with different polarities was investigated.
Experimental
Materials
All materials were used as received without further purification. ELO (Lankroflex L) was obtained from Akcros Chemicals (oxirane oxygen = 9%). Bio-derived dicarboxylic acid (Pripol 1009) was kindly supplied by Croda. 4-N,N-Dimethylaminopyridine (DMAP) was purchased from Fluka. Toluene and ethanol were obtained from Fisher Scientific.Preparation of Starbon®
Starbon® fillers were synthesised as previously reported in the literature.19 The preparation method comprises three key stages: gelation of starch, retrogradation and water exchange with a lower surface tension solvent, followed by oven-drying to yield a predominantly mesoporous material. In the final stage the mesoporous starch was doped with a catalytic amount of an organic acid (e.g. 0.1 mmol of p-toluenesulfonic acid per 1 g of starch) and heated under vacuum to yield at 300 °C and 800 °C the materials S300 and S800 respectively.Characterisation of Starbon®
Nitrogen adsorption/desorption isotherms were conducted at 77 K using a Micromeritics ASAP 2010 volumetric adsorption analyser. Prior to analysis, the samples were outgassed at 65 °C for at least 3 h under reduced pressure and mass differences were corrected after the experiment. The surface area of samples was automatically calculated by the Micromeritics ASAP 2010 volumetric adsorption analyser. The isotherm data were used to obtain the BET specific surface area data (SBET) and the Barret–Joyner–Halenda (BJH) pore size distribution was calculated from the adsorption branch of the N2 physisorption isotherms.Preparation of thermoset composites
A vigorously stirred mixture of epoxidised linseed oil (ELO) (3.5 g), Pripol 1009 (3.5 g) and 4-(dimethylamino) pyridine (DMAP) (0.5% by weight of the total resin weight) was heated to 130 °C for 3 minutes. Subsequently, Starbon® (S300 or S800) was added into the mixture which was maintained at 130 °C with stirring for a further 3 minutes. The mixture was then poured into a circular aluminium tray (inner diameter = 70 mm) and cured in an oven at 140 °C for 20 h.MDSC of the reaction mixtures
Reaction mixtures were investigated using a TA Q2000 modulated differential scanning calorimeter (MDSC). The mixtures were accurately weighed (10 mg) into high-pressure stainless steel pans, sealed and subjected to a heat/cool cycle from −80 °C to 250 °C at 1 °C min−1 with a modulation cycle of ±0.5 °C every 200 seconds.Thermal degradation temperature
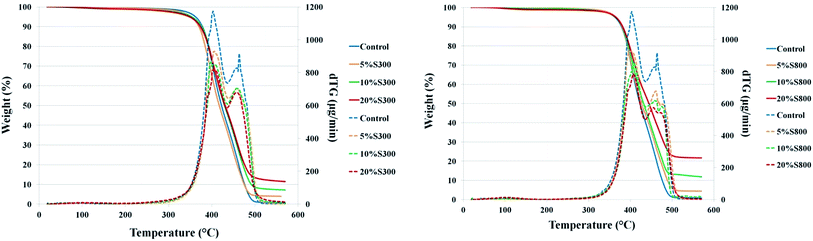
TG-DTA curves were performed on a Perkin Elmer Pyris Diamond TG-DTA instrument using α-alumina (α-Al2O3) as a standard material. The samples were heated from 25–600 °C at 10 °C min−1 under a nitrogen atmosphere. The initial sample weight was approximately 10–15 mg.Infrared spectroscopy
In order to study the curing reaction for each formulation, spectra of the samples before and after curing were recorded using a Bruker Vertex 70 spectrophotometer in Attenuated Total Reflectance (ATR) mode with a resolution of 2 cm−1 and 32 scans. All spectra were normalised using OPUS 5.0 software as provided by the instrument manufacturer.Scanning electron microscopy
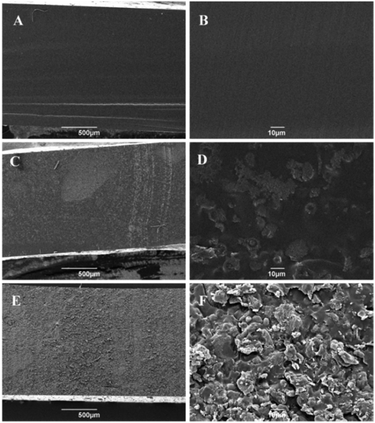
Film samples were cut into 2 × 10 mm pieces, and then mounted onto alumina sample holders using double-sided tape. The samples were positioned so that the cross-section of the films can be seen on the top view. Prior to analysis, approximately 5 nm Au/Pd coating was applied to the samples using a high-resolution sputter SC-7640 coating device at a sputtering rate of 1500 V min−1. The samples were viewed using a JEOL JSM-6490LV (JEOL, Japan).Swelling behaviour7
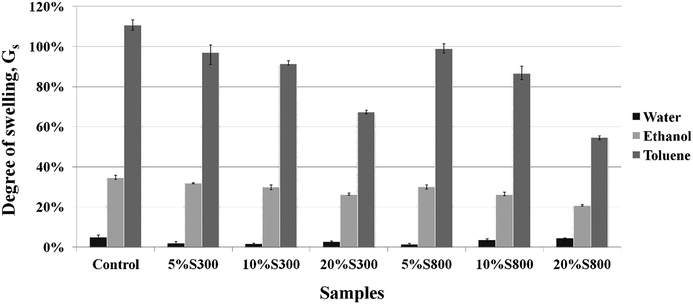
Composite films of approximate 20 mg weight were immersed in water, ethanol or toluene at room temperature for 24 hours and then removed from the solvent and blotted with a tissue to remove excess liquid before being re-weighed. The average degree of swelling (Gs) was determined from at least three experiments and calculated using the formulation:where, Wi represents the initial weight of the film and Wf represents the final weight of the film after drying.
Dynamic mechanical analysis
Dynamic mechanical analyser (DMA Q800, TA) in three point bending mode was used for all samples. The dimensions of the samples were 20 × 4 × 1.25 mm3 (length, width, thickness); measurements were carried out at 10 Hz, 20 μm of strain amplitude in bending and a heating rate of 1 °C min−1.Mechanical test
The composite films were cut into standard dumb-bell shapes with the average thickness in the region of 1–1.5 mm. Tensile studies were conducted using an Instron V.5567A universal testing machine fitted with 1000 N capacity load cell. The initial grip separation was set at 20 mm and the crosshead speed was 20 mm min−1. The mechanical properties reported for each sample were the average of three measurements.Results and discussion
Characterisation of Starbon®
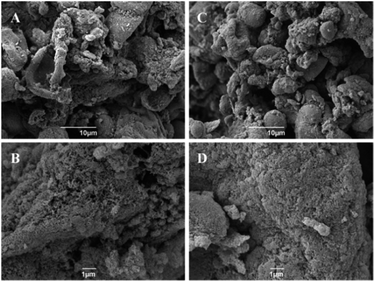
The textural properties of the Starbon® fillers were quantitatively analysed using N2 adsorption, and are presented in Table 1. It was found that they show type IV behaviour, characteristic of mesoporous materials. Though, with increasing preparation temperature from 300 to 800 °C BET surface areas increased from 115 to 445 m2 g−1, correlating with an increase in micropore formation from 4.5 to 21.6%.21 Furthermore, the formation of an increasingly macroporous structure can also be observed in the presented SEM images of the Starbon® fillers (Fig. 2). Previous findings using FT-IR, 13C-MAS NMR and XPS have also demonstrated that significant differences are observed in the functionality of the Starbon® material dependent on the temperature of preparation, from initially predominately hydroxyl-rich to aliphatic/alkene groups at 300 °C, through to highly aromatic at 800 °C.26,29,30| Properties | S300 | S800 |
|---|---|---|
| SBET (m2 g−1) | 115 | 445 |
| Total pore volume (cm3 g−1) | 0.67 | 0.74 |
| Average pore diameter (nm) desorption | 29.3 | 23.5 |
| Microporous volume (cm3 g−1) | 0.03 | 0.16 |
| % microporosity | 4.5 | 21.6 |
Curing study of the reactive mixtures using FT-IR
ATR-IR analyses of the reactive mixtures prior to and after heating were carried out in order to determine the potential mechanism of curing (Fig. 3). The disappearance of the peak at 820 cm−1 and the appearance of a band at 3440 cm−1 associated with hydroxyl functionality after curing suggested epoxide ring opening had occurred during the reaction. Furthermore, a new peak at 1016 cm−1 was observed denoting the presence of ether functionality, indicating possible etherification of the alkoxide group, which can then continue to react with other epoxides and form the crosslinked network. During this time reactions involving the diacid crosslinker are also evident from the disappearance of νas(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1709 cm−1 corresponding to the carboxylic acid. Furthermore, a band at 1741 cm−1 associated with the C
O) at 1709 cm−1 corresponding to the carboxylic acid. Furthermore, a band at 1741 cm−1 associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the ester group presented in the triglyceride was observed in the spectra of uncured mixture. After curing, a band at 1736 cm−1 could be noted, suggesting esterification. Thus it can be seen that etherification and esterification occurred during the curing process. The addition of Starbon® fillers scarcely altered the spectra of both reactive mixtures and cured films (see ESI†); however, the ester band at 1736 cm−1 of the cured control sample showed a slightly higher intensity than the ones with fillers, suggesting a greater degree of crosslinking as also confirmed by DSC.
O of the ester group presented in the triglyceride was observed in the spectra of uncured mixture. After curing, a band at 1736 cm−1 could be noted, suggesting esterification. Thus it can be seen that etherification and esterification occurred during the curing process. The addition of Starbon® fillers scarcely altered the spectra of both reactive mixtures and cured films (see ESI†); however, the ester band at 1736 cm−1 of the cured control sample showed a slightly higher intensity than the ones with fillers, suggesting a greater degree of crosslinking as also confirmed by DSC.
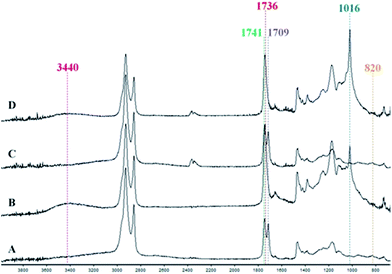 | ||
| Fig. 3 FT-IR spectra of (A) control reactive mixture, (B) control thermoset composite, (C) 20% S800 reactive mixture, (D) 20% S800 thermoset composite. | ||
Thermal analysis of the reactive mixtures
Modulated DSC was used to investigate thermal events that occurred during the curing process of the reactive mixtures. The control formulation was comprised of ELO, Pripol 1009 and DMAP (0.5% of total weight). In other formulations, various amounts of either S300 or S800 (5–20 wt%) was included. Non-reversible heat flow traces of all formulations showed an exothermic transition which corresponded to the curing reaction (Fig. 4). The formulations with Starbon® showed similar or lower total heats of reaction compared to that of the control sample, dependent on the amount of filler added. This was more pronounced for the formulations containing the S300 as the filler. This could be explained by the larger particle size of S300 (Fig. 2) that would inhibit the polymer chains to crosslink, hence the more significant drop in heat of reaction. However, this contradicts the result obtained from a previous study that used starch as filler as the formulation with starch demonstrated higher heat of reaction comparing to ones without.31 In this case it was suggested that the presence of starch might promote additional crosslinking. This postulation was highlighted as being a result of the hydrophilic hydroxyl groups present in the structure of the polysaccharide, which promoted the oxirane ring opening of ELO.31 The carbonisation of the mesoporous starch to obtain Starbon® increases its hydrophobicity and reduces the presence of hydroxyl functionality. At 300 °C, the dominant functionalities present are aliphatic and alkene functionalities. At temperatures of >600 °C aromatic and graphite-type structures are present.21 As such, Starbon® which possesses a lower number of hydroxyl groups (S800) cannot promote the ring-opening as presented in the case of starch, thus resulting in a lower observed heat of reaction.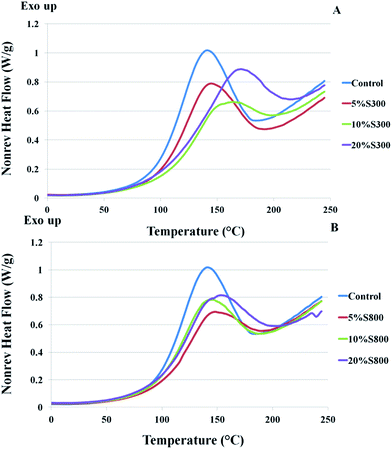 | ||
| Fig. 4 DSC thermograms of formulations comprised of ELO, Pripol, DMAP and different amounts of S300 (A) and S800 (B) – 5, 10 and 20%wt. | ||
The presence of S300 increases the onset and peak temperatures of the exothermic transition with the amounts of the filler, whilst the S800 formulations demonstrated close values to those of the control. Higher peak temperatures and longer curing time were observed for the Starbon® filled samples, as compared to samples with no filler. This suggests that the addition of Starbon® retarded the curing reaction. Similar observation was reported in carbon nanotubes reinforced polymers.32–34 The Starbon® retardation of cure could relate to the adsorption of polymer chains onto carbonaceous mesoporous surfaces due to a CH–π interaction. Such effects were also observed in a case of carbon nanofiber reinforced bio-based polyester reported by Tang et al.35 The cause of the delay in curing of the polymer was due to the physical hindrance of Starbon® to the mobility of the matrix. Furthermore, a study by Xu et al. revealed that serious retardation indicated better dispersion of a carbonaceous filler in the polymer matrix.32 This results in a better quality of dispersion for the Starbon® materials throughout the matrix and allows more interface area between the filler and polymer, thus increasing the physical hindrance caused by filler particles to the mobility of the polymer chains. In previous studies, starch was also found to cause retardation of the curing process, which may be due to slow diffusion of the molecules whilst starch granules were present in the reactive mixtures.31 It is also noteworthy that the curing curve of the formulation with starch showed two overlapping exothermic transitions between 75 °C to 200 °C, however the formulation with Starbon® gave only one transition in the same region. In a polymeric system, the observation of two transitions is generally associated with the presence of two phases,36 the second transition at higher temperature could correspond to the region that was more influenced by the presence of starch, hence the curing was delayed. The fact that Starbon® fillers are more hydrophobic relative to starch allows better diffusion of the filler particles. The uniform distribution of Starbon® particles results in single curing transition with no evident sign of post-cure in second heating cycle (see ESI†).
The glass transition temperature (Tg) of the S300 filled samples exhibited lower Tg as the loading of the filler increased, whilst the more graphitic S800 filled samples showed a slight increase of Tg compared to that of the control sample. The increase in Tg can be anticipated in composites and this has been reported in numerous studies in the case of carbonaceous filler-polymer composites.37–39 This augmentation of Tg can be associated with a reduction of the mobility of the polymer matrix around Starbon® particles due to the interfacial interactions between the polymer chains and the filler. As such, the segmental motion of the polymer chains is reduced.
However, the decrease in Tg of the more hydrophilic S300 samples may be explained by bundling tendency as observed in composites with single wall carbon nanotubes.40 The tendency of these particles to form close-packed bundles and consequently increase a free volume which provides more mobility of polymer chains, resulting in a drop-off of Tg.40 Also, the heat of reactions for the S300 samples was disrupted to a greater extent compared to the materials containing S800 and as a consequence their subsequent crosslink densities will be lower. It is therefore suggested that a combination of these events led to reduced Tg for the S300 samples compared to the S800 counterparts.
Thermoset composites characterisations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ results (ESI†). This provides an indication of lower crosslink density due to lower cure grade and worse scattered E′.
δ results (ESI†). This provides an indication of lower crosslink density due to lower cure grade and worse scattered E′.
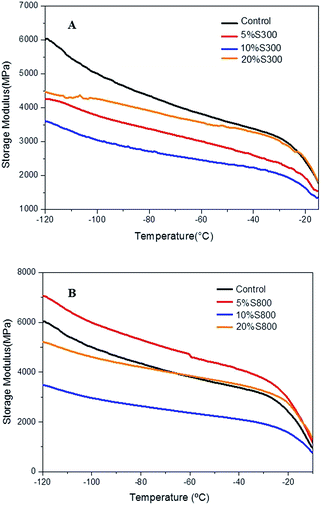 | ||
| Fig. 7 Storage modulus of the thermoset films with S300 (A) and S800 (B) at different loadings – 5, 10, 20 wt% as a function of temperature. | ||
Though the measurement of the storage modulus is influenced by a number of factors, overall it seems that incorporation of the hydrophobic more graphitic S800 into the resin matrix imparts better mechanical performance for the composites in comparison with the more hydrophilic S300-reinforced materials. Changes in the functionality of starch upon carbonisation from hydroxyl-rich polysaccharides to carboxyl, ether groups at S300 and further into highly aromatic materials with S800 may also explain this different reinforcing effect.25,31 Secondly, higher surface area of the S800 filler would provide a better environment into which the resin could easily adhere, thereby yielding a composite with better dispersion of the filler within the polymer as previously seen on SEM micrographs (Fig. 6D–F).
It is noted that swelling behaviour of the thermoset composites highlights the potential for such materials to be utilised in the development of membranes for water–ethanol separation through pervaporation. This method is considered a promising process in separating azeotropic mixtures. During the process, the penetrants should swell the membrane and as a result its microscopic structure is changed. This phenomenon consequently improves diffusion rates of the swollen membrane as compared with those of non-swollen ones.46 The diffusion of ethanol into a non-swollen membrane is normally difficult due to its large size while the swollen membrane can facilitate its diffusion.47 These thermoset composites should allow good diffusion of ethanol through the membrane and therefore increase the ethanol flux. Also, the production of such materials from bio-based resources which include the incorporation of tuneable mesoporous materials such as Starbon® may open new doors in the separation of higher value products as part of a biorefinery concept and will be the focus of future investigations.
Conclusions
Fully bio-derived thermoset composites were synthesised from epoxidised linseed oil, bio-derived curing agent and carbonaceous starch. The curing was confirmed by ATR-IR and MDSC. Thermal analysis demonstrated that the composites prepared with the more graphitic S800 had a higher Tg than that of the material without filler, implying more interfacial interactions between the polymer chains and the filler. However, for the hydrophilic S300 the bundling tendency seemed to influence the mobility of polymer chains, resulting in the decrease of Tg. Even dispersion of Starbon® filler into the resin imparts good mechanical performance for the composite, with S800 being more pronounced than S300. Starbon® fillers aided in reinforcing the materials, with increased maximum tensile strength and Young's modulus of 260% and 400% respectively. The resulting materials were found to be thermally stable until 300 °C, which enables use in high temperature applications. The use of Starbon® technology enables the incorporation of carbonaceous materials into the composite, with tuneable surface functionality (from hydrophilic to hydrophobic). The inclusion of this carbonaceous material with hierarchical pore structure and high BET surface area may further aid in these composites finding use in a range of applications as a bio-based thermoset material. The swelling tests of the bio-based composites suggested that these materials can potentially be utilised as membranes and will be the focus of future studies.Acknowledgements
N. S. would like to thank the National Research University Project for their financial support through Biofuels Research Cluster at Khon Kaen University (NRU59006). N. S. and A. J. H. would like to thank the financial support of the Newton Mobility Grant (Royal Society). C. S. gratefully acknowledges funding through The Oil Refinery Contract Contribution Fund and the Ministry of Energy, Thailand. P. S. S. gratefully acknowledges the Spanish Ministry Economy and Competitivity (MINECO) for a Ramón y Cajal senior research fellowship (RYC-2014-16759) and a proyecto de I + D + I para jóvenes investigadores (MAT2014-59674-JIN). H. Y. thanks the financial support from China Scholarship Council. H. Y. is also thankful to the China Postdoctoral Science Foundation. J. P. F.-B. is grateful for the financial support from “Marie Curie” Amarout Europe Programme.Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- V. L. Budarin, P. S. Shuttleworth, J. R. Dodson, A. J. Hunt, B. Lanigan, R. Marriott, K. J. Milkowski, A. J. Wilson, S. W. Breeden, J. Fan, E. H. K. Sin and J. H. Clark, Energy Environ. Sci., 2011, 4, 471–479 CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- J. H. Clark, L. A. Pfaltzgraff, V. L. Budarin, A. J. Hunt, M. Gronnow, A. S. Matharu, D. J. Macquarrie and J. R. Sherwood, Pure Appl. Chem., 2013, 85, 1625–1631 CrossRef CAS.
- P. Gogoi, M. Boruah, S. Sharma and S. K. Dolui, ACS Sustainable Chem. Eng., 2015, 3, 261–268 CrossRef CAS.
- T. Tsujimoto, T. Takayama and H. Uyama, Polymers, 2015, 7, 2165–2174 CrossRef CAS.
- P. Gogoi, H. Horo, M. Khannam and S. K. Dolui, Ind. Crops Prod., 2015, 76, 346–354 CrossRef CAS.
- M. D. Samper, R. Petrucci, L. Sánchez-Nacher, R. Balart and J. M. Kenn, Composites, Part B, 2015, 71, 203–209 CrossRef CAS.
- J. M. Raquez, M. Deléglise, M. F. Lacrampe and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487–509 CrossRef CAS.
- M. Galià, L. Montero de Espinosa, J. C. Ronda, G. Lligadas and V. Cádiz, Eur. J. Lipid Sci. Technol., 2010, 112, 87–96 CrossRef.
- N. Supanchaiyamat, P. S. Shuttleworth, A. J. Hunt, J. H. Clark and A. S. Matharu, Green Chem., 2012, 14, 1759–1765 RSC.
- A. O'Donnell, M. A. Dweib and R. P. Wool, Compos. Sci. Technol., 2004, 64, 1135–1145 CrossRef.
- Z. Liu, S. Z. Erhan, D. E. Akin and F. E. Barton, J. Agric. Food Chem., 2006, 54, 2134–2137 CrossRef CAS PubMed.
- N. Boquillon, J. Appl. Polym. Sci., 2006, 101, 4037–4043 CrossRef CAS.
- M. Yin, J. A. Koutsky, T. L. Bard, N. M. Rodriguez, R. T. K. Baker and L. Klebanov, Chem. Mater., 1993, 5, 1024–1031 CrossRef CAS.
- I. Sedat Gunes, G. A. Jimenez and S. C. Jana, Carbon, 2009, 47, 981–997 CrossRef.
- K. Hilarius, D. Lellinger, I. Alig, T. Villmow, S. Pegel and P. Pötschke, Polymer, 2013, 54, 5865–5874 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- V. L. Budarin, J. H. Clark, J. J. E. Hardy, R. Luque, K. Milkowski, S. J. Tavener and A. J. Wilson, Angew. Chem., Int. Ed., 2006, 45, 3782–3786 CrossRef CAS PubMed.
- R. J. White, V. L. Budarin, R. Luque, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 3401–3418 RSC.
- V. L. Budarin, J. H. Clark, F. E. I. Deswarte, J. J. E. Hardy, A. J. Hunt and F. M. Kerton, Chem. Commun., 2005, 23, 2903–2905 RSC.
- A. Muñoz García, A. J. Hunt, V. L. Budarin, H. L. Parker, P. S. Shuttleworth, G. J. Ellis and J. H. Clark, Green Chem., 2015, 17, 2146–2149 RSC.
- J. C. Colmenares, P. Lisowski and D. Łomot, RSC Adv., 2013, 3, 20186–20192 RSC.
- H. L. Parker, J. R. Dodson, V. L. Budarin, J. H. Clark and A. J. Hunt, Green Chem., 2015, 17, 2200–2207 RSC.
- A. S. Marriott, E. Bergström, A. J. Hunt, J. Thomas-Oates and J. H. Clark, RSC Adv., 2014, 4, 222–228 RSC.
- H. L. Parker, A. J. Hunt, V. L. Budarin, P. S. Shuttleworth and J. H. Clark, RSC Adv., 2012, 2, 8992–8997 RSC.
- H. L. Parker, V. L. Budarin, J. H. Clark and A. J. Hunt, ACS Sustainable Chem. Eng., 2013, 1, 1311–1318 CrossRef CAS.
- D. Gema, V. L. Budarin, J. A. Castro-Osma, P. S. Shuttleworth, S. C. Z. Quek, J. H. Clark and M. North, Angew. Chem., Int. Ed., 2016, 55, 9173–9177 CrossRef PubMed.
- A. S. Marriott, A. J. Hunt, E. Bergström, K. Wilson, V. L. Budarin, J. Thomas-Oates, J. H. Clark and R. Brydson, Carbon, 2014, 67, 514–524 CrossRef CAS.
- P. S. Shuttleworth, V. L. Budarin, R. J. White, V. M. Gun'ko, R. Luque and J. H. Clark, Chem.–Eur. J., 2013, 19, 9351–9357 CrossRef CAS PubMed.
- N. Supanchaiyamat, A. J. Hunt, P. S. Shuttleworth, C. Ding, J. H. Clark and A. S. Matharu, RSC Adv., 2014, 4, 23304–23313 RSC.
- J. Xu, K. M. Razeeb and S. Roy, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1845–1852 CrossRef CAS.
- A. Beigbeder, M. Linares, M. Devalckenaere, P. Degée, M. Claes, D. Beljonne, R. Lazzaroni and P. Dubois, Adv. Mater., 2008, 20, 1003–1007 CrossRef CAS.
- D. Yue, Y. Liu and Z. Shen, J. Mater. Sci., 2006, 41, 2541–2544 CrossRef CAS.
- Z. Tang, D. Sun, D. Yang, B. Guo, L. Zhang and D. Jia, Compos. Sci. Technol., 2013, 75, 15–21 CrossRef CAS.
- F. H. Gojny and K. Schulte, Compos. Sci. Technol., 2004, 64, 2303–2308 CrossRef CAS.
- Z. Jin, K. P. Pramoda, G. Xu and S. H. Goh, Chem. Phys. Lett., 2001, 337, 43–47 CrossRef CAS.
- J. Q. Pham, C. A. Mitchell, J. L. Bahr, J. M. Tour, R. Krishanamoorti and P. F. Green, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 3339–3345 CrossRef CAS.
- A. Allaoui and N. El Bounia, eXPRESS Polym. Lett., 2009, 3, 588–594 CrossRef CAS.
- A. Apicella, C. Migliaresi, L. Nicodemo, L. Nicolais, L. Iaccarino and S. Roccotelli, Composites, 1982, 13, 406–410 CrossRef CAS.
- C. Ding, P. S. Shuttleworth, S. Makin, J. H. Clark and A. S. Matharu, Green Chem., 2015, 17, 4000–4008 RSC.
- W. Liu, K. Xu, C. Wang, B. Qian, Y. Sun, Y. Zhang, H. Xie and R. Cheng, J. Therm. Anal. Calorim., 2016, 123, 2459–2468 CrossRef CAS.
- S. Jeong, S. Yeo and S. Yi, J. Mater. Sci., 2005, 40, 5407–5411 CrossRef CAS.
- P. Hambleton, Support Materials and Solvents, in High Performance Liquid Chromatography: Fundamental Principles and Practice, ed. W. J. Lough and I. W. Wainer, Blackie Academic and Professional, Glasgow, 1996, p. 93 Search PubMed.
- A. Zlatanić, C. Lava, W. Zhang and Z. S. Petrović, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 809–819 CrossRef.
- Y. K. Ong, G. M. Shi, N. L. Le, Y. P. Tang, J. Zuo, S. P. Nunes and T.-S. Chung, Prog. Polym. Sci., 2016, 57, 1–31 CrossRef CAS.
- R. Jiraratananon, A. Chanachai, R. Y. M. Huang and D. Uttapap, J. Membr. Sci., 2002, 195, 143–151 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02837g |
| This journal is © The Royal Society of Chemistry 2017 |