Open Access Article
Open Access ArticleSynthesis of anisotropic silica colloids†
Qiyu Yu *a,
Kun Wangb,
Jing Zhangb,
Mingyang Liub,
Yuanyuan Liub and
Chao Cheng*c
*a,
Kun Wangb,
Jing Zhangb,
Mingyang Liub,
Yuanyuan Liub and
Chao Cheng*c
aSchool of Materials Science and Engineering, Sichuan University of Science and Engineering, Zigong 643000, China. E-mail: yuqiyu2008@163.com
bInstitute of Atomic and Molecular Physics, Sichuan University, Chengdu 610064, China
cModern Experiment Technology Center, Anhui University, Hefei 230601, China. E-mail: chengc@ahu.edu.cn
First published on 28th July 2017
Abstract
A water-in-alcohol emulsion strategy was exploited for the preparation of various anisotropic silica colloids. Sodium citrate and highly concentrated polyvinylpyrrolidone (in the water-rich emulsion droplets) were utilized to costabilize the emulsion system. Simply by choosing proper alcohols and/or the mixing ratios of the alcohol mixtures, a wide range of shape- and structure-selective silica micro-/nanostructures, including hollow structures, were prepared. Some binary alcohol combinations, e.g. 1-heptanol/ethanol, can provide superb versatility via tuning the mixing ratio. In particular, the formation process of the anisotropic silica particles was examined. Tentatively, a “salting-out” mechanism was proposed to explain the formation of a Janus-like silica-droplet structure at the initial silica condensation stage, which is essential for the present anisotropic growth mode. The effect of the interfacial tensions between silica and the emulsion system was discussed. Interestingly, one-end-opened hollow structures, e.g. silica “tubes”, can be achieved by arresting the anisotropic growth of hollow silica particles midway.
Introduction
Colloidal silica micro-/nanomaterials have attracted tremendous attention because of the ease of production, low cost, highly flexible silica chemistry and excellent physical properties.1 The development of shape- and structure-controlled synthetic methods can certainly promote the exertion of this kind of materials. Especially, shape-anisotropic silica particles with non-centrosymmetric features can sometimes provide enhanced and even new properties owing to the anisotropic effects.2–6 Besides, anisotropic silica particles can also be useful templating or supporting materials to enhance and tune the properties of other materials.7 Unfortunately, the frequently used Stöber method8 and modified methods are not suitable for such synthesis, since amorphous silica colloids incline to form a sphere-like shape via isotropic polymerization of the siloxane precursor to minimize surface free energy.Usually, hard templates or shape-directing agents were exploited to control the morphology of silica particles. A straightforward method is to utilize other anisotropic templates to replicate silica shells with similar morphology. Therefore, as-prepared core-silica shells are usually used instead for fundamental and application studies.9,10 These methods require complex synthetic procedures and may bring in some limitations due to the presence of the templates or voids in the particles. Besides, a lot of attention has also been paid to biomimetic synthesis of anisotropic silica particles.11 However, the shape-directing mechanism is elusive and the rational design of the silica structure is difficult.
To date, synthesis of shape-anisotropic silica colloids without using hard templates remains a great challenge.12,13 In 2008, Zhang et al.14 reported an interesting emulsion strategy which realized shape-controlled growth of silica nanostructures. This method adopted a water-in-alcohol system composed of 1-pentanol, ethanol, polyvinylpyrrolidone (PVP) and an aqueous sodium citrate (Na3Cit)-protected gold colloid. Ammonia and tetraethyl orthosilicate (TEOS) were added into the system to initiate the growth of silica particles. It was suggested that, highly concentrated PVP in water-rich droplets, forming a PVP–water species, was able to stabilize the emulsion system, and that the complexes of PVP with the gold nanoparticles acted as soft templates to direct the shape-controlled growth of silica particles. Later, Kuijk et al.2 simplified the emulsion system by using Na3Cit instead of the gold nanoparticles. They observed the growing process of the rod-like silica particles in the emulsion system, and proposed a one-dimensional growth mode similar to the vapor–liquid–solid mechanism.15 That is, the silica rods growth occurred at the emulsion interface and the one-dimensional growth was supported by the one-sided precursor supply from the droplets.
The realization of the one-dimensional growth mode invokes great interest in the shape-controlled synthesis of silica micro-/nanostructures in this PVP- and Na3Cit-stabilized water-in-alcohol emulsion system. Various anisotropic silica structures were prepared under different experimental parameters, such as the choice of precursor, relative concentration of the reagents and reaction temperature.16–21 Particularly, more complex, segmented silica structures and related hybrid structures were prepared by simply regulating the reaction parameters during the one-dimensional growth.22–27 This water-in-alcohol emulsion system has shown incomparable versatility in the controlled synthesis of anisotropic silica micro-/nanostructures. Current study mostly focuses on the formation of rod-like structures in 1-pentanol/ethanol alcohol system, while other interesting silica materials, such as anisotropic hollow silica particles, are rarely studied. Most importantly, the knowledge about the mechanism behind the one dimensional growth mode, such as the driving force for anisotropic precursor supply, is rather limited. The exact roles of the alcohol phase, PVP and Na3Cit in the anisotropic growth of silica particles still remain elusive. Herein, we investigated the synthesis of silica colloids in a range of water-in-alcohol systems adopting different alcohols. We made an effort to elucidate the anisotropic growth mechanism in this emulsion strategy, and give plausible explanations for the effect of alcohol solvent on the shape and structure of as-prepared products, especially for the formation of hollow anisotropic silica colloids.
Experimental
Chemicals
1-octanol, 1-heptanol, 1-hexanol, 1-pentanol, 1-butanol, 1-propanol, 2-propanol, absolute ethanol, benzyl alcohol, tetraethyl orthosilicate (TEOS), citric acid, LD-tartaric acid, trisodium citrate dihydrate, ammonium chloride and ammonium nitrate are either of the analytical grade or of the highest purity grade available commercially. Polyvinylpyrrolidone (PVP, Mn = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Fisher. Ammonia solution (28%) and Milli-Q water were used for all the experiments.
000) was purchased from Fisher. Ammonia solution (28%) and Milli-Q water were used for all the experiments.
Synthesis of silica particles with method I
The synthesis of tadpole-like silica hollow particles in a 1-heptanol/ethanol mixture was used as an example to introduce the synthesis. In a plastic tube (with screw cap), 1 g PVP was dissolved in a mixture of 5 mL 1-heptanol and 6 mL ethanol. Sonication was applied during this process if necessary. Then 0.4 mL sodium citrate aqueous solution (9 g L−1) and 0.2 mL ammonia were added. The tube was dramatically shaken by hand to mix the chemicals. Then 0.1 mL TEOS was added and the system was shaken again and finally was left standing in the ambient condition (about 25 °C) overnight for the growth of silica colloids. The product was isolated by centrifugation. Especially, the synthesis can be quenched midway by centrifugation at a certain reaction time.Synthesis of silica particles with method II
This method is the same to method I except that the system was preheated to 60 °C before TEOS addition. After TEOS addition, the reaction system was left standing in ambient condition for the growth of silica colloids.Note that the reaction tube was sealed to prevent volatilization of the solvent or ammonia. Single alcohols (11 mL) or alcohol/ethanol binary combinations (11 mL in total, with various mixing ratios) were also used as solvent for the synthesis of different silica samples. Upscaling the synthesis from 11 mL alcohol batch up to 55 mL batch was also successful. The silica particles were isolated by centrifugation at 2000g for 5 min and then washed with ethanol and water for two times. In a study, citric acid (5.7 g L−1), LD-tartaric acid (4.5 g L−1), NH4Cl (1.6 g L−1) and NH4NO3 (2.4 g L−1) aqueous solution were used instead of the sodium citrate solution.
Characterization
The transmission electron microscopy (TEM) images of the silica particles were taken on a Tecnai T12 trans-mission electron microscope operating at 120 kV and a JEOL JEM2100 microscope at 200 kV. Scanning electron microscopy (SEM) images were taken on a FEI Quanta 600 field-emission microscope.Results and discussion
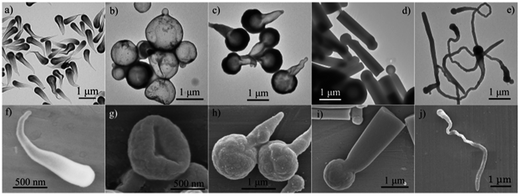
Other than the frequently adopted 1-pentanol/ethanol alcohol system, our synthesis also utilized the binary combinations of a relatively longer alcohol, such as 1-heptanol and 1-hexanol, with ethanol to construct the PVP- and Na3Cit-stabilized emulsion system. Some single alcohol systems also proved suitable for the synthesis. Our syntheses can be classified into two methods according to different ways of temperature control. In method I, the syntheses were carried out at room temperature all along. In method II, the emulsion system was preheated to 60 °C, and then, after TEOS addition, the growth of silica was allowed to proceed in the ambient condition (see Experimental). Without specification, method I was used for synthesis in the context.Besides the mostly reported rod-like particles, several anisotropic silica particles with hollow interiors can be prepared using our methods. Fig. 1 shows electron microscopy images of several silica particles prepared by using different alcohols or alcohol mixtures as the continuous phase. Three typical hollow structures (Fig. 1a–c and f–h), with tadpole-, balloon-, and bottle-like shapes, are readily witnessed in our syntheses in various alcohol systems. Note that the balloon-like hollow particles (Fig. 1b) typically has a small bulge outwards, which differs from normal centrosymmetric hollow silica spheres. Besides, they have very thin shells, and tend to deflate (Fig. 1g). Obviously, the rod-like silica particles (Fig. 1d and i) prepared in 1-hexanol/ethanol (v/v = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), with an additional round head, are also distinct from the mostly reported ones.2 We called them “matchstick-like” particles. The synthesis in 1-butanol/ethanol (v/v = 10
3), with an additional round head, are also distinct from the mostly reported ones.2 We called them “matchstick-like” particles. The synthesis in 1-butanol/ethanol (v/v = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using method II gave snake-like particles with slightly hollow interiors (Fig. 1e and j). The formation mechanism of the hollow structures will be discussed later. The dimensions of these silica particles generally range from one hundred nanometers to a few micrometers. In the context, only primary linear alcohols were investigated, although other kinds of alcohols can also be used for synthesis (Fig. S1†). Very similar silica particles were obtained in these alcohol systems by adjusting the alcohol/ethanol mixing ratio. Thereafter, only SEM images were exhibited for the products with similar morphology and inner structures to those showed in Fig. 1.
1) using method II gave snake-like particles with slightly hollow interiors (Fig. 1e and j). The formation mechanism of the hollow structures will be discussed later. The dimensions of these silica particles generally range from one hundred nanometers to a few micrometers. In the context, only primary linear alcohols were investigated, although other kinds of alcohols can also be used for synthesis (Fig. S1†). Very similar silica particles were obtained in these alcohol systems by adjusting the alcohol/ethanol mixing ratio. Thereafter, only SEM images were exhibited for the products with similar morphology and inner structures to those showed in Fig. 1.
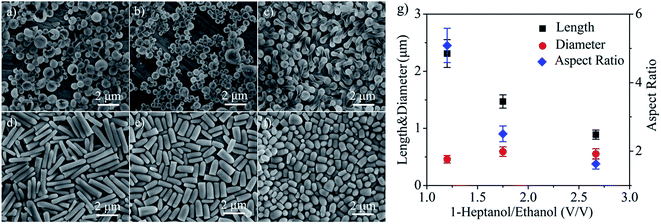
The results showed in Fig. 1 suggested that the nature of the alcohol solvent has great influence on the shape and structure of as-prepared silica particles. It is found that the combination of ethanol and a relatively longer alcohol, such as 1-hexanol and 1-heptanol, can offer versatile tunability on the morphology of as prepared silica particles simply by changing the mixing ratio. Fig. 2 gives the typical silica particles prepared with method I in six different 1-heptanol/ethanol systems. By adjusting the mixing ratio of the two alcohols, a wide range of anisotropic silica particles were prepared. With the increase of the 1-heptanol/ethanol ratio from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 5
8 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, hollow anisotropic structures were obtained and the “head” part of the particles become less and less prominent (Fig. 2a–c). Further increasing the proportion of 1-heptanol led to rod-like particles at mixing ratios of 6
6, hollow anisotropic structures were obtained and the “head” part of the particles become less and less prominent (Fig. 2a–c). Further increasing the proportion of 1-heptanol led to rod-like particles at mixing ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 8
4 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, with a gradually decreasing aspect ratio (Fig. 2d–g). Note that the diameter of the silica rods keeps at around 500 nm with relatively small changes (Fig. 2g). The results in Fig. 2 suggest two things: (1) the shape and structure of the silica product is highly tunable over a wide range of 1-heptanol/ethanol mixing ratios, and (2) too much usage of ethanol or 1-heptanol in the alcohol mixture results in decreased anisotropicity of the products. Actually, the use of pure ethanol or 1-heptanol give almost spherical silica particles (vide infra). Similar tendency can also be observed when using other binary alcohol combinations (Fig. S2–S4†).
3, with a gradually decreasing aspect ratio (Fig. 2d–g). Note that the diameter of the silica rods keeps at around 500 nm with relatively small changes (Fig. 2g). The results in Fig. 2 suggest two things: (1) the shape and structure of the silica product is highly tunable over a wide range of 1-heptanol/ethanol mixing ratios, and (2) too much usage of ethanol or 1-heptanol in the alcohol mixture results in decreased anisotropicity of the products. Actually, the use of pure ethanol or 1-heptanol give almost spherical silica particles (vide infra). Similar tendency can also be observed when using other binary alcohol combinations (Fig. S2–S4†).
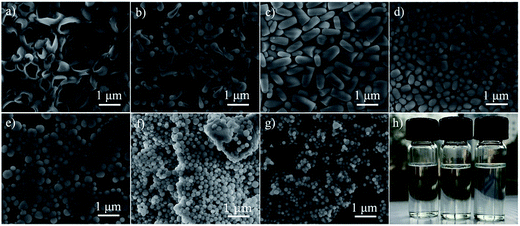
The synthesis of silica in some single alcohol systems was also investigated. Fig. 3a–g gives the images of the silica particles prepared with method I in linear primary alcohols from C2 to C8. The use of ethanol generated relatively big hollow silica particles with almost spherical morphology (Fig. 3a). The syntheses using alcohols with medium chain lengths of C3, C4, and C5 led to several anisotropic particles, hollow “tadpoles”, rods, and shorter rods respectively (Fig. 3b–d). Longer alcohols of C6, C7 and C8 lead to almost isotropic solid silica spheres (Fig. 3e–g). In fact, even combined with a considerable amount of ethanol, two long alcohols as solvent may also lead to solid sphere-like silica particles (Fig. S5†).
We further examined the formation of stable water-in-alcohol emulsions in single alcohol systems. Fig. 3h is a photograph picture showing three solvent systems (without TEOS addition step), using 1-butanol as solvent. It is found that the coexistence of PVP and Na3Cit made a slightly cloudy emulsion, which is visible with the naked eye. The absence of PVP or Na3Cit resulted in clear homogeneous solution. This phenomenon, also observed in 1-pentanol/ethanol,2 is reminiscent of the salting-out effect. Likely, there exists a lot of synergy between PVP and Na3Cit in the water-binding event, which results in the “salting-out” of most alcohol molecules and the stabilization of the emulsion. These results also suggest that the addition of ethanol as a cosolvent is not a necessity for the emulsion-assisted anisotropic silica growth.
Although the synthesis adopting pure ethanol or pure long chain alcohols failed to prepare anisotropic particles, it appears that the detrimental effect of ethanol and long chain alcohols would cancel each other out during the synthesis using binary alcohol/ethanol combinations. The longer the chain length of alcohol, the higher ratio of ethanol is needed for synthesis with similar effect. As a result, similar silica products can possibly be prepared in several different alcohol systems by choosing proper alcohol/ethanol mixing ratios to match the alcohol chain length. For instances, the syntheses with 1-butanol and 1-hexanol/ethanol (v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) generated similar rod-like products; 1-pentanol/ethanol (v/v = 5
4) generated similar rod-like products; 1-pentanol/ethanol (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and 1-hexanol/ethanol (v/v = 9
6) and 1-hexanol/ethanol (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13) led to bottle-like hollow particles; 1-propanol, 1-pentanol/ethanol (v/v = 6
13) led to bottle-like hollow particles; 1-propanol, 1-pentanol/ethanol (v/v = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), and 1-heptanol/ethanol (v/v = 5
5), and 1-heptanol/ethanol (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) led to tadpole-like hollow particles (Fig. S2–S4 and S6†). Arbitrarily tunable mixing ratio makes the synthesis using alcohol/ethanol more versatile than single alcohol systems.
6) led to tadpole-like hollow particles (Fig. S2–S4 and S6†). Arbitrarily tunable mixing ratio makes the synthesis using alcohol/ethanol more versatile than single alcohol systems.
It is quite interesting that the nature of the alcohol solvent can accurately control the shape, structure and aspect ratio of as-prepared silica particles. By comparing Fig. 3 with 2, it is safe to conclude that the increase of alcohol chain length in single alcohol systems has similar effect to the increase of the proportion of long chain alcohol in binary alcohol/ethanol systems. We estimate that it is the hydrophobicity-related properties of the alcohol phase that governs the emulsion-assisted silica growth.
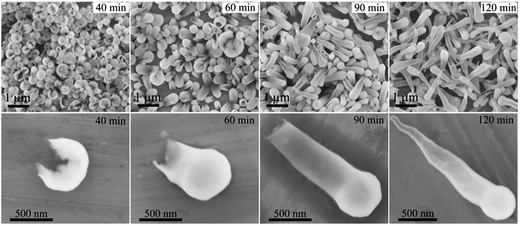
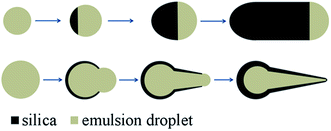
The one-dimensional growth process of rod-like silica was studied by Kuijk et al.2 Herein, we examined the growth process of the hollow anisotropic silica particles. The synthesis of tadpole-like silica particles, as an example, was followed at different growth stages by quenching the synthesis at different reaction times. As illustrated in Fig. 4, the “tadpole” evolves its head first, just like a broken half of an egg shell, and then a gradually shrinking tail derives from the opening of the head, with a closed end. Obviously, this formation process of the anisotropic hollow silica particles is very different from the reported solid-to-hollow etching process or the emulsion-templated process,28–30 where no shell opening can be observed for the intermediate products. Thanks to this unique anisotropic growth process, this emulsion strategy can be used to prepare one-end-opened hollow anisotropic silica particles by quenching the reaction before the formation of a closed end. Fig. 5 gives the TEM images of a typical intermediate product of the synthesis of tadpole-like silica particles. These tube- or flask-like silica particles can be used for cargo delivery and as excellent nanoreactors.31
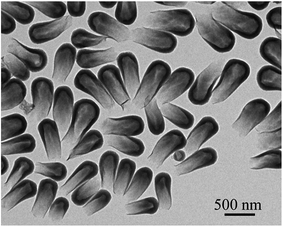 | ||
Fig. 5 Typical silica “nanotubes” prepared with method I in 1-heptanol/ethanol (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) by quenching the reaction at 80 min. 6) by quenching the reaction at 80 min. | ||
Based on the above observation and previous reported results,2 the emulsion-assisted anisotropic growth of the silica particles, solid and hollow, can be illustrated in Fig. 6. The silica formation process can be divided into two stages: phase separation and anisotropic growth. At the first stage, the hydrolyzed TEOS monomers condense from the water-rich emulsion droplet to form a Janus-like solid (silica)-solution (emulsion droplet) structure, which resembles the preparation of “dimpled” polymer particles by a temperature-controlled swelling process.32 The round “heads” of the hollow particles take shape at this stage. Flat silica-droplet interfaces lead to solid rod-like particles, while curved interfaces lead to hollow silica particles. High reaction temperature will lead to faster precursor hydrolysis and silica condensation. In method II, the selective use of a higher temperature at the initial stage of silica growth will reinforce the “head” part of the silica particles, at least partly due to accelerated reaction. Under otherwise the same conditions, the use of method II leads to silica rods with a matchstick-like shape, and the hollow silica particles produced with method II have a more prominent “head” (Fig. 1, S3 and S6†). It is believed that, besides the reaction kinetics, the interfacial tensions between silica particles and the emulsion system control the phase separation stage and guide the morphological evolution of the silica particles during following anisotropic growth process.2 Under this growth mechanism, it is possible to prepare more complex silica particles with multi-block or segmented structures by varying the reaction parameters during growth.22–26
The localization of the growing silica particles at the emulsion interfaces is a prerequisite for such anisotropic growth (Fig. S7†). Tentatively, we estimate that the effect of Na3Cit and highly concentrated PVP in the emulsion droplet can desolvate the in situ formed silanol-terminated silica particles. Owing to the resulted big interfacial tension, the droplet will repel the desolvated silica particle as well as alcohol, while the outer alcohol phase with relatively low hydrophobicity has good compatibility with the silica particle. As a result, the silica particle will be “salted out” from the droplet at the phase separation stage. Note that the salting-out effect or soluting-out effect for polymers has already been reported.33,34 However, the reason for the adhesion of the silica particles to the droplets is not known. It was reported that the silica particles prepared with this strategy contained a little proportion of PVP.35 Possibly, the embedded PVP molecules help to make a sticky silica surface, which keeps the silica particle in place.
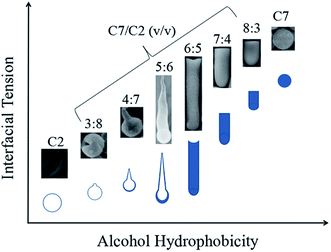
Based on above discussions, we try to give an explanation for the morphological and structural variations of the silica products upon increasing the 1-heptanol/ethanol volume ratio (Fig. 2, 3a and f). Upon raising the ratio of 1-heptanol, the alcohol phase gains more and more hydrophobicity, which will result in a gradual increase of the silica–alcohol interfacial tension. On the other hand, the silica-droplet interfacial tension also increases due to decreased solubility of alcohol in the droplets. At the phase separation stage, the increasing interfacial tensions should lead to an increasing silica-droplet contact angle and a decreasing silica surface area, and therefore a less and less curved silica-droplet interface can be anticipated.
Our experimental results, summarized in Fig. 7, fit this explanation very well. In pure ethanol, the emulsion droplets act as a template to yield almost full spherical shells. As the 1-heptanol/ethanol mixing ratio increases from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 5
8 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the head part of the hollow particles become smaller and thus have a wider opening, from which the particle derived an increasingly bigger tail. Further increasing the 1-heptanol ratio, the hollow particles gradually change to solid rod-like particles due to the increasing of silica-droplet contact angle. As a transitional feature, rod-like particles with an end surface slightly curved inward were obtained at 1-heptanol/ethanol mixing ratio of 6
6, the head part of the hollow particles become smaller and thus have a wider opening, from which the particle derived an increasingly bigger tail. Further increasing the 1-heptanol ratio, the hollow particles gradually change to solid rod-like particles due to the increasing of silica-droplet contact angle. As a transitional feature, rod-like particles with an end surface slightly curved inward were obtained at 1-heptanol/ethanol mixing ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The deceasing tendency of the length of the rod-like particles (at mixing ratios of 7
5. The deceasing tendency of the length of the rod-like particles (at mixing ratios of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 8
4 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) might be ascribed to slowing reaction rate, owing to decreased alcohol content in the droplet. In pure 1-heptanol, the hydrophobicity of the outer alcohol phase is too high that silica could not be repelled out from the droplets, giving solid spherical particles.
3) might be ascribed to slowing reaction rate, owing to decreased alcohol content in the droplet. In pure 1-heptanol, the hydrophobicity of the outer alcohol phase is too high that silica could not be repelled out from the droplets, giving solid spherical particles.
The formation of a Janus-like silica-droplet structure is essential for the present anisotropic growth of silica particles. Corresponding mechanism is very complex and more investigations are needed for a full understanding of the roles of Na3Cit and PVP. Our further experiments demonstrate that some reagents such as tartaric acid and citric acid can be used instead of Na3Cit (Fig. S8†), while some salts we examined, such as NH4Cl and NH4NO3, prove not suitable for such replacement due to demulsification (Fig. S9†). The study is still ongoing.
Conclusions
In summary, the synthesis of anisotropic silica colloids in various water-in-alcohol emulsion systems was investigated. The coexistence of sodium citrate and highly concentrated PVP in the water-rich emulsion droplets contributes to the phase separation of alcohol and water, and thus the formation of the emulsion systems, possibly via a mechanism similar to the salting-out effect. The formation of the anisotropic silica particles in the emulsion systems can be divided into two stages: phase separation and anisotropic growth. Particularly, the formation of a Janus-like silica-droplet structure at the phase separation stage is crucial for the whole synthesis. Curved silica-droplet interfaces generally lead to hollow particles, and flat or slightly curved interfaces lead to solid rod-like particles.It is believed that the interfacial tensions between silica and the emulsion system largely control the phase separation stage and thus determine the morphology and micro-/nanostructure of as-prepared silica particles. In our synthesis, the interfacial tensions can be well tuned by adjusting the hydrophobicity of the alcohol phase. Besides, a relatively higher temperature at the initial stage can also be adopted to influence the phase separation event.
A series of morphology-controlled anisotropic silica particles, solid or hollow, was prepared by using different alcohol systems. Binary alcohol systems, such as 1-heptanol/ethanol, can offer excellent tunability by adjusting the mixing ratio. A “salting-out” mechanism was proposed to explain the shape- and structure-controlled synthesis in different alcohol systems. Our investigation demonstrates that the water-in-alcohol emulsion strategy holds great potentials in the synthesis of various functional anisotropic silica particles, such as one-end-opened silica tubes and segmented silica micro-/nanostructures. Note that our mechanistic study is very preliminary, and more insightful research should be carried out to depict a clear picture for the anisotropic growth process.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We acknowledge the financial support from a startup fund (2015SCU11077). Q. Y. sincerely thanks Prof. Peng Wang for valuable discussions.Notes and references
- N. J. Halas, ACS Nano, 2008, 2, 179–183 CrossRef CAS PubMed.
- A. Kuijk, A. van Blaaderen and A. Imhof, J. Am. Chem. Soc., 2011, 133, 2346–2349 CrossRef CAS PubMed.
- A. Kuijk, D. V. Byelov, A. V. Petukhov, A. van Blaaderen and A. Imhof, Faraday Discuss., 2012, 159, 181–199 RSC.
- C. Rosu, S. Jacobeen, K. Park, E. Reichmanis, P. Yunker and P. S. Russo, Langmuir, 2016, 32, 13137–13148 CrossRef CAS PubMed.
- R. P. Murphy, K. Hong and N. J. Wagner, Langmuir, 2016, 32, 8424–8435 CrossRef CAS PubMed.
- H. E. Bakker, T. H. Besseling, J. E. G. J. Wijnhoven, P. H. Helfferich, A. van Blaaderen and A. Imhof, Langmuir, 2017, 33, 881–890 CrossRef CAS PubMed.
- E. Prodan and P. Nordlander, Nano Lett., 2003, 3, 543–547 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- M. P. B. van Bruggen, Langmuir, 1998, 14, 2245–2255 CrossRef CAS.
- N. Hijnen and P. S. Clegg, Chem. Mater., 2012, 24, 3449–3457 CrossRef CAS.
- J. J. Yuan and R. H. Jin, Adv. Mater., 2005, 17, 885–888 CrossRef CAS.
- H. Zhang, T. J. Bandosz and D. L. Akins, Chem. Commun., 2011, 47, 7791–7793 RSC.
- S. Shen, T. Gu, D. Mao, X. Xiao, P. Yuan, M. Yu, L. Xia, Q. Ji, L. Meng, W. Song, C. Yu and G. Lu, Chem. Mater., 2012, 24, 230–235 CrossRef CAS.
- J. Zhang, H. Liu, Z. Wang and N. Ming, Chem.–Eur. J., 2008, 14, 4374–4380 CrossRef CAS PubMed.
- K. A. Dick, Prog. Cryst. Growth Charact. Mater., 2008, 54, 138–173 CrossRef CAS.
- A. Q. Zhang, H. J. Li, D. J. Qian and M. Chen, Nanotechnology, 2014, 25, 135608 CrossRef PubMed.
- P. Datskos, J. Chen and J. Sharma, Chem. Commun., 2014, 50, 7277–7279 RSC.
- J. Wang and Y. Lu, Mater. Des., 2016, 111, 206–212 CrossRef CAS.
- F. Hagemans, E. B. van der Wee, A. van Blaaderen and A. Imhof, Langmuir, 2016, 32, 3970–3976 CrossRef CAS PubMed.
- A. R. Morgan, A. B. Dawson, H. S. Mckenzie, T. S. Skelhon, R. Beanland, H. P. W. Franks and S. A. F. Bon, Mater. Horiz., 2014, 1, 65–68 RSC.
- B. W. Longbottom, L. A. Rochford, R. Beanland and S. A. F. Bon, Langmuir, 2015, 31, 9017–9025 CrossRef CAS PubMed.
- J. He, B. Yu, M. J. Hourwitz, Y. Liu, M. T. Perez, J. Yang and Z. Nie, Angew. Chem., Int. Ed., 2012, 51, 3628–3633 CrossRef CAS PubMed.
- P. Datskos and J. Sharma, Angew. Chem., Int. Ed., 2014, 53, 451–454 CrossRef CAS PubMed.
- P. Datskos, J. Chen and J. Sharma, RSC Adv., 2014, 4, 2291–2294 RSC.
- Y. Yang, G. Chen, L. J. Martinez-Miranda, H. Yu, K. Liu and Z. Nie, J. Am. Chem. Soc., 2016, 138, 68–71 CrossRef CAS PubMed.
- P. Datskos, G. Polizos, D. A. Cullen, M. Bhandari and J. Sharma, Chem.–Eur. J., 2016, 22, 18700–18704 CrossRef CAS PubMed.
- P. Datskos, D. A. Cullen and J. Sharma, Angew. Chem., Int. Ed., 2015, 54, 9011–9015 CrossRef CAS PubMed.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- Q. Yu, P. Wang, S. Hu, J. Hui, J. Zhuang and X. Wang, Langmuir, 2011, 27, 7185–7191 CrossRef CAS PubMed.
- C. Zoldesi and A. Imhof, Adv. Mater., 2005, 17, 924–928 CrossRef CAS.
- D. Yi, Q. Zhang, Y. Liu, J. Song, Y. Tang, F. Caruso and Y. Wang, Angew. Chem., Int. Ed., 2016, 55, 14733–14737 CrossRef CAS PubMed.
- S. H. Kim, A. D. Hollingthworth, S. Sacanna, S. J. Chang, G. Lee, D. J. Pine and G. R. Yi, J. Am. Chem. Soc., 2012, 134, 16115–16118 CrossRef CAS PubMed.
- R. Sadeghi and F. Jahani, J. Phys. Chem. B, 2012, 116, 5234–5241 CrossRef CAS PubMed.
- R. Sadeghi, N. Ebrahimi and M. D. Tehrani, Polymer, 2016, 98, 365–377 CrossRef CAS.
- A. Kuijk, A. Imhof, M. H. W. Verkuijlen, T. H. Besseling, E. R. H. van Eck and A. van Blaaderen, Part. Part. Syst. Charact., 2014, 31, 706–713 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More experimental results about the silica particles prepared in different water-in-alcohol emulsion systems. See DOI: 10.1039/c7ra02835k |
| This journal is © The Royal Society of Chemistry 2017 |