Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effect of rigid phenoxyl substituent on the NH3-sensing properties of tetra-α-(4-tert-butylphenoxyl)-metallophthalocyanine/reduced graphene oxide hybrids†
Zheying Yua,
Bin Wang *a,
Yong Lia,
Di Kanga,
Zhimin Chena and
Yiqun Wu*ab
*a,
Yong Lia,
Di Kanga,
Zhimin Chena and
Yiqun Wu*ab
aKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, P. R. China. E-mail: wangbin@hlju.edu.cn
bShanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai 201800, P. R. China. E-mail: yqwu@siom.ac.cn
First published on 24th April 2017
Abstract
Herein, we report a type of enhanced ammonia (NH3) sensing materials formed by the functionalization of reduced graphene oxide (RGO) with 1,8,15,22-tetra-(4-tert-butylphenoxyl)-metallophthalocyanine (TBPOMPc, M = Cu, Ni, and Pb) via a solution self-assembly method based on π–π stacking interactions. The RGO/TBPOMPc hybrids exhibit excellent sensitivity, high response value, and fast response and recovery at room temperature, especially the RGO/TBPOPbPc sensor. The enhancement of the NH3-sensing performance by TBPOMPc with a rigid phenoxyl-substituted group is attributed to the self-assembly behavior of TBPOMPc molecules. The rigid structure of TBPOMPc effectively prevents the intermolecular aggregation behavior. On the one hand, it expands the specific surface area of the RGO/TBPOMPc hybrids, which is propitious for the physical adsorption and diffusion of NH3 molecules and reduces the response and recovery time. On the other hand, it weakens the electronic interaction between TBPOMPc molecules and results in reducing the resistance of charge transfer from NH3 to TBPOMPc. Moreover, TBPOMPc is beneficial to enhance the sensitivity and selectivity of the RGO/TBPOMPc sensors towards NH3. By contrast, the response of various RGO/TBPOMPc sensors decreases in the order of RGO/TBPOPbPc > RGO/TBPOCuPc > RGO/TBPONiPc. Furthermore, the rigid structure and central metals of TBPOMPc play a critical role in the sensitivity of NH3, as evidenced from the scanning tunneling microscopy, current–voltage characteristics, and electrochemical impedance spectra.
1. Introduction
NH3 as a highly toxic gas is widely used in refrigeration, refining, manufacturing, cleaning, and nitrogenous fertilizers.1,2 Exposure to high concentrations of NH3 (ca. > 300 ppm) can cause serious health problems in humans.3 To date, a number of studies have been reported on NH3 sensors such as inorganic, inorganic oxide/dioxide, and conducting polymers.4–7 However, most of these sensors cause high power consumption and exhibit low response to low concentration of NH3, poor selectivity, and slow recovery time at room temperature. Therefore, an NH3 sensor that causes lower power consumption and exhibits higher sensitivity, better selectivity, and fast recovery time at room temperature is urgent and significant for human demand.Reduced graphene oxide (RGO) as a novel carbon material has a broad prospect in the field of gas sensors due to its high electrical conductivity, high chemical activity, and large specific surface area, which is beneficial for charge transfer and gas adsorption and desorption.8–10 RGO gas sensors have shown higher sensitivity for the detection of polar gases such as NO2, NO, NH3, CO2, H2S, etc.11–19 However, these sensors require improvement in terms of their selectivity, recovery time, and response to low concentration of gases. Therefore, functionalization of RGO is important to improve its sensitivity, selectivity, and speed up the recovery time at room temperature.
The surface of RGO retains partially polar functional groups such as –COOH, –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –O−, etc. All of these endow RGO with good dispersibility in the solvent and make the functionalization of RGO easy.20–24 Metallophthalocyanine (MPc) is a well-known organic semiconductor material that has been identified as a promising gas sensor due to its macrocyclic π–π conjugated system with an interesting structure, physical properties, and convenient chemical modification. When the central metal and the peripherally substituted groups are changed, MPc sensor exhibits high sensitivity, good selectivity, steady reproducibility, and fast response and recovery at room temperature in the detection of NO2, NH3, CO, H2, etc.25–33 However, the MPc molecules tend to aggregate during the film formation process, which is not conducive to the charge transfer and physical adsorption between gas molecules and MPc.34–36 Therefore, considering the advantages of RGO and MPc, a gas sensor containing both RGO and MPc combined via π–π non-covalent hybridization can be obtained. Our group has reported a type of NH3 sensors obtained via the functionalizing of RGO with tetra-α-iso-pentyloxymetallophthalocyanine (TIPOMPc, M = Cu, Ni, and Pb). The RGO/TIPOMPc hybrids exhibited superior NH3-sensing performance.37 To date, to the best of our knowledge, there are few reports related to the influence of central metals and the substituted group of MPc on the NH3-sensing property of RGO/MPc hybrids. Herein, we demonstrated a type of NH3 sensors that contains RGO modified by MPc with a rigid substituent group (4-tert-butylphenoxyl) and different central metals. Compared to the flexible chain substituted TIPOMPc, our results indicated that TBPOMPc with a rigid substituted group more effectively prevented the intermolecular aggregation formation. Its influence on the NH3-sensing behavior was due to the following two aspects: one is the specific surface area of RGO/MPc hybrids and the other is the charge transfer resistance of RGO/MPc hybrids. RGO/TBPOMPc hybrids have larger specific surface area but smaller charge transfer resistance than RGO/TIPOMPc hybrids. The former is conducive to the physical adsorption and diffusion of gas molecules, thus reducing the response and recovery time;38 the latter is beneficial to the electron transfer from NH3 to TBPOMPc and the decrease of hole carrier, resulting in the enhancement of sensitivity and selectivity to NH3.39
O, –O−, etc. All of these endow RGO with good dispersibility in the solvent and make the functionalization of RGO easy.20–24 Metallophthalocyanine (MPc) is a well-known organic semiconductor material that has been identified as a promising gas sensor due to its macrocyclic π–π conjugated system with an interesting structure, physical properties, and convenient chemical modification. When the central metal and the peripherally substituted groups are changed, MPc sensor exhibits high sensitivity, good selectivity, steady reproducibility, and fast response and recovery at room temperature in the detection of NO2, NH3, CO, H2, etc.25–33 However, the MPc molecules tend to aggregate during the film formation process, which is not conducive to the charge transfer and physical adsorption between gas molecules and MPc.34–36 Therefore, considering the advantages of RGO and MPc, a gas sensor containing both RGO and MPc combined via π–π non-covalent hybridization can be obtained. Our group has reported a type of NH3 sensors obtained via the functionalizing of RGO with tetra-α-iso-pentyloxymetallophthalocyanine (TIPOMPc, M = Cu, Ni, and Pb). The RGO/TIPOMPc hybrids exhibited superior NH3-sensing performance.37 To date, to the best of our knowledge, there are few reports related to the influence of central metals and the substituted group of MPc on the NH3-sensing property of RGO/MPc hybrids. Herein, we demonstrated a type of NH3 sensors that contains RGO modified by MPc with a rigid substituent group (4-tert-butylphenoxyl) and different central metals. Compared to the flexible chain substituted TIPOMPc, our results indicated that TBPOMPc with a rigid substituted group more effectively prevented the intermolecular aggregation formation. Its influence on the NH3-sensing behavior was due to the following two aspects: one is the specific surface area of RGO/MPc hybrids and the other is the charge transfer resistance of RGO/MPc hybrids. RGO/TBPOMPc hybrids have larger specific surface area but smaller charge transfer resistance than RGO/TIPOMPc hybrids. The former is conducive to the physical adsorption and diffusion of gas molecules, thus reducing the response and recovery time;38 the latter is beneficial to the electron transfer from NH3 to TBPOMPc and the decrease of hole carrier, resulting in the enhancement of sensitivity and selectivity to NH3.39
2. Experimental
2.1 Preparation of the RGO/TBPOMPc hybrids (M = Cu, Ni, and Pb)
Preparation of GO and TBPOMPc (M = Cu, Ni, and Pb). We synthesized micrometer-sized GO flakes from graphite powder using a modified Hummers method.40 TBPOMPc was synthesized by refluxing (p-tert-butylphenoxyl)phthalonitrile with DBU and anhydrous CuCl2, NiCl2, and PbCl2 in anhydrous isopentanol, followed by column chromatography separation according to an established procedure.41 For a detailed description, see GO and TBPOMPc synthesis section in the ESI.†Preparation of RGO/TBPOMPc (M = Cu, Ni, and Pb). The schematic of the interaction process for the preparation of RGO/TBPOMPc hybrids is illustrated in Fig. S1B.† The prepared GO was sonicated in DMF, and the TBPOMPc DMF solution was added to the abovementioned solvent, followed by sonication in the dark for about 2.0 h and continuous stirring for 24 h. After this, the final product was purified through filtration and centrifugation. Finally, the hybrid was obtained and dried in vacuum for 24 h. The GO/TBPOMPc hybrid was added to a solution of NaOH (pH = 11) and ultrasonicated for 90 min. Hydrazine hydrate (N2H4) was added to the abovementioned solution and the mixed solution was heated at 90 °C for 2.0 h followed by centrifugation at 9000 rpm. Finally, the product was washed with DI water and ethyl alcohol and dried in a vacuum oven at 80 °C for 24 h. For comparison, RGO was also prepared via the similar procedures without TBPOMPc.
2.2 Structural characterization
RGO/TBPOMPc hybrids were characterized via Fourier transform infrared spectroscopy (FTIR, PE instruments, Spectrum One FTIR spectrometer), Raman spectroscopy (JobinYvon HR800 with excitation from the 450 nm laser source), ultraviolet-visible spectroscopy (UV-vis, Perkin-Elmer Lambda 900 spectrophotometer), thermogravimetric analysis (TGA, Perkin-Elmer TGA-7 system), and X-ray photoelectron spectroscopy (XPS, Kratos AXIS Ultra DLD system using monochromatic Al Kα X-ray source 1486.6 eV). The surface morphology and thickness of the films deposited on the silicon wafer were investigated via atomic force microscopy (AFM, Digital Instrument Nanoscope IIIa), transmission electron microscopy (TEM, JEM 2100 instrument at 200 kV), and scanning electron microscopy (SEM, Hitachi S4800). The film thicknesses of RGO/TBPOMPc placed on the electrodes were investigated via a stylus surface profilometer (KLA-Tencor, Alpha-step D-100 Stylus Profiler).2.3 NH3-sensing test
The sensing device was a 5 × 5 mm interdigitated electrode; it was fabricated using beam lithography on Al2O3 substrate through sputtering Au as a conductor layer, and the RGO/TBPOMPc hybrid was dispersed in DMF to create a suspension (0.5 mg mL−1, 10 mL) by ultrasonication for 2 h. Then, a 2 μL suspension was spotted on the gold electrodes. After annealing treatment at 100 °C for 1 h in an Ar flow to remove the residual DMF, the RGO/TBPOMPc hybrid was connected with the gold fingers. Fig. S2† shows film thicknesses of RGO/TBPOMPc (M = Cu, Ni, and Pb) placed on the electrodes. The results show that the film thicknesses are about 80 nm. For comparison, a gas sensor of free RGO was also fabricated via similar procedures.The sensor testing was carried out under practical conditions (i.e. room temperature 25 °C, relative humidity 50% RH; NH3 and N2 (99.9%) were purchased from GuangMing Research and Design Institute of Chemical Industry, PR China) using a homemade gas sensing measurement system.37,51 One typical sensing test cycle has three steps in a sequential manner: first, N2 (99.999% purity) was introduced into the sensing chamber (2 L min−1) to obtain a baseline until the resistance remained unchanged for about 30 min. Second, different concentrations of NH3, varying from 0.3 ppm to 3200 ppm, were injected into the chamber and the fan inside the chamber was turned on to make the gas uniform. We observed and obtained the resistance value when it remained unchanged for about 10 min. Finally, 99.999% purity N2 (2 L min−1) was introduced again for sensor recovery. Multiple testing cycles were performed by continuously repeating the same test several times. The room temperature dynamic sensing response sensitivity S was defined as ΔR/R = (Rg − R)/R, where R is the average sensor resistance in the presence of N2 and Rg is the sensor resistance after exposure to NH3 gas. The response and recovery time were defined as the times needed to reach 90% of the final resistance.
2.4 The influence of MPc with a rigid substituted group on the NH3-sensing behavior
The self-assembly behavior of TBPOMPc and TIPOMPc on the surface of RGO was investigated via scanning tunneling microscopy (STM, UNISOKU USM1400SA); the specific surface area of both RGO/TBPOMPc and RGO/TIPOMPc were investigated by N2 adsorption and desorption analysis (Micromeritics TriStar II), and the samples were out gassed for 18 h at 90 °C under vacuum before carrying out the measurements. The pore size distribution was calculated using the Barrett–Joyner–Halenda (BJH) method. The current–voltage (I–V) characteristics of the fabricated sensors were measured using a Keithley 4200 semiconductor parameter analyzer with a two-point probe setup via sweeping the potential between −2 and +2 V under a 0.01 V s−1 scan rate. Electrochemical impedance spectra (EIS) were obtained using an IM6e electrochemical working station at room temperature (frequency range from 0.01 Hz to 100 kHz at the open circuit potential with a 5 mV amplitude).3. Results and discussion
3.1 Structural characterization
The FTIR spectra of RGO before and after modification by TBPOMPc are shown in Fig. 1(A) and S3.† The typical peaks of RGO are located at 3427 cm−1 and 1059 cm−1, which correspond to the O–H stretching and vibration mode of absorbed water. The peak around 1507 cm−1 corresponds to the ν(C–O–C) frequency. After modification, the characteristic peaks of TBPOMPc and RGO were found in the RGO/TBPOMPc hybrids (such as RGO/TBPOCuPc) at about 2955 cm−1, 1507 cm−1, 1252 cm−1, 581 cm−1, and 543 cm−1. All these observations indicate that RGO/TBPOMPc hybrids were successfully obtained.The UV-vis absorption spectra of TBPOMPc (2.0 × 10−3 mol L−1, 25 °C), RGO, and RGO/TBPOMPc hybrids (0.1 mg mL−1, 25 °C) in DMF solutions are given in Fig. 1(B) and S4.† TBPOMPcs show typical electronic absorption spectra with two strong absorption regions. The first region at about 335 nm (B-band) originates from the a2u (HOMO) → aeg (LUMO) transition, and the second region at about 600–700 nm (Q-band) is attributed to the transition from the a1u (HOMO) to the aeg (LUMO). For the typical electronic absorption region of RGO at about 255 nm, the weak absorbance is attributed to the poor dispersion in DMF. After RGO was modified with TBPOMPc, the characteristic peak of RGO red-shifted at about 300 nm, and the Q-band of TBPOMPc (M = Cu, Ni, and Pb) broadened with the decreasing intensity and red-shifted at about 26 nm, 53 nm, and 5 nm, respectively. All of them indicate the electron transfer from the electron donor TBPOMPc to the electron accepter RGO, which proves the strong π–π interaction between RGO and TBPOMPc.42,43
Fig. 2(A–C) shows the Raman spectra of RGO before and after modification by TBPOMPc. RGO shows a (G) peak at 1591 cm−1 and a (D) peak at 1367 cm−1, which are the characteristic peaks of sp2 and sp3 hybridized carbon atoms in graphene that distinguish the order and disorder/defect in the structure, respectively.44 After modification, the (G) peak and (D) peak appeared in RGO/TBPOMPc as well, which evidently indicated that RGO/TBPOMPc hybrids were obtained. The G band (∼1591 cm−1) corresponds to the first-order scattering of the E2g mode observed for the sp2 carbon domain, whereas the D band (∼1367 cm−1) is attributed to a common feature of the sp3 defect in carbon. As a consequence, the intensity ratio ID/IG is usually a measure of the disorder/defect in graphene and further explains the covalent or non-covalent modification of graphene.45 After modification, no obvious difference in the ID/IG of RGO/TBPOMPc (0.90) and the ID/IG of RGO (0.91) was observed, which evidently indicated that RGO/TBPOMPc hybrids were obtained via non-covalent hybridization. The (G) peak of RGO/TBPOMPc (M = Cu, Ni, and Pb) shifted to a short wavelength by about 4 cm−1, 9 cm−1, and 7 cm−1, respectively; moreover, the intensity of pyrrole C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (about 1530 cm−1), C–N stretching (about 1350 cm−1), and isoindole ring stretching (about 1450 cm−1) of TBPOMPc obviously decreased. All these observations indicate strong π–π electronic interaction between RGO and TBPOMPc. TBPOMPc provides abundant charge carrier to RGO and raises the Fermi level.46–48
C stretching (about 1530 cm−1), C–N stretching (about 1350 cm−1), and isoindole ring stretching (about 1450 cm−1) of TBPOMPc obviously decreased. All these observations indicate strong π–π electronic interaction between RGO and TBPOMPc. TBPOMPc provides abundant charge carrier to RGO and raises the Fermi level.46–48
 | ||
| Fig. 2 (A–C) Raman spectra of RGO, TBPOMPc, and RGO/TBPOMPc hybrids, respectively, and (D) thermogravimetric analysis results of RGO, TBPOMPc, and RGO/TBPOMPc hybrids. | ||
The thermogravimetric analyses (TGA) results of RGO, TBPOMPc, and RGO/TBPOMPc hybrids are shown in Fig. 2(D). For the analyses, the samples were vacuum dried at 90 °C, the temperature was increased from 20 °C to 800 °C at 20 °C min−1, and the flow rate of oxygen was 100 mL min−1. The results show that the weight loss rate of RGO was about 2% when the temperature was below 600 °C, and it is probably due to water and solvent evaporation, which were adsorbed on the surface of RGO. When the temperature was increased to 600 °C, the weight loss rate of RGO was about 10%, which was caused by the thermal decomposition of oxygen-containing groups on the sheet edge of RGO. The decomposition temperature of TBPOMPc was above 400 °C. When the temperature was increased above 650 °C, TBPOMPc completely decomposed, and the final decomposition product was MO. The weight loss rates are 91% (TBPOCuPc), 93% (TBPONiPc), and 80% (TBPOPbPc), which is consistent with the theoretical weight loss rate of TBPOCuPc (93.2%), TBPONiPc (93.6%), and TBPOPbPc (83.4%), respectively. The decomposition temperature of RGO/TBPOMPc hybrids is above 350 °C, ranging from 350 °C to 700 °C, and all the hybrids have a fast weight loss rate, which indicates that TBPOMPc were combined with RGO via π–π* non-conjugated.49,50 Above 700 °C, the RGO/TBPOMPc hybrids completely decomposed, and the weight loss rate of RGO/TBPOCuPc, RGO/TBPONiPc, and RGO/TBPOPbPc was 27%, 28%, and 24%, respectively. Fig. S5† shows the thermogravimetric analysis results of RGO, TIPOMPc, and RGO/TIPOMPc hybrids. Similar to RGO/TBPOMPc, TG analyses implied that TIPOMPc content in the hybrids ranged from 16.9 to 17.8 wt%.
XPS was also employed to confirm the successful attachment of RGO/TBPOMPc hybrids and demonstrate charge transfer from TBPOMPc to RGO. As shown in Fig. 3(A), RGO/TBPOMPc hybrids exhibited the characteristic peaks of C 1s, N 1s, and O 1s and the characteristic peaks of Cu 2p (934.9 eV), Ni 2p (855.6 eV), and Pb 4f (138.6 eV). As shown in Fig. 4(E), the N 1s peaks of RGOTBPO/MPc hybrids consist of two split peaks, which are attributed to two groups of four nitrogen atoms in different chemical environments in the molecule and further suggests that the RGO/TBPOMPc hybrids were successfully prepared. Fig. 3(B–D) show that compared with the Cu 2p: 934.5 eV, Ni 2p: 855.5 eV, and Pb 4f: 137.8 eV peaks of TBPOMPc, the Cu 2p (934.8 eV), Ni 2p (855.6 eV), and Pb 4f (138.6 eV) peaks of RGO/TBPOMPc red-shifted by about 0.4 eV, 0.1 eV, and 0.8 eV, respectively; this also agrees with the charge transfer phenomena of the RGO/TBPOMPc hybrids and their N 1s XPS peak shifts to higher binding energy compared with that of TBPOMPc (as shown in Fig. 3(E)). These phenomena indicate charge transfer from TBPOMPc to RGO in the hybrids. The binding energy is correlated to the electron density around the nucleus (the lower the electronic density, the higher the binding energy).44
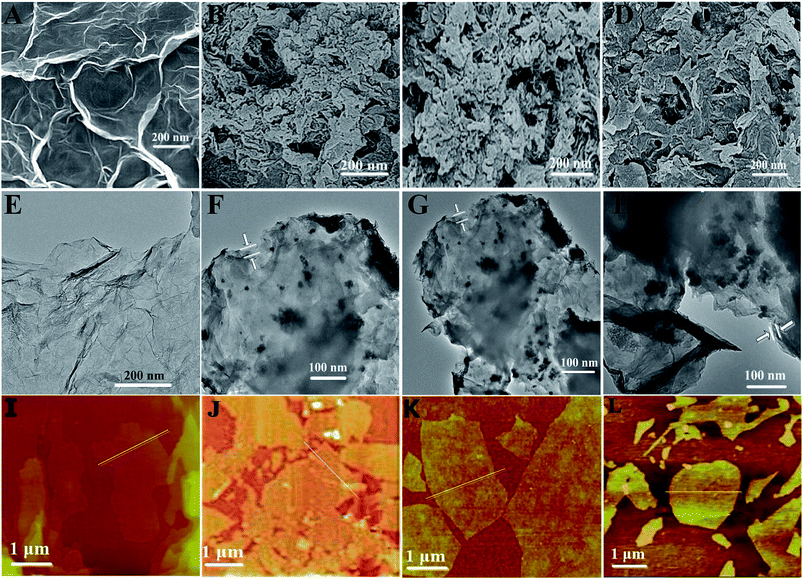
3.2 Morphology analysis of the RGO/TBPOMPc hybrids
The morphologies of RGO and RGO/TBPOMPc hybrids were investigated via AFM, SEM, and TEM. The AFM analysis result is shown in Fig. 4(I–L). The cross-section analysis indicates that the average thickness of the RGO film is around 5 nm (Fig. S6A†), and the theoretical thickness of the single-layer graphene is about 0.35 nm,45 which implies that the film is composed of ten layers. The thickness of the RGO/TBPOMPc hybrids is about 6 nm (Fig. S6B–D†); therefore, the AFM height confirms the non-covalent attachment of the TBPOMPc molecule to the RGO basal plane through π–π interaction.The SEM images of RGO and RGO/TBPOMPc hybrids are shown in Fig. 4(A–D). RGO exhibits the flake-like morphology with a slightly folded edge and flat surface, which is a typical characteristic of a nanosheet-like structure. After hybridization of RGO with TBPOMPc, TBPOMPc overlaid the surface of RGO, and the interface between the layers became fuzzy. The SEM images of RGO/TBPOMPc show that RGO/TBPOMPc has a porous surface morphology, which is helpful for the adsorption and desorption of gas molecules.
Fig. 4(E–H) show the TEM images and morphology analysis results of the RGO/TBPOMPc thin films. The TEM images indicate that RGO retained its lamellar structure and smooth surface; the layers overlapped each other, and the sheet thickness was about 5 nm. In contrast, RGO/TBPOMPc hybrids were obviously observed to have some cluster aggregates that contained TBPOMPc covered on the surface of RGO and formed a blurry rough layer. This further confirmed RGO/TBPOMPc formation via π–π non-covalent hybridization.
3.3 NH3-sensing properties of the RGO/TBPOMPc hybrids
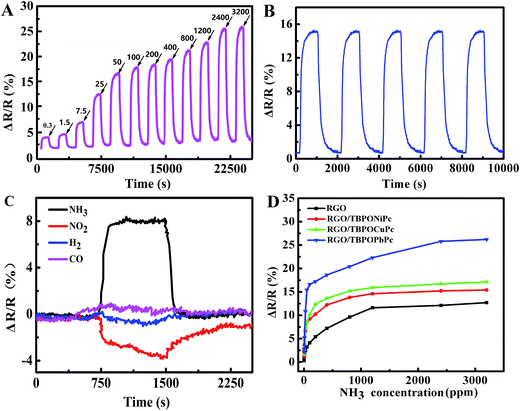
Fig. 5(A) and S7(A–C)† show the variations of the film resistance with time in the presence of NH3 (0.3–3200 ppm) at room temperature. The results showed that the resistance of sensors dramatically increased with the increasing concentration of NH3, and all the RGO/TBPOMPc sensors recovered to the original resistance value in the absence of NH3. For pure RGO, it is hard to recover the resistance of the RGO sensor to the original resistance with the increasing concentration of NH3. It indicates the improved recovery performance of the RGO/TBPOMPc hybrids. For 400 ppm NH3, the response/recovery times are as follows: RGO/TBPOCuPc (639 s/578 s), RGO/TBPONiPc (807 s/755 s), and RGO/TBPOPbPc (540 s/422 s), which suggest that the response/recovery of these sensors is faster than that of RGO/TIPOCuPc (849 s/687 s), RGO/TIPONiPc (916 s/833 s), and RGO/TIPOPbPc (887 s/447 s) sensors.37,51 It indicates that RGO/TBPOMPc hybrids are beneficial for the adsorption and diffusion of NH3 molecules.Fig. S7(D)† shows the response of RGO and RGO/TBPOMPc hybrids to NH3 (0.3–3200 ppm) at room temperature. The inset plot implies the response to the low concentration of NH3 (0.3–50 ppm). It indicates that RGO/TBPOMPc has better sensitivity (∼2%) towards NH3 (0.3 ppm), and excellent linear response in the range of 0.3–50 ppm and saturation response towards NH3 above 2000 ppm. For the RGO/TBPOMPc hybrid, the sensitivity to NH3 (400 ppm) was as follows: RGO/TBPOCuPc (12.2%), RGO/TBPONiPc (13.6%), and RGO/TBPOPbPc (18.6%), which is higher than those of RGO (7.2%), RGO/TIPOCuPc (11.5%), RGO/TIPONiPc (10.2%), and RGO/TIPOPbPc (11.4%). As the concentration of NH3 was decreased to 0.3 ppm, the sensitivity to NH3 (0.3 ppm) was as follows: RGO/TBPOCuPc (1.2%), RGO/TBPONiPc (1.6%), and RGO/TBPOPbPc (2.2%), which is still higher than that of RGO (0.3%), RGO/TIPOCuPc (1.8%), RGO/TIPONiPc (1.0%), and RGO/TIPOPbPc (1.4%).51
The RGO/TBPOMPc hybrids exhibit higher sensitivity than RGO/TIPOMPc hybrids towards the lower concentration of NH3, especially the RGO/TBPOPbPc sensor that shows excellent gas sensing performance. Most of the MPc hybrids, such as CuPc and NiPc, present planar configuration; Pb2+ with a larger atom radius will be located on the plane of the Pc ring and form four pyramid structures due to the limitation of the central space of Pc.32 The structure of the TBPOPbPc molecule is similar to those of PbPc52 and PbNc53 molecules, which are not planar. This molecular structure weakens the conjugating power of the phthalocyanine macrocycle, which is beneficial for the absorption of gases and central metal and makes it easier for the gas molecules to deeply adsorb;54 thus, the RGO/TBPOPbPc sensor shows excellent gas sensing performance.
Reversibility is one of the most important characteristics for gas sensors. Fig. 5(B) shows the resistance of the RGO/TBPOPbPc hybrid exposed to 50 ppm NH3 (five cycles). The results show that the films have good repeatability and no obvious degradation after consecutive measurements was observed. Moreover, under the atmospheric condition of 50% RH, the films were rather stable, and the resistance was almost unchanged within two weeks. After being exposed to air for about two months, the change in the resistance was within 2%. The response to NH3 changed to only about 5%.
Selectivity is also an important property of gas sensors. We measured the sensing response of the RGO/TBPOPbPc sensor to several other gases including reducing gases, such as H2 and CO, and an oxidizing gas such as NO2. The sensing test cycle is the same as that for NH3. Our results show that the RGO/TBPOPbPc sensor has an excellent selectivity for 25 ppm NH3 among all the test gases. As shown in Fig. 5(C), the sensor has negligible response to both H2 and CO (100 ppm). The result indicates that NH3 is preferable for the RGO/TBPOMPc sensor among common reducing gases, which acted as an electron donor. NO2 is an oxidization gas and an electron acceptor. The resistance of RGO/TBPOPbPc to 300 ppm NO2 decreased, suggesting charge transfer from TBPOPbPc to the NO2 molecule.
3.4 The influence of rigid phenoxyl substituted MPc on NH3-sensing property
Both RGO and MPc are p-type semiconductor materials, and the carriers are holes. NH3 as an electron donor tends to increase the electron density of RGO or MPc; thus, the number of hole carriers is reduced, and the resistance of RGO or MPc is increased.15,55 The gas sensing mechanism is mainly influenced by a physical process and a chemical process. The physical process includes the physical adsorption of gas molecules on the surface of a gas sensor and also the gas diffusion inside the sensing material, which affects the response and recovery times. The chemical process includes the charge transfer between gas and the sensing material, and the production of a carrier, which affects the sensitivity and selectivity.56,57 Therefore, the influence of phenoxyl substituted MPc on NH3 sensing property was discussed based on this theory.The different surface area As of RGO and RGO/MPc were determined by N2 adsorption and desorption isotherms. The As was calculated by Brunauer–Emmett–Teller (BET) at the relative pressure of 0.05–0.30, and the pore size distribution was calculated using the Barrett–Joyner–Halenda (BJH) method for isotherm desorption branch, and the results are shown in Fig. S10.† It indicates that all the adsorption isotherms are typical IV type with an H4 hysteresis loop, which are characteristic of a mesoporous structure. The curve slowly increased at the low P/P0 region (0.0–0.1) due to a small amount of microporous adsorbent, and as the pressure increased (0.3–0.8), the isotherm slope increased, exhibited a mesoporous adsorbent; at the high P/P0 region (0.9–1.0), the isotherm slope that was still increasing exhibited macroporous adsorption, which is consistent with the SEM images. As N2 condensation took place in the mesoporous channels, the desorption–adsorption isotherm is not coincidental, showing a typical H4 hysteresis loop, which is characteristic of a typical layered mesoporous structure. At the P/P0 of 0.5–0.8, adsorption capacity rapidly increased; the P/P0 location reflects pore sizes, and the changing range of P/P0 can be used as a measure of the mesoporous uniformity. Under the condition of the same central metal, the specific surface areas of RGO/TBPOMPc films are bigger than those of RGO/TIPOMPc films because the rigid substituted TBPOMPc effectively prevented the aggregate formation and increased the molecular spacing, whereas a flexible chain substituted TIPOMPc has a tendency to locate adjacent to each other for less steric hindrance. Large specific surface area is favorable for the adsorption and desorption of gas molecules. From the inset images shown in Fig. S10,† it was observed that the mesopores of RGO/TBPOMPc are mainly distributed in the vicinity of 5–30 nm, the average pore diameter is about 12.1 nm, and mesoporous distribution is centered at about 12 nm. Comparison of the pore volume of both RGO/TBPOMPc and RGO/TIPOMPc in the 2.5–5.0 nm range at the same P/P0 indicates that RGO/TBPOMPc has a bigger pore volume. TIPOMPc molecules are closely adjacent to each other and overlap with each other; thus, it has a higher capacity to shadow and block the micropores, thus making the pore volume smaller. Hence, RGO/TBPOMPc hybrids show better sensing performance towards NH3 than the RGO/TIPOMPc hybrids (Fig. 5(D)) (Table 1).
| Sample | RGO | RGO/TBPOCuPc | RGO/TIPOCuPc |
|---|---|---|---|
| As (m2 g−1) | 447.9 | 205.4 | 151.2 |
| Vm (cm3 g−1) | 0.57 | 0.33 | 0.31 |
| Sample | RGO | RGO/TBPONiPc | RGO/TIPONiPc |
|---|---|---|---|
| As (m2 g−1) | 447.9 | 197.0 | 171.4 |
| Vm (cm3 g−1) | 0.57 | 0.33 | 0.29 |
| Sample | RGO | RGO/TBPOPbPc | RGO/TIPOPbPc |
|---|---|---|---|
| As (m2 g−1) | 447.9 | 277.1 | 190.1 |
| Vm (cm3 g−1) | 0.57 | 0.41 | 0.32 |
 | ||
| Fig. 7 I–V curves of RGO and RGO/TBPOPbPc and RGO/TIPOPbPc hybrids (A) and EIS measurements of RGO, RGO/TIPOPbPc, RGO/TBPOPbPc, TIPOPbPc, and TBPOPbPc electrodes in a 6 M KOH aqueous solution (B). | ||
Electrochemical impedance spectroscopy (EIS) is a principal method to examine the fundamental behavior, such as charge transfer impedance (Rct), solution impedance (Rs), etc., of modified electrode materials. The impedances for MPc, RGO, and RGO/MPc were tested in the frequency range from 0.01 Hz to 100 kHz at the open circuit potential with 5 mV amplitude. The results are shown in Fig. 7(B) and S12.† The radius of the semicircle impedance loop in the high frequency region could reflect the resistance to mass transfer/diffusion rate of ions from the electrode to the modified material. The equivalent circuit model exhibits that the entire capacitor circuit is constituted by Rs, Zw, Rct, C, and Q, as shown in Fig. S12C.† Rs is the sum of the contact resistance and material resistance, which is related to the ionic conductivity of the electrolyte and electronic conductivity of the electrodes and current collectors. Zw is the Warburg resistance (45° inclined curve) related to the ion diffusion/transport in the electrolyte. Rct is the resistance for blocking the ions from entering the pores of the electrode materials and also for blocking the movement of the ions into the solution and separator. C and Q present the capacitor layers, which are formed during the charge–discharge process. The values of impedance calculated using ZSimpWin software are summarized in Table 2. Obviously, the Rct value reflects the conductivity that has a great influence on the charge transfer from NH3 to gas sensitive material. As we expected, the Rct value of the RGO/TBPOMPc hybrid is higher than that of RGO but lower than that of TBPOMPc, and for the RGO/TIPOMPc hybrid, it is the same as that for the RGO/TBPOMPc hybrid. On the one hand, RGO has poor selectivity and low sensitivity towards the low concentration of NH3 due to its excellent conductivity. Too fast charge mobility could lead to weak electronic interaction effect between RGO and NH3 or similar reducing gas (low concentration); thus, the response value is hard to detect. The Rct value of the RGO/MPc hybrids shows a marked increase than that of RGO, which indicates that an electron is first transferred from NH3 to MPc and then it enhances the electronic interaction effect between NH3 and RGO. Therefore, the selectivity and sensitivity of the RGO/MPc sensor will be enhanced over those of the RGO sensor. On the other hand, RGO/MPc hybrids effectively improve the conductivity of MPc, which favors the electron transfer from NH3 to MPc, thus changing the electron density of MPc and the number of hole carriers. Therefore, the sensitivity of RGO/MPc to NH3 is higher than that of MPc. Under the conditions of the same central metal, the Rct value follows the order TBPOMPc < TIPOMPc and RGO/TBPOMPc < RGO/TIPOMPc. The reason for this is the rigid phenoxyl substituent of TBPOMPc that effectively prevents the aggregation formation of MPc; thus, the intermolecular electron cloud does not effectively overlap and the electronic interaction effect of TBPOMPc is lower than that of TIPOMPc. The lower the values of Rct, the stronger the electron transport. As a result, the resistance of charge transfer from NH3 to TBPOMPc is reduced, and the conductivity of TBPOMPc is enhanced. Therefore, the NH3 sensitivity of RGO/TBPOMPc is higher than that of RGO/TIPOMPc.
| Sample | RGO | TBPOCuPc | TIPOCuPc | RGO/TBPOCuPc | RGO/TIPOCuPc |
|---|---|---|---|---|---|
| Rct (Ω) | 0.565 | 2.662 | 3.736 | 1.355 | 1.901 |
| Sample | RGO | TBPONiPc | TIPONiPc | RGO/TBPONiPc | RGO/TIPONiPc |
|---|---|---|---|---|---|
| Rct (Ω) | 0.565 | 3.176 | 4.226 | 1.654 | 2.134 |
| Sample | RGO | TBPOPbPc | TIPOPbPc | RGO/TBPOPbPc | RGO/TIPOPbPc |
|---|---|---|---|---|---|
| Rct (Ω) | 0.565 | 2.560 | 4.154 | 1.332 | 2.219 |
4. Conclusions
In summary, we demonstrated the fabrication and application of RGO/TBPOMPc (M = Cu, Ni, and Pb) in NH3 gas sensors at room temperature. RGO/TBPOMPc hybrids with rigid phenoxyl substituted groups were synthesized by noncovalent assembly. Compared to RGO/TIPOMPc sensors, the obtained RGO/TBPOMPc sensors, especially the RGO/TBPOPbPc sensor, show high sensitivity (15.5% to 50 ppm NH3 with a LOD of 300 ppb), excellent stability and reproducibility, fast response/recovery, and excellent selectivity. The significant enhancement in NH3 sensing is associated with the combination of TBPOMPc with a rigid phenoxyl substituted group and RGO. First, the rigid phenoxyl substituted MPc prevents the aggregation behavior of TBPOMPc molecules on the surface of RGO. As a result, RGO/TBPOMPc hybrids have larger specific area and lower charge transfer resistance, which is propitious for the adsorption and diffusion of gas molecules and charge transfer from NH3 to TBPOMPc. Second, as a conductive agent, RGO provides continuous pathways for charge transportation, leading to a fast and sensitive response. More importantly, charge transfer between TBPOMPc and RGO can be achieved via π–π stacking interaction. In this system, the rigid structure and central metals of TBPOMPc have a great influence on the NH3-sensing performance. It is suggested that through changing the peripherally substituted group and central metals of MPc, we could regulate its molecular aggregation behavior on the surface of RGO to adjust the NH3-sensing behavior.Acknowledgements
We gratefully acknowledge the financial support received from the National Natural Science Foundation of China (51202061 and 51002046), the Natural Science Foundation for the Returned Overseas Scholars of Heilongjiang Province (LC2012C02), the Natural Science of Heilongjiang Province (B201308), the Postdoctoral Science-Research Developmental Foundation of Heilongjiang Province (LBH-Q11015) and the Innovative Talents Program for the Returned Overseas Scholars of Harbin (2012RFLXG031), and the Opening Project Foundation of Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education.References
- S. M. Cui, H. H. Pu, G. H. Lu, Z. H. Wen, E. C. Mattson, C. J. Hirschmugl, M. Gajdardziska-Josifovska, M. Weinert and J. H. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4898 CAS.
- X. L. Huang, N. T. Hu, R. G. Gao, Y. Yu, Y. Y. Wang, Z. Yang, E. Siu-Wai Kong, H. Wei and Y. F. Zhang, J. Mater. Chem., 2012, 22, 22488 RSC.
- K. P. Yoo, K. H. Kwon, N. K. Min, M. J. Lee and C. J. Lee, Sens. Actuators, B, 2009, 143, 333 CrossRef.
- D. J. Late, Y. K. Huang, B. Liu, J. Acharya, S. N. Shirodkar and J. J. Luo, et al., ACS Nano, 2013, 7, 4879 CrossRef CAS PubMed.
- S. Chen and G. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6473 CAS.
- P. K. Kannan and R. Saraswathi, J. Mater. Chem. A, 2014, 2, 394 Search PubMed.
- O. S. Kwon, S. J. Park, H. Yoon and J. Jang, Chem. Commun., 2012, 48, 10526 RSC.
- S. Mao, G. H. Lu and J. H. Chen, J. Mater. Chem. A, 2014, 2, 5573 CAS.
- W. J. Yuan and G. Q. Shi, J. Mater. Chem. A, 2013, 1, 10078 CAS.
- T. Wang, D. Huang, Z. Yang, S. S. Xu, G. L. He, X. L. Li, N. T. Hu, G. L. Yin, D. N. He and L. Y. Zhang, Nano-Micro Lett., 2016, 8, 95 CrossRef.
- N. Hu, Z. Yang, Y. Wang, L. Zhang, Y. Wang, X. Huang, H. Wei, L. Wei and Y. Zhang, Nanotechnology, 2014, 25, 025502 CrossRef PubMed.
- Y. Wang, L. Zhang, N. Hu, Y. Wang, Y. Zhang, Z. Zhou, Y. Liu, S. Shen and C. Peng, Nanoscale Res. Lett., 2014, 9, 251 CrossRef PubMed.
- Y. P. Dan, Y. Lu, N. J. Kybert, Z. T. Luo and A. T. C. Johnson, Nano Lett., 2009, 9, 1472 CrossRef CAS PubMed.
- P. A. Pandey, N. R. Wilson and J. A. Covington, Sens. Actuators, B, 2013, 183, 478 CrossRef CAS.
- S. S. Varghese, S. Lonkar, K. K. Singh, S. Swaminathan and A. Abdala, Sens. Actuators, B, 2015, 218, 160 CrossRef CAS.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32 CrossRef CAS.
- H. Meng, W. Yang, K. Ding, L. Feng and Y. F. Guan, J. Mater. Chem. A, 2015, 3, 1174 CAS.
- S. M. Hafiz, R. Ritikos, T. J. Whitcher, N. M. Razib and D. C. S. Bien, et al., Sens. Actuators, B, 2014, 193, 692 CrossRef CAS.
- S.-J. Choi, B.-H. Jang, S.-J. Lee, B. K. Min, A. Rothschild and I.-D. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 2588 CAS.
- H. J. Salavagione, A. M. Díez-Pascual, E. Lázaro, S. Verab and M. A. Gómez-Fatoua, J. Mater. Chem. A, 2014, 2, 14289 CAS.
- Z. B. Ye, Y. D. Jiang, H. L. Tai and Z. Yuan, Integr. Ferroelectr., 2014, 154, 73 CrossRef CAS.
- S. Yoo, X. Li, Y. Wu, W. H. Liu, X. L. Wang and W. H. Yi, J. Nanomater., 2014, 2014(7), 1 CrossRef.
- P. G. Su and H. C. Shieh, Sens. Actuators, B, 2014, 190, 865 CrossRef CAS.
- L. Zhou, F. Shen, X. Tian, D. Wang, T. Zhang and W. Chen, Nanoscale, 2013, 5, 1564 RSC.
- F. I. Bohrer, C. N. Colesniuc, J. Park, M. E. Ruidiaz, I. K. Schuller, A. C. Kummel and W. C. Trogler, J. Am. Chem. Soc., 2009, 131, 478 CrossRef CAS PubMed.
- B. Wang, X. Q. Zhou, Y. Q. Wu, Z. M. Chen, C. Y. He and X. Zuo, Sens. Actuators, B, 2012, 161, 498 CrossRef CAS.
- T. Sizun, M. Bouvetn and J. Suisse, Talanta, 2012, 97, 318 CrossRef CAS PubMed.
- X. J. Wang, S. L. Ji, H. B. Wang and D. H. Yan, Sens. Actuators, B, 2011, 160, 115 CrossRef CAS.
- A. Singh, S. Samanta, A. Kumar, A. K. Debnath, R. Prasad, P. Veerender, V. Balouria, D. K. Aswal and S. K. Gupta, Org. Electron., 2012, 13, 2600 CrossRef CAS.
- S. Pochekailov, J. Nožár, S. Nešpůrek, J. Rakušan and M. Karásková, Sens. Actuators, B, 2012, 169, 1 CrossRef CAS.
- T. Sizun, T. Patois, M. Bouvet and B. Lakard, J. Mater. Chem., 2012, 22, 25246 RSC.
- G. Guillaud, J. Simon and J. P. Germain, Coord. Chem. Rev., 1998, 178–180, 1433 CrossRef CAS.
- B. Wang, Y. Q. Wu, X. L. Wang, Z. M. Chen and C. Y. He, Sens. Actuators, B, 2014, 190, 157 CrossRef CAS.
- X. H. Liang, Z. M. Chen, H. Wu, L. X. Guo, C. Y. He, B. Wang and Y. Q. Wu, Carbon, 2014, 80, 268 CrossRef CAS.
- Y. Y. Wang, N. T. Hu, Z. H. Zhou, D. Xu, Z. Wang, Z. Yang, H. Wei, E. S. W. Kong and Y. F. Zhang, J. Mater. Chem., 2011, 21, 3779 RSC.
- J. H. Park, J. E. Royer, E. Chagarov, T. Kaufman-Osborn, M. Edmonds and T. Kent, et al., J. Am. Chem. Soc., 2013, 135, 14600 CrossRef CAS PubMed.
- X. Q. Zhou, X. L. Wang, B. Wang, Z. M. Chen, C. Y. He and Y. Q. Wu, Sens. Actuators, B, 2014, 193, 340 CrossRef CAS.
- F. Schedin, A. K. Geim and S. V. Morozov, et al., Nat. Mater., 2007, 6, 652 CrossRef CAS PubMed.
- P. Li, Y. Ding, A. Wang, L. Zhou, S. H. Wei and Y. M. Zhou, et al., ACS Appl. Mater. Interfaces, 2013, 5, 2255 CAS.
- S. Park, J. An, R. D. Piner, I. Jung, D. Yang, A. Velamakanni, S. T. Nguyen and R. S. Ruoff, Chem. Mater., 2008, 20, 6592 CrossRef CAS.
- K. Moriya, H. Enomoto and Y. Nakamura, Sens. Actuators, B, 1993, 13–14, 412 CrossRef.
- H. Z. Zhao, Y. Zhang, B. Zhao, Y. Y. Chang and Z. S. Li, Environ. Sci. Technol., 2012, 46, 5198 CrossRef CAS PubMed.
- X. Ling, J. X. Wu, W. G. Xu and J. Zhang, Small, 2012, 8(9), 1365 CrossRef CAS PubMed.
- A. Chunder, T. Pal, S. I. Khondaker and L. Zhai, J. Phys. Chem. C, 2010, 114, 15129 CAS.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS PubMed.
- H. J. Shin, S. M. Kim, S. M. Yoon, A. Benayad, K. K. Kim, S. J. Kim, H. K. Park, J. Y. Choi and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 2062 CrossRef CAS PubMed.
- V. Z. Poenitzsch, D. C. Winters, H. Xie, G. R. Dieckmann, A. B. Dalton and I. H. Musselman, J. Am. Chem. Soc., 2007, 129, 14724 CrossRef CAS PubMed.
- A. Das, S. Pisana, B. Chakraborty, S. Piscanec, S. K. Saha, U. V. Waghmare, K. S. Novoselov, H. R. Krishnamurthy, A. K. Geim, A. C. Ferrari and A. K. Sood, Nat. Nanotechnol., 2008, 3, 210 CrossRef CAS PubMed.
- N. Karousis, J. Ortiz, K. Ohkubo, T. Hasobe, S. Fukuzumi, A. Sastre-Santos and N. Tagmatarchis, J. Phys. Chem. C, 2012, 116, 20564 CAS.
- A. Ndiaye, P. Bonnet, A. Pauly, M. Dubois, J. Brunet, C. Varenne, K. Guerin and B. Lauron, J. Phys. Chem. C, 2013, 117, 20217 CAS.
- X. C. Li, B. Wang, X. L. Wang, X. Q. Zhou, Z. M. Chen, C. Y. He, Z. Y. Yu and Y. Q. Wu, Nanoscale Res. Lett., 2015, 10, 373 CrossRef PubMed.
- Y. Iyechika, K. Yakushi, I. Ikemoto and H. Kuroda, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 766–770 CrossRef.
- R. E. C. Clavijo, M. M. Campos-Vallette, M. S. Saavedra, A. Alvarado and G. E. Diaz, Vib. Spectrosc., 1997, 14, 79–85 CrossRef.
- J. D. Wright, Prog. Surf. Sci., 1981, 31, 1–60 CrossRef.
- C. W. Bauschlicher Jr and A. Ricca, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 115409 CrossRef.
- H. Chang, D. J. Lee, S. M. Lee and Y. H. Lee, Appl. Phys. Lett., 2001, 79, 3863 CrossRef CAS.
- J. J. Zhao, A. Buldum, J. Han and J. P. Lu, Nanotechnology, 2002, 13, 195 CrossRef CAS.
- D. R. Kauffman, O. Kuzmych and A. Star, J. Phys. Chem. C, 2007, 111, 3539 CAS.
- H. Wu, Z. M. Chen, J. L. Zhang, F. Wu, C. Y. He, B. Wang, Y. Q. Wu and Z. Y. Ren, J. Mater. Chem. A, 2016, 4, 1096 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02740k |
| This journal is © The Royal Society of Chemistry 2017 |