Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterogeneous Fenton-like degradation of phenanthrene catalyzed by schwertmannite biosynthesized using Acidithiobacillus ferrooxidans†
Xiaoqing Menga,
Su Yana,
Wenzhu Wuc,
Guanyu Zheng *ab and
Lixiang Zhouab
*ab and
Lixiang Zhouab
aDepartment of Environmental Engineering, College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing 210095, China. E-mail: gyzheng@njau.edu.cn; Fax: +86 25 84395160; Tel: +86 25 84395160
bJiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing 210095, China
cNanjing Institute of Environmental Science, Ministry of Environmental Protection of PRC, Nanjing 210042, China
First published on 18th April 2017
Abstract
Heterogeneous Fenton-like degradation of phenanthrene in aqueous solution was investigated using schwertmannite biosynthesized by Acidithiobacillus ferrooxidans LX5 as a catalyst. The effects of different reaction parameters including catalyst loading, H2O2 concentration, initial solution pH and inorganic anions on the Fenton-like degradation of phenanthrene were studied. Results showed that the biosynthesized schwertmannite had an effective catalytic ability on phenanthrene degradation. The degradation efficiency of phenanthrene was 99.0% within 3–5 h reaction under conditions of H2O2 200 mg L−1, schwertmannite 1 g L−1, phenanthrene 1 mg L−1 and pH 3.0–4.5. The degradation was mainly via a surface mechanism, in which phenanthrene was readily adsorbed on the surface of schwertmannite and then oxidized by ˙OH produced from H2O2 decomposition. The XPS results of schwertmannite before and after Fenton-like degradation of phenanthrene revealed the change of Fe2+/Fe3+ species on the surface of schwertmannite. Moreover, phthalates, octadecanoic acid and 9,10-phenanthraquinone were identified by GC-MS analyses as the main intermediate compounds during phenanthrene degradation, and all the intermediates were finally mineralized. The repeated use of biosynthesized schwertmannite for phenanthrene degradation illustrated its stability and reusability as a Fenton-like catalyst. Therefore, schwertmannite biosynthesized using A. ferrooxidans is an excellent catalyst for the degradation of phenanthrene in heterogeneous Fenton-like reactions.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs), organic compounds consisting of at least two fused benzene rings, are highly toxic, carcinogenic and resistant to natural degradation, subsequently causing a serious threat to human health through the food chain.1–3 The European Community and the U.S. Environmental Protection Agency have listed them as priority pollutants. Wastewater contaminated with PAHs is usually enriched at the sites of manufactured gas plants, refineries and many other chemical industries.4,5 Owing to their strong persistence and ubiquitous occurrence, substantial studies have been devoted to seek a highly efficient treatment to remove PAHs from industrial wastewater.Advanced oxidation processes (AOPs) including Fenton-catalysis, photo-catalysis and ozonation have become important technologies for the degradation of persistent organic pollutants (POPs). Among the AOPs, Fenton treatment shows great oxidative potential,6,7 which is based on the catalyzed decomposition of hydrogen peroxide (H2O2) by ferrous ion to generate the strong oxidant hydroxyl radicals. Conventional Fenton treatment is frequently limited by the required optimal pH of around 3.0 to prevent the precipitation of ferrous ion, and the treatment process would produce a large amount of ferric hydroxide sludge during the neutralization stage.8,9 Moreover, the oxidation efficiency of Fenton process can be significantly decreased by the presence of various inorganic anions, consequently limiting its usage in the treatment of high salinity wastewater.10,11 Thus, heterogeneous Fenton-like oxidation is developed to overcome the limitations associated with conventional Fenton treatment.12,13
Heterogeneous Fenton-like oxidation is initiated by hydrogen peroxide, and hydroxyl radicals can be produced on the surface of the catalysts or in bulk solution.13,14 Over the last two decades, heterogeneous Fenton-like oxidation catalyzed by goethite, magnetite, ferrihydrite or limonite, etc., has been used to treat water or soils contaminated by organic pollutants.9 Pinto et al.15 showed that the oxidation of methylene blue was caused by hydroxyl radicals produced on the surface of δ-FeOOH, and the contribution of soluble Fe on methylene degradation was negligible. Xue et al.16 revealed that the Fenton-like oxidation of pentachlorophenol (PCP) catalyzed by magnetite was dominated by the reactions on the catalyst surface, and in details PCP was readily adsorbed on a fixed number of surface active sites and then attacked by hydroxyl radicals produced from the decomposition of H2O2. Toda et al.17 used sulfurized limonite as a catalyst to decompose methylene blue and found that ˙OH was formed by a heterogeneous reaction on the surface of sulfurized limonite and a Fenton reaction catalyzed by dissolved ferrous ion.
Schwertmannite is a Fe(III)-hydroxysulfate with poorly crystalline structure and high surface area, which is abundant in nature because of its extensive presence in iron- and sulfate-rich mine drainage18,19 or sludge bioleaching environment.20 The chemical formula of schwertmannite is Fe8O8(OH)8−2x(SO4)x, (1 ≤ x ≤ 1.75),21 and its unit cell always consists of eight FeO3(OH)3 octahedra forming double chains which are shared over edges and run parallel to the b-axis.22 Schwertmannite can be synthesized through either chemical methods, including a dialysis technique18,23,24 and a chemically oxidative synthesis method,23–25 or a biological method using iron-oxidizing bacterium Acidithiobacillus ferrooxidans. The dialysis technique usually needs a dialysis period of more than 30 days,18,23,24 which is time-consuming and inefficient for schwertmannite synthesis.20 The chemically oxidative synthesis of schwertmannite through adding H2O2 into FeSO4 solution takes one to several days to complete, and the specific surface areas of the produced schwertmannite are 4–14 m2 g or 2.06–16.30 m2 g.23,25 Recently, the biological synthesis of schwertmannite receives much more attention, because schwertmannite can be biosynthesized by A. ferrooxidans within only 2–3 days and the specific surface areas of the produced schwertmannite are much larger than that synthesized using chemically oxidative synthesis method.24,26 For instances, Li et al.26 reported that the specific surface area of biosynthesized schwertmannite using A. ferrooxidans was 45.63 m2 g−1, larger than 3.17 m2 g−1 of chemically synthesized samples. In addition, Li et al.26 and Liu et al.25 found that the schwertmannite yield of biological method is comparatively or much higher than that of chemically oxidative synthesis method. Therefore, the biosynthesis method using A. ferrooxidans is a more attractive approach over chemical methods for schwertmannite production, in terms of the specific surface area and synthesis yield of produced schwertmannite.
Recently, some studies attempted to use chemosynthetic schwertmannite as a catalyst in the heterogeneous Fenton-like oxidation to oxidize nitrobenzene and phenol.27,28 However, the catalytic capacity of biosynthetic schwertmannite and the associated mechanism are still unclear in the heterogeneous Fenton-like oxidation of hydrophobic organic pollutants. Especially, previous studies reported that the catalysts with much larger specific surface areas usually would have much higher catalytic activities, because of their large numbers of active sites for H2O2 decomposition.29 Therefore, the objectives of the present study are to (1) investigate the effect of reaction conditions including the loading of biosynthetic schwertmannite, concentration of H2O2, solution initial pH, and the presence of inorganic anions on the degradation of phenanthrene catalyzed by biosynthetic schwertmannite and H2O2, (2) reveal the associated catalytic degradation mechanism and possible transformation pathway of phenanthrene in biosynthetic schwertmannite/H2O2 system, and (3) study the stability of biosynthetic schwertmannite during its repeated use in the catalytic degradation of phenanthrene.
2. Materials and methods
2.1. Reagents
Phenanthrene (≥99%) was purchased from Aladdin Co., Ltd. Acetonitrile, methanol and n-hexane were purchased from Sigma Aldrich Co. Ltd at HPLC grade, and dichloromethane and acetone were supplied by Aladdin Co., Ltd at HPLC grade. Hydrogen peroxide (30% solution) and other reagents were obtained from Sinopharm Chemical Regent Beijing Co., Ltd and used as received. All solutions and suspensions were prepared by using deionized water.2.2. Preparation of catalysts
Biosynthetic schwertmannite was prepared in the laboratory according to the method described in previous studies.20 Briefly, 20 mL of freshly prepared A. ferrooxidans LX5 cell suspensions with a cell density of 3 × 108 cells per mL were introduced into 1000 mL Erlenmeyer flask containing 480 mL of ferrous sulfate solution with ferrous ion concentration of 0.144 M. The flasks were then incubated for 2 days at 180 rpm and 28 °C in a rotary shaker. The precipitates formed in the flasks were harvested by filtering through a Whatman no. 4 filter paper, sequentially washed with acidified water with pH 2.0 and deionized water for three times to remove soluble impurities, and dried at 50 °C to a constant weight. The preparation of chemosynthetic schwertmannite and chemosynthetic goethite followed the methods of Liu et al.25 and Manning et al.30 The detailed procedures can be found in the ESI.†2.3. Characterization of catalysts
The morphology of biosynthetic schwertmannite was examined by scanning electron microscope (SEM, Hitachi S-4700) at 20 kV accelerating voltage. The crystal structure of biosynthetic schwertmannite was determined by X-ray powder diffraction (XRD, ThermoFisher XTRA) using an X-ray diffractometer fitted with Cu Kα radiation (40 kV and 40 mA), and samples were scanned from 2θ = 10°–70° with a 2θ = 0.02° step-size. The functional groups of biosynthetic schwertmannite were determined by Fourier transform infrared spectroscopy (FTIR) with a wavelength range of 400–4000 cm−1, which was scanned 32 times and resolution ratio of 0.09 cm−1. The specific surface area of biosynthetic schwertmannite was measured by using the Brunauer–Emmett–Teller (BET) through N2 adsorption–desorption method (BET, Tristar 3000, Micromeritics).2.4. Catalytic degradation of phenanthrene
To prepare 1 mg L−1 phenanthrene solution, phenanthrene was firstly dissolved in acetone to make a solution with a concentration of 1000 mg L−1, and then 1 mL prepared solution was diluted to a concentration of 1 mg L−1 using deionized water.31The phenanthrene degradation experiments were conducted in 35 mL glass vessels with screw caps having a PTFE-lined septum. In each vessel, the reaction medium was prepared by adding a certain amount of schwertmannite to 10 mL phenanthrene solution with a concentration of 1 mg L−1, and the solution initial pH was adjusted to defined values using 1 M HClO4 or 1 M NaOH.32 The reaction was then initiated by adding a certain amount of H2O2 into the glass vessels. All experiments were carried out under dark conditions and shaken in a rotary shaker at 180 rpm and 28 °C. At the given reaction time intervals, vessels were taken out and added with 3 mL methanol to quench the reaction. Vessels taken out were added with ten drops of 1 M H2SO4 to totally dissolve schwertmannite. Phenanthrene in the resulting solution was extracted using dichloromethane for quantitative analysis of phenanthrene degradation efficiency using a high performance liquid chromatography (HPLC), and alternatively it was extracted using n-hexane to indentify the intermediate products during the phenanthrene degradation using a gas chromatography mass spectrometer (GC-MS). Meanwhile, parallel samples in withdrawn vessels were filtered through a 0.22 μm glass fiber filter paper, and the obtained supernatant was used for the determination of the concentrations of total Fe, Fe2+ and Fe3+, H2O2 concentration and total organic carbon (TOC) content. To determine the removal efficiency of phenanthrene from the solution, the supernatant was directly extracted using dichloromethane and quantified using HPLC, which is without the procedure of dissolving schwertmannite. Initial loading content of schwertmannite was changed from 0.1 to 2 g L−1 to test the loading of catalyst on the catalytic degradation efficiency of phenanthrene. To verify the presence of ˙OH in the degradation system, methanol was used as a scavenger to give a volume percentage ranging from 1% to 10%,27,28 which was added before adding H2O2 to the solution. Under the optimum reaction condition, the schwertmannite was collected at the end of the experiment, washed with the deionized water, and dried to a constant weight. The oxidation states of iron species on the schwertmannite surface was measured by the X-ray photoelectron spectrum (XPS), which was performed in a Kratos AXIS His, mono Al Kα system (Energy 1486.7 eV, Kratos Analytical, Japan).
To explore the presence of inorganic anions on the effect of phenanthrene degradation, SO42−, H2PO4−, NO3− and Cl− with the respective concentration ranging from 50 to 200 mg L−1 were introduced to the degradation system which contained 1 mg L−1 phenanthrene, 1 g L−1 schwertmannite and 200 mg L−1 H2O2, at pH 3.0. The stability and reusability of schwertmannite was also investigated in a multi-cycle experiment. In each cycle, the initial concentrations of phenanthrene, H2O2 and schwertmannite were 1 mg L−1, 200 mg L−1 and 1 g L−1, respectively, and the reaction time and solution initial pH were 12 h and 3.0. The used catalyst was collected at the end of each oxidation experiment, washed with the deionized water, dried enough, and then used in the next cycle experiment. In each cycle experiment, the degradation efficiency of phenanthrene and TFe concentration in the solution were measured. Biosynthetic schwertmannite was characterized by using X-ray powder diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) after 12 times of repeated use.
2.5. Analytical methods
The concentration of phenanthrene was analyzed using a HPLC (HPLC-1260, Agilent) equipped with UV detection wavelength of 254 nm. Waters PAH C18 column (5 μm, 4.6 mm × 250 mm) was used for the separation of phenanthrene. Samples were injected at 20 μL at a column temperature of 27 °C, and the mobile phase was the mixture of water (40%) and acetonitrile (60%). Phenanthrene and its intermediate products were identified using a SHIMADZU gas chromatograph (GC-2010) incorporated with a mass spectrometer operated on a full scan mode (50–550 amu), where the MS capillary column (250 μm, 0.25 μm × 30 m) was used. Helium was used as carrier gas at a flow rate of 1.0 mL min−1. The initial oven temperature was 80 °C (5 min holding) and continued to rise temperature to 300 °C at a speed of 10 °C min−1 (10 min holding). The concentration of dissolved Fe was determined by using Inductively Coupled Plasma (ICP, Agilent 5100). The concentration of H2O2 and the content of total organic carbon were measured using the titanium sulfate method and TOC analyzer (TOC-5000, Shimadzu), respectively.3. Results and discussion
3.1. Characterization of biosynthetic schwertmannite and its catalytic capability
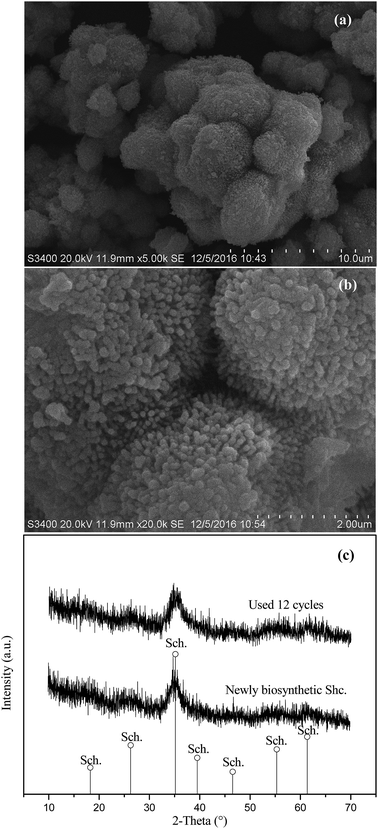
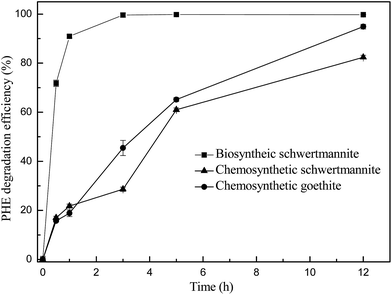
Oxidation of ferrous sulfate by A. ferrooxidans LX5 cell suspensions resulted in the formation of iron precipitates. The iron precipitates formed were visualized by SEM. As shown in Fig. 1a and b, they were consisted of aggregates of spherical particles, and the surface of spherical particles showed a “hedge-hog” morphology which is typical for schwertmannite.19 The XRD patterns of iron precipitates (Fig. 1c) displayed a weak crystalline structure with seven typical broad characteristic peaks (2θ: 18.24, 26.27, 35.16, 39.49, 46.53, 55.29, 61.34°), and all diffraction peaks well matched those of the standard diffraction data for schwertmannite (JCPDS no. 47-1775), suggesting that the iron precipitates formed are pure schwertmannite particles. The BET analysis revealed that the specific surface area of the biosynthesized schwertmannite was 54.5 m2 g−1.The degradation of 1 mg L−1 phenanthrene catalyzed by biosynthetic schwertmannite, chemosynthetic schwertmannite and chemosynthetic goethite was evaluated under the same experimental conditions of 1.0 g L−1 catalyst, and 200 mg L−1 H2O2 at pH 3.0, and results are shown in Fig. 2. It can be found that as high as 99% of phenanthrene degradation was achieved within only 3 h in Fenton-like reaction catalyzed by biosynthetic schwertmannite. However, within the same reaction period (3 h), less than 28.6% and 45.4% of phenanthrene was degraded in Fenton-like reaction catalyzed by chemosynthetic schwertmannite and chemosynthetic goethite, respectively. These results clearly indicated that the catalytic activity of biosynthetic schwertmannite in Fenton-like system was much higher than that of either chemosynthetic schwertmannite or chemosynthetic goethite. The much poorer performance of chemosynthetic schwertmannite in the catalytic degradation of phenanthrene may be related to its relatively low specific surface area (6.84 m2 g−1), because of lack of “hedge-hog” morphology on its particle surfaces (as shown in Fig. S1†), and the discrepancy of catalytic capabilities between biosynthetic schwertmannite and goethite probably resulted from much less surface active sites of goethite.29,33,34
3.2. Effect of schwertmannite loading, H2O2 concentration, and solution initial pH on the degradation of phenanthrene
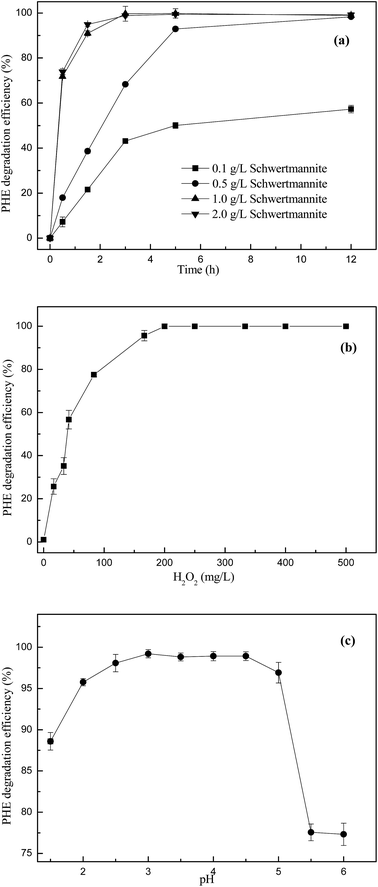
The effect of biosynthetic schwertmannite loading on the catalytic degradation of phenanthrene is shown in Fig. 3a. The loading of 0.1 g L−1 biosynthetic schwertmannite only resulted in 57.3% degradation of 1 mg L−1 phenanthrene within 12 h of reaction, and 12 h was consumed to degrade 98.3% of phenanthrene when the loading of biosynthetic schwertmannite was 0.5 g L−1. However, phenanthrene was totally degraded within only 3–5 h at the schwertmannite loading of 1 g L−1, and the degradation of phenanthrene was not further improved when the loading of biosynthetic schwertmannite was increased to 2.0 g L−1. Thus, 1.0 g L−1 of schwertmannite loading is suitable for the degradation of phenanthrene in the Fenton-like reaction catalyzed by biosynthetic schwertmannite.The concentration of H2O2 is an important factor influencing the Fenton or Fenton-like reaction, since it directly relates to the amount of hydroxyl radicals produced in the catalytic reactions.29 The effect of H2O2 concentration on the degradation of phenanthrene by Fenton-like reaction catalyzed by biosynthetic schwertmannite was investigated by changing H2O2 concentration from 16.7 to 500 mg L−1 at pH 3.0, with the schwertmannite loading of 1 g L−1 and phenanthrene concentration of 1 mg L−1. As shown in Fig. 3b, the degradation efficiency of phenanthrene within 5 h of Fenton-like reaction catalyzed by biosynthetic schwertmannite was enhanced from 25.7% to 99.9% when increasing H2O2 concentration from 16.7 to 200 mg L−1. Actually, when the concentration of H2O2 was 200 mg L−1, about 99.6% of phenanthrene can be degraded in only 3 h of reaction (Fig. 3a). Previous studies found that higher concentration of H2O2 may scavenge the produced reactive oxidation species (like ˙OH) to form HO2˙, thus lowering the degradation efficiency of organics, because the oxidation potential of HO2˙ was much lower than that of ˙OH.27 However, the degradation of phenanthrene was not lowered when further increasing H2O2 concentration beyond 200 mg L−1. Since too much H2O2 would definitely raise the cost of treatment, the optimum H2O2 concentration was 200 mg L−1 for the efficient degradation of 1 mg L−1 phenanthrene within 3–5 h of reaction catalyzed by biosynthetic schwertmannite.
Many studies reported that the initial pH of solution seriously affects the performance of the Fenton-like process in degrading pollutants, because of the role of solution pH in controlling the catalytic activity and the hydrogen peroxide stability.35–37 In addition, an acidic condition is usually beneficial for the degradation of organics in Fenton-like reactions catalyzed by iron oxide minerals including magnetite, hematite and goethite because the dissolution of iron oxide minerals can easily occur at acidic conditions to release dissolve iron to catalyze homogenous Fenton-like reactions.29 The effect of solution initial pH on the degradation of phenanthrene was determined within a pH range of 1.5–6.0. As shown in Fig. 3c, the degradation efficiency of phenanthrene was enhanced from 88.6% to 99.2% when increasing the solution initial pH from 1.5 to 3.0. Obviously, the maximum degradation efficiency of phenanthrene was achieved in the solution initial pH range of 3.0–4.5, which was higher than 99.0%. When the solution initial pH was higher than 4.5, the degradation of phenanthrene decreased rapidly. Especially, the degradation efficiency of phenanthrene was only 77.6% when the solution initial pH was 5.5. Therefore, the optimum pH for the degradation of phenanthrene catalyzed biosynthetic schwertmannite is 3.0–4.5, at which pH values more than 99.0% of 1 mg L−1 phenanthrene can be degraded effectively.
3.3. Mechanism of phenanthrene degradation
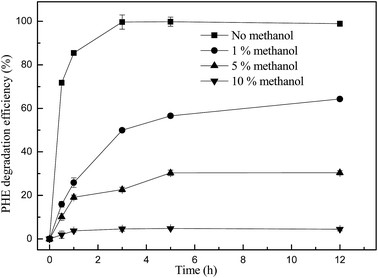
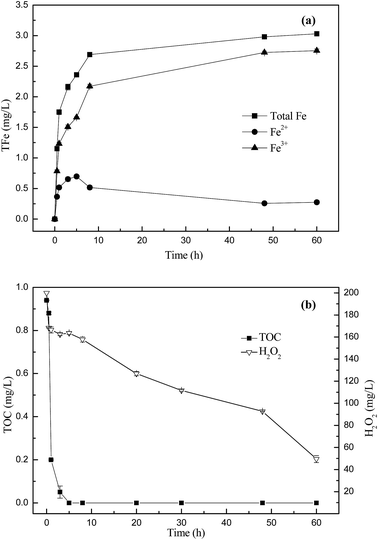
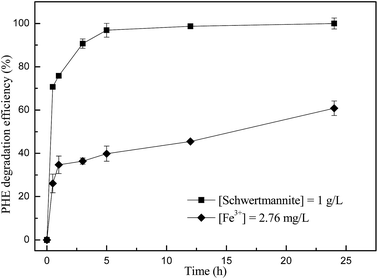
In order to verify the presence of ˙OH in the Fenton-like reaction catalyzed by biosynthetic schwertmannite, methanol was chosen as a ˙OH scavenger during the catalytic degradation of phenanthrene. It can be seen from Fig. 4 that the degradation of phenanthrene was rapidly lowered by the presence of methanol in the system. Only 64.3% and 30.4% of phenanthrene was degraded within 12 h of reaction when 1% or 5% of methanol was added, respectively. In addition, the degradation of phenanthrene was totally inhibited by the presence of 10% methanol. These results clearly indicated that phenanthrene was catalytically degraded by ˙OH generated by H2O2 decomposition in the Fenton-like reaction catalyzed by biosynthetic schwertmannite.15,28 To further reveal the mechanism responsible for phenanthrene degradation, dissolved iron concentration, H2O2 concentration and TOC content in the solution were monitored during the degradation of 1 mg L−1 phenanthrene catalyzed by 1 g L−1 of schwertmannite and 200 mg L−1 H2O2 at pH 3.0. As shown in Fig. 5a, the ferrous concentration reached a peak value of 0.70 mg L−1 at 5 h when phenanthrene was completely degraded, and then its concentration decreased to 0.28 mg L−1 at 60 h of reaction, which could be ascribed to the oxidation of ferrous ions into ferric ions by remaining ˙OH and H2O2 in the solution. After 8 h of reaction, the concentrations of ferric and total dissolved iron attained 2.17 and 2.70 mg L−1, respectively, which suggests that Fe inside the structure of schwertmannite or adsorbed on the surface of schwertmannite were released into the solution during the reaction. Furthermore, about 3.03 mg L−1 of iron dissolved into solution after 60 h of reaction, only equivalently 0.68% of total iron of 1.0 g L−1 catalyst used, which shows a very slow leaching of iron from biosynthetic schwertmannite. As shown in Fig. 5b, the TOC content decreased sharply from 0.94 mg L−1 to an undetectable level within the first 5 h of reaction, which clearly illuminated that 1 mg L−1 of phenanthrene was completely mineralized by the reaction catalyzed by schwertmannite and H2O2. Correspondingly, the concentration of H2O2 also declined from 200 to 163.6 mg L−1 within the first 5 h of reaction, and then the excessive H2O2 was run out gradually.To clarify the contribution of homogenous Fenton reaction catalyzed by dissolved ferric ion on the degradation of phenanthrene, the highest dissolved ferric ion concentration during the degradation of 1 mg L−1 phenanthrene catalyzed by 1 g L−1 of schwertmannite and 200 mg L−1 H2O2 at pH 3.0, 2.76 mg L−1 of ferric ion, was used to catalyze a homogenous Fenton reaction to degrade phenanthrene. As shown in Fig. 6, only 39.8% of phenanthrene could be degraded by the homogenous Fenton reaction catalyzed by 2.76 mg L−1 of dissolved ferric ion and 200 mg L−1 H2O2 at pH 3.0 after 5 h, but as high as 100% of phenanthrene was degraded in 5 h by the reaction catalyzed by 1 g L−1 schwertmannite and 200 mg L−1 H2O2 at pH 3.0. It should be taking into account that 2.76 mg L−1 Fe3+ was not available from the beginning of the Fenton-like reaction catalyzed by schwertmannite, and thus the degradation of phenanthrene by the homogenous Fenton reaction should be much lower than 39.8%. This result clearly indicated that the degradation of phenanthrene did not merely result from the homogenous Fenton reaction catalyzed by dissolved ferric ion, and both homogenous Fenton reaction catalyzed by dissolved ferric ion and heterogeneous Fenton-like reaction catalyzed schwertmannite played important roles in degrading phenanthrene. In addition, it seems that the heterogeneous Fenton-like reaction catalyzed by schwertmannite was much more effective than the homogenous Fenton reaction catalyzed by dissolved ferric ion in degrading phenanthrene during the degradation of 1 mg L−1 of phenanthrene catalyzed by schwertmannite and H2O2.
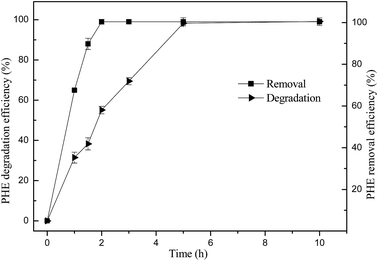
Although phenanthrene could be degraded in only 5 h by the reaction catalyzed by 1 g L−1 of schwertmannite and 200 mg L−1 H2O2 at pH 3.0, its removal from the solution seems much faster. Here, the degradation referred to that phenanthrene in the reaction system was oxidized into small molecular compounds, or even CO2 and H2O, while the removal means the disappearance of phenanthrene from the reaction solution through both oxidation and adsorption processes. As shown in Fig. 7, 1 mg L−1 of phenanthrene was completely removed from the solution in only 2 hours when the degradation efficiency of phenanthrene was only 55.1%. This result obviously indicated that phenanthrene was readily adsorbed onto the surface of schwertmannite particles probably because of strong sorption capacity of schwertmannite for hydrophobic organic pollutants such as phenanthrene. In fact, adsorption experiments revealed that the sorption capacity of biosynthetic schwertmannite for phenanthrene is as high as 0.74 mg g−1 (data not shown). The strong sorption capacity of biosynthetic schwertmannite for phenanthrene clearly indicates that the degradation of phenanthrene by biosynthetic schwertmannite/H2O2 reaction was mainly via a surface mechanism, in which phenanthrene can be readily adsorbed on the surface of schwertmannite and then oxidized by hydroxyl radical produced from H2O2 decomposition.
The XPS results of biosynthetic schwertmannite before and after Fenton-like degradation of phenanthrene are shown in Fig. 8 and Table S1.† The Fe 2p lines are broad and can be deconvoluted into two components with line position at 711.1 and 712.8 eV, respectively. These two components correspond to the Fe3+ and Fe2+ species.38 Before the Fenton-like reaction, the major surface iron specie of biosynthetic schwertmannite is found to be Fe3+, which contributes to 58.2% of the total surface iron atoms, and 41.8% of the total iron surface atoms was Fe2+. For biosynthetic schwertmannite after the Fenton-like reaction, the intensity ratio of Fe2+/Fe3+ species was changed to 54.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.5. The curve fitting was carried out using the Gaussian–Lorentzian ratio = 20 (G
45.5. The curve fitting was carried out using the Gaussian–Lorentzian ratio = 20 (G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 20
L = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80), the symmetry factor of 0.4, and the χ2 of 2.05. The binding energy of Fe 2p, and Fe2+ and Fe3+ surface concentration on the biosynthetic schwertmannite catalyst before and after phenanthrene degradation were listed in Table S1.† The change of Fe2+/Fe3+ species on the surface of biosynthetic schwertmannite implies that the biosynthetic schwertmannite take part in oxidation–reduction reaction during phenanthrene degradation and the Fenton-like degradation of phenanthrene mainly occurred on the surface of biosynthetic schwertmannite.
80), the symmetry factor of 0.4, and the χ2 of 2.05. The binding energy of Fe 2p, and Fe2+ and Fe3+ surface concentration on the biosynthetic schwertmannite catalyst before and after phenanthrene degradation were listed in Table S1.† The change of Fe2+/Fe3+ species on the surface of biosynthetic schwertmannite implies that the biosynthetic schwertmannite take part in oxidation–reduction reaction during phenanthrene degradation and the Fenton-like degradation of phenanthrene mainly occurred on the surface of biosynthetic schwertmannite.
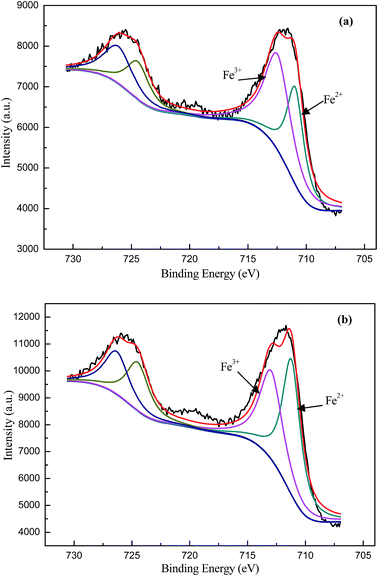 | ||
| Fig. 8 XPS spectrums of the biosynthetic schwertmannite before (a) and after (b) phenanthrene degradation (Fe 2p line). | ||
Thus, the Fenton-like degradation of phenanthrene catalyzed by biosynthetic schwertmannite can be explained by the following mechanisms:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII + phenanthrene → FeIII + phenanthrene → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII − phenanthrene FeIII − phenanthrene
| (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII + H2O2 → FeIII + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIIIH2O2 FeIIIH2O2
| (2) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIIIH2O2 → FeIIIH2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII + ˙HO2 + H+ FeII + ˙HO2 + H+
| (3) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII + ˙HO2 → FeIII + ˙HO2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII + O2 + H+ FeII + O2 + H+
| (4) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII + H2O2 → FeII + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII + ˙OH + OH− FeIII + ˙OH + OH−
| (5) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII − phenanthrene + ˙OH → FeIII − phenanthrene + ˙OH → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII + degradation products → …CO2 + H2O FeIII + degradation products → …CO2 + H2O
| (6) |
At the beginning of Fenton-like degradation of phenanthrene catalyzed by biosynthetic schwertmannite, phenanthrene molecules were readily adsorbed on the surface of schwertmannite to form ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII − phenanthrene (eqn (1)), where
FeIII − phenanthrene (eqn (1)), where ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII represents FeIII sites on the catalyst surface.39 The heterogeneous Fenton-like reaction was activated by the formation of a complex between
FeIII represents FeIII sites on the catalyst surface.39 The heterogeneous Fenton-like reaction was activated by the formation of a complex between ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII and H2O2, being assigned as
FeIII and H2O2, being assigned as ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIIIH2O2 (eqn (2)). Then the generated
FeIIIH2O2 (eqn (2)). Then the generated ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIIIH2O2 species are converted to
FeIIIH2O2 species are converted to ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII species and ˙HO2 (eqn (3)), and the generated ˙HO2 may further react with
FeII species and ˙HO2 (eqn (3)), and the generated ˙HO2 may further react with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIII to produce
FeIII to produce ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII species (eqn (4)). The above formed
FeII species (eqn (4)). The above formed ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeII (eqn (3) and (4)) can react with H2O2 to generate ˙OH radicals (eqn (5)). The ˙OH radicals thus attack the phenanthrene molecules adsorbed on the surface of schwertmannite and realize the degradation and/or mineralization (eqn (6)). This mechanism is analogous to previous studies on the heterogeneous Fenton-like reactions of the iron-oxide/H2O2 system.29,38
FeII (eqn (3) and (4)) can react with H2O2 to generate ˙OH radicals (eqn (5)). The ˙OH radicals thus attack the phenanthrene molecules adsorbed on the surface of schwertmannite and realize the degradation and/or mineralization (eqn (6)). This mechanism is analogous to previous studies on the heterogeneous Fenton-like reactions of the iron-oxide/H2O2 system.29,38
3.4. Transformation pathway of phenanthrene
The intermediates of phenanthrene degradation were identified by using GC-MS to further reveal its transformation pathway in the Fenton-like reaction catalyzed by biosynthetic schwertmannite. As shown in Fig. S2,† some intermediates were found in the sample with 1 h reaction time, and the characteristics peaks were summarized in Table 1.| Peak | Retention time | m/z | Empirical formula | Compound |
|---|---|---|---|---|
| A | 18.120 | 178 | C14H10 | Phenanthrene |
| B | 19.717 | 281 | C18H32O2 | Octadecanoic acid |
| C | 20.842 | 208 | C14H8O2 | 9,10-Phenanthraquinone |
| D | 22.300 | 390 | C24H38O4 | Dioctyl phthalate |
| E | 22.742 | 281 | C18H32O2 | Octadecanoic acid |
| F1 | 24.542 | 390 | C24H38O4 | Dioctyl phthalate |
| F2 | 24.810 | 390 | ||
| F3 | 25.085 | 390 | ||
| G1 | 26.192 | 405 | C25H40O4 | Nonyl octyl phthalate |
| G2 | 26.342 | 405 |
According to the standard spectra from the NIST library database, the peak B and E can be assigned to the compound of octadecanoic acid. Correspondingly, the peak C can be assigned to 9,10-phenanthraquinone, and the peak D, F (F1, F2 and F3) and G (G1 and G2) were assigned to the compound phthalate by analyzing the fragmentation pattern of mass spectra, which was not observed before reaction. It was noted that peak G (G1 and G2) was identified as nonyl octyl phthalate, which gives the maximum m/z value of 405 (Fig. S3†). The above stated intermediates are consistent with the previous reports.40,41 However, no products appeared in the sample with 5 h reaction time, which clearly indicates that all intermediates were finally mineralized into CO2 and H2O, consistent with the result of TOC removal.
The above mentioned products were only portion of the reaction intermediates, since some intermediates had too short lifetime and could not be detected by using the current GC-MS analysis. Considering the above products identified and some reported phenanthrene transformation pathways,41,42 the transformation pathway of phenanthrene in the biosynthetic schwertmannite/H2O2 system was temporarily proposed in Fig. 9. In the main, hydroxyl radicals and singlet oxygen attacked the benzene rings of phenanthrene at positions 9 and 10 to ketonize phenanthrene, leading to substituted products such as 9,10-phenanthraquinone (product C).43 Subsequently, the benzene rings of 9,10-phenanthraquinone can be cleaved and then formed compounds of phthalate. The above phthalate is esterified into dioctyl phthalate (product F1, F2 and F3) and nonyl octyl phthalate (product G1 and G2) in OH-induced oxidation process.41,44 Afterward, the rest benzene ring is further cleaved, and the formed products can be attacked by hydroxyl radicals continuously until complete mineralization.
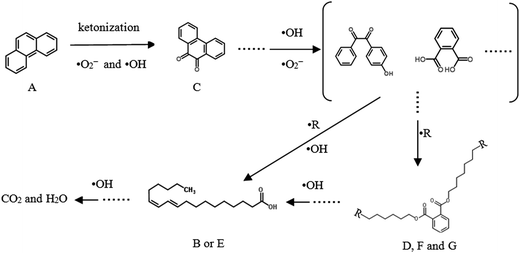 | ||
| Fig. 9 Proposed transformation pathway of phenanthrene catalyzed by biosynthetic schwertmannite/H2O2 reaction. | ||
3.5. Effects of inorganic anions on the degradation of phenanthrene and the stability and reusability of biosynthetic schwertmannite
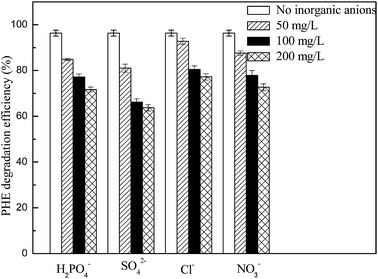
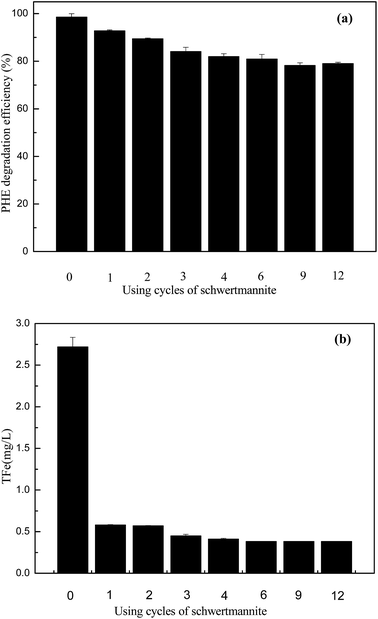
Inorganic anions were always detected in industrial wastewater or underground water, and their existences usually affect the oxidation efficiency of Fenton process.45–47 In the present study, the concentrations of inorganic anions were controlled in a range of 50–200 mg L−1 and the phenanthrene degradation experiments were carried out in 5 h. The experimental results were shown in Fig. 10, and it could be found that the inhibitory effect of SO42−, H2PO4−, NO3− or Cl− on the degradation of phenanthrene was enhanced with the increase of their respective concentration from 50 to 200 mg L−1. However, these inorganic anions presented different potential in respect of inhibiting the degradation of phenanthrene, and the inorganic anions studied suppressed the degradation of phenanthrene in a sequence of SO42− > H2PO4− > NO3− > Cl−. After 5 h reaction, the efficiency of phenanthrene degradation was only 63.74%, 71.67%, 72.71% and 77.24% in the presence of 200 mg L−1 of SO42−, H2PO4−, NO3− and Cl−, respectively. Actually, previous studies reported that SO42− and H2PO4− could easily combine with ferric ion on the surface of iron oxides, and formed sulfate–ferric or phosphoric–ferric complexes, resulting in a very slow decomposition of H2O2.10 Liu et al.47 showed that both SO42− and Cl− had a potential of scavenging hydroxyl radicals and would form SO4˙− (2.43 V), Cl˙ (2.41 V) and Cl2˙− (2.09 V) at pH 3.0, which had lower oxidation ability than hydroxyl radicals. Besides, Cl− could interact with the iron oxide surface through specific adsorption,48 and NO3− may also have the ability to scavenge ˙OH to lower the degradation efficiency of organics.28From the view of application, the long-term stability of biosynthetic schwertmannite should also be considered, and thus its catalytic capacity and the leaching of Fe from schwertmannite were investigated in a multi-cycle experiment. In each cycle, the initial concentrations of phenanthrene, H2O2 and schwertmannite were 1 mg L−1, 200 mg L−1 and 1 g L−1, respectively, and the reaction time and solution initial pH were 12 h and 3.0. As shown in Fig. 11a, the degradation efficiency of phenanthrene decreased from 99.0% to 80.0% when schwertmannite was reused for 12 cycles. This result clearly indicated that schwertmannite retained as high as 80.0% of its catalyzing capacity even after 12 cycles of reuse, which suggested that schwertmannite could be repeatedly used as a Fenton-like catalyst. It could be seen from Fig. 11b that the concentration of leached Fe was 2.72 mg L−1 before recycling schwertmannite. During the 12 cycles of schwertmannite, the leaching amount of Fe remained in a range of 0.38–0.58 mg L−1, which is acceptable in accordance with European Union discharge standards (<2 mg L−1).49
The structure and morphology of schwertmannite after 12 times of repeated use were also investigated. XRD spectra shown in Fig. 2a displayed that the XRD patterns of repeatedly used schwertmannite for 12 times still matched the standard diffraction data for schwertmannite (JCPDS no. 47-1775). The FTIR spectrum of newly biosynthesized schwertmannite was shown in the Fig. S4.† It can be seen that the absorption peaks at wavelength 3217 cm−1 were attributed to the stretching of free/bound hydroxyl groups; the absorption peaks at wavelength 1631 cm−1 were assigned to the O–H vibrations of H2O molecules or structural OH groups; the γ3 and γ1 vibration of SO42− were represented in the wavenumbers of 1117 and 982 cm−1, respectively; the absorption peaks at wavelength 609 cm−1 were caused by the vibration of SO42−; the absorption peaks at wavelength 483 cm−1 were ascribed to Fe–O bond vibration. All functional groups represented in the IR spectra were the same as that of schwertmannite.21,23,38 The FTIR spectrum of repeatedly used schwertmannite for 12 times was in the agreement with those of newly biosynthesized schwertmannite. These results indicated that the Fenton-like reaction barely damaged the structure and functional groups of schwertmannite. Although some studies pointed out that schwertmannite is a metastable mineral and it can easily transform into goethite under oxidation conditions,9 results of the present study showed that schwertmannite is an excellent Fenton-like catalyst for the degradation of phenanthrene and its mineral structure did not change during its repeated use.
4. Conclusion
The schwertmannite biosynthesized by Acidithiobacillus ferrooxidans is a poorly crystalline iron oxyhydroxysulfate with a high specific surface area. This biosynthetic schwertmannite can be used as a catalyst for the degradation of phenanthrene by the heterogeneous Fenton-like reaction. The degradation efficiency of phenanthrene was above 99.0% within 3–5 h reaction, under the reaction conditions of H2O2 200 mg L−1, schwertmannite 1 g L−1, phenanthrene 1 mg L−1 and pH 3.0–4.5. The degradation of phenanthrene by biosynthetic schwertmannite/H2O2 reaction was mainly via a surface mechanism, in which phenanthrene can be readily adsorbed on the surface of schwertmannite and then oxidized by hydroxyl radical produced from H2O2 decomposition. The Fenton-like degradation of phenanthrene resulted in a change of Fe2+/Fe3+ species on the surface of biosynthetic schwertmannite. Moreover, phthalates, octadecanoic acid and 9,10-phenanthraquinone were the main intermediate compounds during the transformation of phenanthrene in biosynthetic schwertmannite/H2O2 system, and all the intermediate compounds can be mineralized finally. In the presence of inorganic anions, phenanthrene degradation was suppressed to some degree in a sequence of SO42− > H2PO4− > NO3− > Cl−. The repeated use of schwertmannite as a Fenton-like catalyst did not significantly change its catalytic activity, mineral structure, and function groups, indicating that biosynthetic schwertmannite had a good stability and reusability as a Fenton-like catalyst for phenanthrene degradation. Therefore, biosynthetic schwertmannite is an excellent catalyst for the degradation of phenanthrene in heterogeneous Fenton-like reactions.Acknowledgements
The authors would like to thank the financial support from National Natural Science Foundation of China (21477055, 21637003, and 21307059).Note and references
- M. S. García-Falcón and J. Simal-Gándara, Food Addit. Contam., 2005, 22, 791–797 CrossRef PubMed.
- L. Rey-Salgueiro, E. Martínez-Carballo, M. S. García-Falcón, C. Gonzalez-Barreiro and J. Simgal-Gandara, Food Chem., 2009, 115, 814–819 CrossRef CAS.
- S. Gan and H. K. Ng, Chem. Eng. J., 2012, 180, 1–8 CrossRef.
- M. S. García-Falcón, B. Soto-González and J. Simal-Gándara, Environ. Sci. Technol., 2006, 40, 759–763 CrossRef.
- M. Usman, P. Faure, C. Ruby and K. Hanna, Appl. Catal., B, 2012, 117, 10–17 CrossRef.
- J. H. Ramirez, F. J. Maldonado-Hódar, A. F. Pérez-Cadenas, C. Moreno-Castilla, C. A. Costa and L. M. Madeira, Appl. Catal., B, 2007, 75, 312–323 CrossRef CAS.
- R. Li, Y. Gao, X. Jin, Z. Chen, M. Megharaj and R. Naidu, J. Colloid Interface Sci., 2015, 438, 87–93 CrossRef CAS PubMed.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- E. D. Burton, S. G. Johnston, K. M. Watling, R. T. Bush, A. F. Keene and L. A. Sullivan, Environ. Sci. Technol., 2010, 44, 2016–2021 CrossRef CAS PubMed.
- J. D. Laat, G. T. Le and B. Legube, Chemosphere, 2004, 55, 715–723 CrossRef PubMed.
- R. Maciel, G. L. Sant'Anna Jr and M. Dezotti, Chemosphere, 2004, 57, 711–719 CrossRef CAS PubMed.
- S. S. Lin and M. D. Gurol, Environ. Sci. Technol., 1998, 32, 1417–1423 CrossRef CAS.
- S. H. Tian, Y. T. Tu, D. S. Chen, X. Chen and Y. Xiong, Chem. Eng. J., 2011, 169, 31–37 CrossRef CAS.
- S. Lomnicki, H. Truong, E. Vejerano and B. Dellinger, Environ. Sci. Technol., 2008, 42, 4982–4988 CrossRef CAS PubMed.
- I. S. X. Pinto, P. H. V. V. Pacheco, J. V. Coelho, E. Lorencon, J. D. Ardisson, J. D. Fabris, P. P. Souza, K. W. H. Krambrock, L. C. A. Oliveira and M. C. Pereira, Appl. Catal., B, 2012, 119, 175–182 CrossRef.
- X. Xue, K. Hanna, M. Abdelmoula and N. Deng, Appl. Catal., B, 2009, 89, 432–440 CrossRef CAS.
- K. Toda, T. Tanaka, Y. Tsuda, M. Ban, E. P. Koveke, M. Koinuma and S. I. Ohira, J. Hazard. Mater., 2014, 278, 426–432 CrossRef CAS PubMed.
- J. M. Bigham, U. Schwertmann, L. Carlson and E. Murad, Geochim. Cosmochim. Acta, 1990, 54, 2743–2758 CrossRef CAS.
- J. M. Bigham, L. Carlson and E. Murad, Mineral. Mag., 1994, 58, 641–648 CAS.
- Y. Liao, L. Zhou, J. Liang and H. Xiong, Mater. Sci. Eng., C, 2009, 29, 211–215 CrossRef CAS.
- J. M. Bigham, U. Schwertmann, S. J. Traina, R. L. Winland and M. Wolf, Geochim. Cosmochim. Acta, 1996, 60, 2111–2121 CrossRef CAS.
- S. Regenspurg and S. Peiffer, Appl. Geochem., 2005, 20, 1226–1239 CrossRef CAS.
- S. Regenspurg, A. Brand and S. Peiffer, Geochim. Cosmochim. Acta, 2004, 68, 1185–1197 CrossRef CAS.
- S. Paikaray, J. Göttlicher and S. Peiffer, Chem. Geol., 2011, 283, 134–142 CrossRef CAS.
- F. Liu, J. Zhou, S. Zhang, L. Liu, L. Zhou and W. Fan, PLoS One, 2015, 10, e0138891 Search PubMed.
- Z. Li, J. Liang, S. Bai and L. Zhou, Acta Sci. Circumstantiae, 2011, 31, 460–467 CAS.
- H. Duan, Y. Liu, X. Yin, J. Bai and J. Qi, Chem. Eng. J., 2016, 283, 873–879 CrossRef CAS.
- W.-M. Wang, J. Song and X. Han, J. Hazard. Mater., 2013, 262, 412–419 CrossRef CAS PubMed.
- L. Xu and J. Wang, Appl. Catal., B, 2012, 123, 117–126 CrossRef.
- B. A. Manning, S. E. Fendorf and S. Goldberg, Environ. Sci. Technol., 1998, 32, 2383–2388 CrossRef CAS.
- P. Nkedi-Kizza, P. S. C. Rao and A. G. Hornsby, Environ. Sci. Technol., 1985, 19, 975–979 CrossRef CAS.
- Z.-R. Lin, L. Zhao and Y.-H. Dong, Chemosphere, 2015, 141, 7–12 CrossRef CAS PubMed.
- C. P. Huang and Y. H. Huang, Appl. Catal., A, 2008, 346, 140–148 CrossRef CAS.
- T. Rhadfi, J. Y. Piquemal, L. Sicard, F. Herbst, E. Briot, M. Benedetti and A. Atlamsani, Appl. Catal., A, 2010, 386, 132–139 CrossRef CAS.
- A. A. Burbano, D. D. Dionysiou, M. T. Suidan and T. L. Richardson, Water Res., 2005, 39, 107–118 CrossRef CAS PubMed.
- A. Georgi, A. Schierz, U. Trommler, C. P. Horwitz, T. J. Collins and F. D. Kopinke, Appl. Catal., B, 2007, 72, 26–36 CrossRef CAS.
- I. A. Katsoyiannis, T. Ruettimann and S. J. Hug, Environ. Sci. Technol., 2008, 42, 7424–7430 CrossRef CAS PubMed.
- Z. Xu, J. Liang and L. Zhou, J. Alloys Compd., 2013, 546, 112–118 CrossRef CAS.
- H. S. Park, Y. C. Lee, B. G. Choi, Y. S. Choi, J. W. Wang and W. H. Hong, Small, 2010, 6, 290–295 CrossRef CAS PubMed.
- B. David and P. Boule, Chemosphere, 1993, 26, 1617–1630 CrossRef CAS.
- H. Jia, J. Zhao, X. Fan, K. Dilimulati and C. Wang, Appl. Catal., B, 2012, 123, 43–51 CrossRef.
- M. Yin, Z. Li, J. Kou and Z. Zou, Environ. Sci. Technol., 2009, 43, 8361–8366 CrossRef CAS PubMed.
- O. T. Woo, W. K. Chung, K. H. Wong, A. T. Chow and P. K. Wong, J. Hazard. Mater., 2009, 168, 1192–1199 CrossRef CAS PubMed.
- T. Cajthaml, P. Erbanová, V. Šašek and M. Moeder, Chemosphere, 2006, 64, 560–564 CrossRef CAS PubMed.
- W. Luo, L. H. Zhu, N. Wang, H. Q. Tang, M. J. Cao and Y. B. She, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- H. Gallard and J. D. Laat, Water Res., 2000, 34, 3107–3116 CrossRef CAS.
- Y. Liu, A. Zhou, Y. Gan and X. Li, J. Hazard. Mater., 2016, 308, 187–191 CrossRef CAS PubMed.
- J. D. Laat and T. G. Le, Appl. Catal., B, 2006, 66, 137–146 CrossRef.
- S. Sabhi and J. Kiwi, Water Res., 2001, 35, 1994–2002 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02713c |
| This journal is © The Royal Society of Chemistry 2017 |