Open Access Article
Open Access ArticleOne pot synthesis of unusual meso-dipyrrinyl corrole†
Laxman Vamshi Krishna Kandala ,
Tejinder Kaur and
Mangalampalli Ravikanth
,
Tejinder Kaur and
Mangalampalli Ravikanth *
*
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India. E-mail: ravikanth@chem.iitb.ac.in
First published on 4th April 2017
Abstract
We have synthesized an unusual meso-dipyrrinyl corrole 1 in one pot reaction using 2 + 1 approach, by condensing pentafluorobenzaldehyde with meso-free dipyrromethane under TFA catalyzed reaction conditions followed by oxidation with a mild oxidant such as p-chloranil. The product was not obtained when we used other aldehydes such as p-nitrobenzaldehyde and p-cyanobenzaldehyde as well as when we changed the oxidant from p-chloranil to DDQ. We elucidated the molecular structure of 1 using detailed 1D and 2D NMR spectroscopy. The absorption spectrum showed typical Soret band and three Q-bands with slight shifts in the peak maxima compared to meso-free corrole. The meso-dipyrrinyl corrole was decently fluorescent and showed emission band at 678 nm. The preliminary study showed that the meso-free carbon of meso-dipyrrinyl corrole was sufficiently reactive for functionalization but the resulted functionalized meso-dipyrrinyl corroles were not stable to isolate. Our attempts to prepare metal complexes of meso-dipyrrinyl corroles remained unsuccessful because of the unstable nature of the resulted coordination complexes.
Introduction
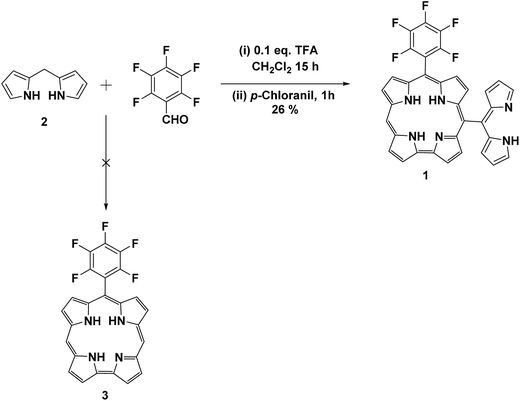
Corroles are tetrapyrrolic aromatic macrocyclic compounds with a direct pyrrole–pyrrole linkage and have one less carbon in their macrocycle compared to tetrapyrrolic porphyrins.1 These macrocycles have a smaller cavity than porphyrins because of the direct pyrrole–pyrrole linkage. Due to one less carbon present in corroles, their symmetry is reduced from D4h as in porphyrins to C2v. Thus, corroles are aromatic 18-π systems like porphyrins and exhibit intense absorptions in the visible region.1 Corroles have four nitrogen atoms in their inner ring, out of which three are of pyrrolic type and one is pyridine type nitrogen. Due to presence of three inner –NH hydrogen atoms in the ring unlike porphyrins which have two ionizable NH atoms, corroles are known to stabilize metals in higher oxidation states compared to their counterpart porphyrins.2 Corroles uniquely have higher acidity as compared to other macrocycles. Corroles have very interesting spectral, photochemical and luminescence properties3 which have potential applications in various fields such as dye sensitized solar cells,4 photodynamic therapy,5 energy transfer systems6 and organic catalysts.7 Inspite of their novel features, the chemistry of corroles are relatively less developed compared to their porphyrin analogues because of the difficulties encountered in the synthesis of corroles. However, in recent past the research on the development of corrole chemistry has gained momentum after the landmark discovery of one pot synthesis of meso-aryl corroles independently by Gross,8 Smith and Paolesse9 and their co-workers in 1999. Although, several methods are available to prepare meso-aryl corroles but the synthesis of meso-free corroles still remain challenging because of their unstable nature. The meso-free carbon of meso-free corroles is very reactive and can be functionalized easily to prepare the meso-functionalized corroles which are useful building blocks to construct large delocalized π-conjugated systems. This area of research is however less explored as there are scarce synthetic protocols for meso-free corroles and these corroles are quite unstable for further chemistry. Herein we report, one pot facile synthesis of meso-dipyrrinyl corrole containing one free meso-carbon using readily available precursors under simple reaction conditions. The meso-free carbon is highly reactive and readily undergoes functionalization but the resulted functionalized meso-dipyrrinyl corroles were found to be very unstable to isolate. The metalation of corrole was also led to decomposition of corrole macrocycle.Experimental section
General
THF and toluene were dried over sodium benzophenone ketyl, BF3·OEt2, p-chloranil and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone(DDQ) obtained from Spectrochem (India) were used as obtained. All other chemicals used for the synthesis were reagent grade unless otherwise specified. Column chromatography was performed on silica (60–120 mesh). 1H NMR spectra (δ in ppm) were recorded using Bruker 400 MHz spectrometer. 13C NMR spectra were recorded on Bruker operating at 101 MHz. TMS was used as an internal reference for recording 1H (of residual proton; δ 7.26) in CDCl3 and 13C (of residual carbon; δ 39.51 signal) in DMSO-d6. Absorption and steady-state fluorescence spectra were obtained with Varian and PC1 Photon Counting Spectrofluorometer manufactured by ISS, USA instruments respectively. Fluorescence spectra were recorded at 25 °C in a 1 cm quartz fluorescence cuvette. The fluorescence quantum yields (ϕf) were estimated from the emission and absorption spectra by comparative method at the excitation wavelength of 440 nm using H2TPP (ϕf = 0.11) as standard.10 The time-resolved fluorescence decay measurements were carried out at the magic angle using a picosecond-diode-laser-based, time-correlated, single-photon-counting (TCSPC) fluorescence spectrometer from IBH, UK. All the decays were fitted to single exponential unless specified. The HRMS spectra were recorded with Bruker maxis impact 282001.00081 using electron spray ionization method and TOF analyser.Synthesis of 1
Samples of pentafluorobenzaldehyde (200 mg, 1.02 mmol) and meso-free dipyrromethane (596 mg, 4.08 mmol) was dissolved in dichloromethane and stirred under nitrogen atmosphere for 5 min. Followed by, addition of catalytic amount of TFA (31 μL, 0.41 mmol) and stirring was continued for 15 h in inert atmosphere. The oxidant p-chloranil was added and stirring was continued for additional 1 h in open air. Solvent was removed under reduced pressure and the crude reaction mixture was subjected to silica gel column chromatographic purification. The compound 1 was eluted using petroleum ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford the compound in 26% yield as green solid (161 mg); 1H NMR (400 MHz, chloroform-d) δ 10.24 (s, 1H), 9.36 (d, 3JHH = 4.7 Hz, 2H), 9.27 (d, 3JHH = 4.6 Hz, 2H), 9.24 (d, 3JHH = 4.8 Hz, 2H), 8.81 (d, 3JHH = 4.8 Hz, 2H), 7.44 (m, 2H), 7.25 (m, 2H) 6.82 (m, 2H). 13C NMR (101 MHz, DMSO) δ 144.97, 132.78, 132.34, 131.48, 130.32, 130.08, 121.11, 116.61, 112.49, 109.14, 107.45, 29.00. HRMS (ESI): m/z (%): calculated for C34H20F5N6 is 607.1664 (M + H)+, found 607.1651 (M + H)+.
1) as eluent to afford the compound in 26% yield as green solid (161 mg); 1H NMR (400 MHz, chloroform-d) δ 10.24 (s, 1H), 9.36 (d, 3JHH = 4.7 Hz, 2H), 9.27 (d, 3JHH = 4.6 Hz, 2H), 9.24 (d, 3JHH = 4.8 Hz, 2H), 8.81 (d, 3JHH = 4.8 Hz, 2H), 7.44 (m, 2H), 7.25 (m, 2H) 6.82 (m, 2H). 13C NMR (101 MHz, DMSO) δ 144.97, 132.78, 132.34, 131.48, 130.32, 130.08, 121.11, 116.61, 112.49, 109.14, 107.45, 29.00. HRMS (ESI): m/z (%): calculated for C34H20F5N6 is 607.1664 (M + H)+, found 607.1651 (M + H)+.
Results and discussion
The meso-dipyrrinyl corrole containing one free meso-carbon 1 is synthesized as shown in Scheme 1. The required precursor, the meso-unsubstituted dipyrromethane 2 was prepared in multigram quantity by following the reported method.11To prepare the meso-free corrole 3, we initially carried out the condensation by taking 2.5 equivalents of meso-unsubstituted dipyrromethane 2 with one equivalent of pentafluorobenzaldehyde in CH2Cl2 in the presence of catalytic amount of trifluoroacetic acid under nitrogen atmosphere for 3 h followed by oxidation with p-chloranil in open air for additional 1 h. The TLC analysis and absorption spectroscopy indicated the formation of corrole which we assumed to be the meso-free corrole 3. The crude reaction mixture was subjected to silica gel column chromatography and afforded the corrole as green solid in ∼5% yield. The high-resolution mass spectrometry showed a molecular ion peak at 607.1651 which does not correspond to the expected corrole 3 but corresponds to the meso-dipyrrinyl substituted corrole containing one meso-free carbon 1 (Fig. 1a) which we confirmed later by detailed NMR studies (vide infra). We repeated the reaction several times under same conditions to check the reproducibility of the product. To optimize the reaction conditions, we also varied the number of equivalents of meso-unsubstituted dipyrromethane 2, the acid catalyst and oxidant. The best yields of 26% were obtained when we condensed four equivalents of meso-free dipyrromethane 2 with one equivalent of pentafluorobenzaldehyde in CH2Cl2 in the presence of 0.1 equivalent of trifluoroacetic acid under nitrogen atmosphere followed by oxidation with three equivalents of p-chloranil in open air. Interestingly, when we used DDQ as an oxidant, we did not see the formation of corrole 1 indicating that the corrole formed was probably decomposed in the reaction mixture. Thus, only p-chloranil as an oxidant resulted in the formation of meso-dipyrrinyl corrole 1. We also varied the acid catalyst from 0.1 equiv. of TFA to 0.1 equiv. of BF3·Et2O and kept the other reaction conditions constant. Under these reaction conditions, the compound 1 was obtained in 6–8% yield along with traces of meso free porphyrin which was also formed. Furthermore, we also changed the electron withdrawing pentafluorobenzaldehyde to 4-nitrobenzaldehyde and 4-cyanobenzaldehyde, but we could not isolate the corresponding meso-dipyrrinyl substituted corrole.
We made several unsuccessful attempts to obtain the crystal structure of compound 1. However, the molecular structure of compound 1 was deduced by detailed 1D and 2D NMR studies. The 1H NMR spectrum of compound 1 is presented in Fig. 1 whereas 1H–1H COSY and 1H–1H NOESY NMR spectra of compound 1 are presented in Fig. 2. The 1H NMR spectrum showed one singlet in the highly downfield region at 10.24 ppm corresponding to the meso proton. The eight β-pyrrole protons appeared as four sets of doublets at 9.36, 9.27, 9.24 and 8.81 ppm. The six protons of the meso-dipyrromethenyl moiety appeared as multiplets at 7.44, 7.25 and 6.82 ppm corresponding to two protons each and the NH proton of meso-dipyrromethenyl unit appeared as broad resonance at 9.32 ppm. All these protons were identified and assigned based on location, integration, cross-peak correlations in 1H–1H COSY and NOESY spectroscopy. The singlet at 10.24 ppm corresponding to the meso proton (type m) showed cross peak correlation with a doublet resonance at 9.36 ppm (Fig. 2a), which we assigned as type a pyrrole protons. The type a protons in turn showed cross peak correlation with a doublet resonance at 9.27 ppm, which we assigned as type b pyrrole protons. The resonance at 9.32 ppm corresponding to NH proton of dipyrromethene moiety showed NOE connectivity with the multiplet at 7.44 ppm (Fig. 2b), which we identified as type g protons.
The type g protons showed cross peak correlation with multiplet at 6.82 ppm, which was assigned as type f protons. The type f showed cross peak correlation with multiplet at 7.25 ppm, which was assigned as type e proton. As we didn't observe any NOE connectivity with the type e proton, out of two doublet resonances observed at 8.81 ppm or 9.24 ppm, we tentatively assigned the doublet at 8.81 ppm as type c resonance, as it is placed nearer to the electron withdrawing meso-pentafluoro phenyl group and the resonance at 9.24 ppm was assigned to type d protons. Thus, 1D and 2D NMR helped in deducing the molecular structure of compound 1. We also recorded 1H NMR spectrum of compound in polar solvent such as DMSO-d6 in which all protons of compound 1 experienced slight shifts compared to CDCl3 (Fig. 3). However, the dipyrromethene NH of compound 1 experienced significant downfield shift (3 ppm) in DMSO-d6 and appeared at 12.4 ppm due to strong hydrogen bonding interaction between compound 1 and solvent. We attempted to record the protonated 1H NMR spectrum of compound 1 by adding a drop of trifluoroacetic acid. The resulted protonated corrole 1.2H2+ showed broad resonances in 1H NMR but the number of resonances were remained same and experienced slight shifts. For example, the dipyrrin NH proton of compound 1 which appeared as broad singlet at 9.3 ppm in CDCl3 was shifted to downfield and appeared at 10.8 ppm. However, we did not observe the inner NH protons of corrole ring of 1 and its protonated derivative 1.2H2+ in 1H NMR at room temperature as well as at low temperature (−40 °C). We did not record at further low temperature due to lack of facility.
 | ||
| Fig. 3 (i) 1H NMR spectra of 1 recorded in DMSO-d6 and (ii) CDCl3 at RT and (iii) 1H NMR spectrum of 1 recorded after protonation (with TFA) in CDCl3 at −40 °C. | ||
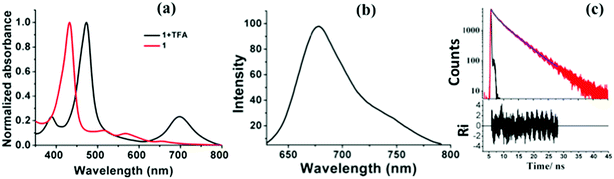
The absorption properties of compound 1 were studied in five different solvents and the relevant data is presented in Table 1. The comparison of absorption spectra of compound 1 and its protonated derivative 1.2H2+ recorded in CH2Cl2 is shown in Fig. 4a. The compound 1 showed strong Soret band at 432 nm and three ill defined Q-bands at 516 nm, 567 nm and 667 nm. Upon protonation by addition of dilute solution of TFA to compound 1, the resulted protonated compound 1.2H2+ showed bathochromically shifted Soret band at 472 nm and one broad Q-band at 699 nm (Fig. 4a).
| Solvent | λabs in nm (ε in L mol−1 cm−1) | ϕf | Lifetime (relative amplitudes) | ||||
|---|---|---|---|---|---|---|---|
| τ1 in ns | τ2 in ns | ||||||
| THF | 431 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544) 544) |
513 (2821) | 571 (2447) | 656 (1385) | 0.129 | 6.57 (100) | — |
| Toluene | 432 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 297) 297) |
514 (2154) | 567 (1824) | 657 (960) | 0.096 | 5.95 (100) | — |
| Chloroform | 430 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022) 022) |
514 (3173) | 573 (2601) | 653 (1524) | 0.069 | 5.52 (82) | 1.90 (18) |
| ACN | 426 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522) 522) |
512 (1912) | 568 (1685) | 655 (887) | 0.059 | 6.31 (86) | 1.30 (14) |
| DMSO | 415 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509) 509) |
511 (2441) | 578 (2183) | 656 (1326) | 0.057 | 4.32 (92) | 0.52 (8) |
The change of solvent from non-polar to polar, the compound 1 exhibited slight shifts in the peak maxima with decrease in extinction coefficients and maximum effects were observed in DMSO.
The fluorescence properties of compound 1 were studied in different solvents by both steady state and time resolved fluorescence techniques and the relevant data is included in Table 1. The fluorescence spectrum recorded in CH2Cl2 is shown in Fig. 4b and the fluorescence decay profile is shown in Fig. 4c. Compound 1 is decently fluorescent and showed one broad emission band at 678 nm with a quantum yield of 0.07. The change of solvent resulted in slight changes in the fluorescence peak maxima and quantum yield. For example, in DMSO, the compound 1 showed one broad emission band at 676 nm with quantum yield of 0.057. The fluorescence decay was fitted to either mono-exponential or bi-exponential and the contribution of second component is less. The observation of two lifetimes for compound 1 in solvents such as DMSO indicates that the compound 1 may be existing in two tautomers and one is predominant over the other as described by Gros, Harvey and co-workers.12 A detailed photophysical studies are required to understand the excited state dynamics of compound 1.
The reactivity of free meso position and the α-positions of the meso-dipyrromethene unit of 1 was tested by carrying out functionalization reactions such as formylation and bromination. In both the cases, the TLC analyses and absorption spectroscopy gave clear indication of the formation of new functionalized products. But we could not isolate the products as the compounds decomposed. Furthermore, we also explored the coordination properties of 1 since the corroles are known to form interesting coordination complexes. We tried phosphorus insertion by reacting compound 1 with POCl3 in pyridine.13 The reaction was completed in 10 min as indicated by color change from green to deep red and the formation was confirmed by mass spectrometry and absorption spectroscopy. However, our attempts to obtain pure phosphorus corrole was unsuccessful as the complex was decomposed. We also tried Pd(II), Co(III) and BF2 complexation, but in all these cases, we could not isolate the corresponding stable pure compound. Further attempts are underway to prepare the coordination complexes of corrole 1.
Conclusion
In conclusion, we synthesized an unusual meso-dipyrrinyl corrole 1 in good yield by TFA catalyzed condensation of pentafluoro benzaldehyde with meso-free dipyrromethane followed by the oxidation with p-chloranil. The unusual meso dipyrrinyl corrole was formed only when we used pentafluorobenzaldehyde as one of the reactant but using the other aldehydes such as 4-nitrobenzaldehyde and 4-cyanobenzaldehyde, the corrole formation was not observed. We also noted that only p-chloranil as an oxidant is compulsory to afford the product whereas the strong oxidizing agent such as DDQ led to decomposition of the product. The meso-dipyrrinyl corrole is quite stable and exhibited interesting absorption and fluorescence properties. The reactivity of the free meso position was tested by subjecting the meso-dipyrrinyl corrole for formylation and bromination reaction conditions. The preliminary results indicated that free meso position was sufficiently reactive to introduce the functional groups such as formyl and bromo groups but the resulted functionalized meso-dipyrrinyl corroles were not sufficiently stable to isolate. The meso-dipyrrinyl corrole also showed an ability to form complexes with metals and non-metals but the resulted complexes decompose during purification or recrystallization. The functionalization and coordination chemistry of such novel unusual meso-dipyrrinyl corrole is presently under investigation in our laboratory.Acknowledgements
We thank Department of Science and Technology, India, IITB-Monash Research Academy and IRCC IIT Bombay for financial support. We thank Mr Sunit Kumar for his help during experimentation.References
- (a) I. Aviv and Z. Gross, Corrole-based applications, Chem. Commun., 2007, 20, 1987–1999 RSC; (b) L. Flamigni and D. T. Gryko, Photoactive corrole-based arrays, Chem. Soc. Rev., 2009, 38, 1635–1646 RSC; (c) T. D. Gryko, Adventures in the synthesis of meso-substituted corroles, J. Porphyrins Phthalocyanines, 2008, 12, 906–917 CrossRef; (d) S. Nardis, D. Monti and R. Paolesse, Novel Aspects of Corrole Chemistry, Mini-Rev. Org. Chem., 2005, 2, 355–374 CrossRef CAS; (e) R. Paolesse, Corrole: The Little Big Porphyrinoid, Synlett, 2008, 15, 2215–2230 CrossRef; (f) D. T. Gryko, Recent Advances in the Synthesis of Corroles and Core-Modified Corroles, Eur. J. Org. Chem., 2002, 1735–1743 CrossRef CAS; (g) Z. Gross and H. B. Gray, Oxidations Catalyzed by Metallocorroles, Adv. Synth. Catal., 2004, 346, 165–170 CrossRef CAS; (h) J. F. B. Barata, M. G. P. M. S. Neves, A. C. Tome and J. A. S. Cavaleiro, Synthesis of new tetrapyrrolic derivatives-porphyrins as dienophiles or dipolarophiles, J. Porphyrins Phthalocyanines, 2009, 13, 532–537 CrossRef; (i) D. T. Gryko, J. P. Fox and D. P. Goldberg, Recent advances in the chemistry of corroles and core-modified corroles, J. Porphyrins Phthalocyanines, 2004, 8, 1091–1105 CrossRef CAS; (j) D. P. Goldberg, Corrolazines: New Frontiers in High-Valent Metalloporphyrinoid Stability and Reactivity, Acc. Chem. Res., 2007, 40, 626–634 CrossRef CAS PubMed; (k) A. W. Johnson and I. T. Kay, Corroles. Part I. Synthesis, J. Chem. Soc., 1965, 1620–1629 RSC; (l) N. S. Genokhova, T. A. Melent'eva and V. M. Berezovskii, Synthesis of Corroles and Octadehydrocorrins, Russ. Chem. Rev., 1980, 49, 1056–1067 CrossRef; (m) T. A. Melent'eva, The Structure and Reactivity of Octadehydrocorrins and Corroles, Russ. Chem. Rev., 1983, 52, 641–661 CrossRef; (n) C. Erben, S. Will and K. M. Kadish, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, Burlington, MA, 2000, vol. 2, p. 233 Search PubMed; (o) R. Paolesse, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, Burlington, MA, 2000, vol. 2, p. 201 Search PubMed; (p) S. Licoccia and R. Paolesse, Struct. Bonding, 1995, 84, 71 CrossRef CAS.
- (a) A. Ghosh, T. Wondimagegn and A. B. J. Parusel, Electronic Structure of Gallium, Copper, and Nickel Complexes of Corrole. High-Valent Transition Metal Centers versus Noninnocent Ligands, J. Am. Chem. Soc., 2000, 122, 5100–5104 CrossRef CAS; (b) A. Ghosh and E. Steene, High-valent transition metal centers and noninnocent ligands in metalloporphyrins and related molecules: a broad overview based on quantum chemical calculations, J. Biol. Inorg Chem., 2001, 6, 739–752 CrossRef CAS PubMed; (c) G. Golubkov, J. Bendix, H. B. Gray, A. Mahammed, I. Goldberg, A. J. DiBilio and Z. Gross, High-Valent Manganese Corroles and the First Perhalogenated Metallocorrole Catalyst, Angew. Chem., Int. Ed., 2001, 40, 2132–2134 CrossRef CAS; (d) I. H. Wasbotten, T. Wondimagegn and A. Ghosh, Electronic Absorption, Resonance Raman, and Electrochemical Studies of Planar and Saddled Copper(III) meso-Triarylcorroles. Highly Substituent-Sensitive Soret Bands as a Distinctive Feature of High-Valent Transition Metal Corroles, J. Am. Chem. Soc., 2002, 124, 8104–8116 CrossRef CAS PubMed; (e) Z. Gross, L. Simkhovich and N. Galili, First catalysis by corrole metal complexes: epoxidation, hydroxylation, and cyclopropanation, Chem. Commun., 1999, 599–600 RSC; (f) Z. Gross, G. Golubkov and L. Simkhovich, Epoxidation Catalysis by a Manganese Corrole and Isolation of an Oxomanganese(V) Corrole, Angew. Chem., Int. Ed., 2000, 39, 4045–4047 CrossRef CAS; (g) A. E. Meier-Callahan, A. J. Di Bilio, L. Simkhovich, A. Mahammed, I. Goldberg, H. B. Gray and Z. Gross, Chromium Corroles in Four Oxidation States, Inorg. Chem., 2001, 40, 6788–6793 CrossRef CAS PubMed; (h) L. Simkhovich and Z. Gross, Iron(IV) corroles are potent catalysts for aziridination of olefins by chloramine-T, Tetrahedron Lett., 2001, 42, 8089–8092 CrossRef CAS; (i) J. Bendix, I. J. Dmochowski, H. B. Gray, A. Mahammed, L. Simkhovich and Z. Gross, Structural, Electrochemical, and Photophysical Properties of Gallium(III) 5,10,15-Tris(pentafluorophenyl)corrole, Angew. Chem., Int. Ed., 2000, 39, 4048–4051 CrossRef CAS.
- (a) T. Ding, E. A. Alemán, D. A. Modarelli and C. J. Ziegler, Photophysical Properties of a Series of Free-Base Corroles, J. Phys. Chem. A, 2005, 109, 7411–7417 CrossRef CAS PubMed; (b) B. Ventura, A. D. Esposti, B. Koszarna, D. T. Gryko and L. Flamigni, Photophysical characterization of free-base corroles, promising chromophores for light energy conversion and singlet oxygen generation, New J. Chem., 2005, 29, 1559–1566 RSC; (c) J. H. Palmer, A. C. Durrell, Z. Gross, J. R. Winkler and H. B. Gray, Near-IR Phosphorescence of Iridium(III) Corroles at Ambient Temperature, J. Am. Chem. Soc., 2010, 132, 9230–9231 CrossRef CAS PubMed; (d) J. Vestfrid, M. Botoshansky, J. H. Palmer, A. C. Durrell, H. B. Gray and Z. Gross, Iodinated Aluminum(III) Corroles with Long-Lived Triplet Excited States, J. Am. Chem. Soc., 2011, 133, 12899–12901 CrossRef CAS PubMed; (e) J. H. Palmer, M. W. Day, A. D. Wilson, L. M. Henling, Z. Gross and H. B. Gray, Iridium Corroles, J. Am. Chem. Soc., 2008, 130, 7786–7787 CrossRef CAS PubMed; (f) J. H. Palmer, A. Mahammed, K. M. Lancaster, Z. Gross and H. B. Gray, Structures and Reactivity Patterns of Group 9 Metallocorroles, Inorg. Chem., 2009, 48, 9308–9315 CrossRef CAS PubMed; (g) J. Poulin, C. Stern, R. Guilard and P. D. Harvey, Photophysical Properties of a Rhodium Tetraphenylporphyrin–tin Corrole Dyad. The First Example of a Through Metal–Metal Bond Energy Transfer, Photochem. Photobiol., 2006, 82, 171–176 CrossRef CAS PubMed; (h) I. Aviv and Z. Gross, Coordination chemistry of corroles with focus on main group elements, Coord. Chem. Rev., 2011, 255, 717–736 CrossRef.
- (a) N. Kobayashi, T. Furuyama and K. Satoh, Rationally Designed Phthalocyanines Having Their Main Absorption Band beyond 1000 nm, J. Am. Chem. Soc., 2011, 133, 19642–19645 CrossRef CAS PubMed; (b) H. Isago and Y. Kagaya, Synthesis, Spectral, and Electrochemical Characterization of the First Arsenic(V)–Phthalocyanines, Inorg. Chem., 2012, 51, 8447–8454 CrossRef CAS PubMed; (c) B. J. Brennan, Y. C. Lam, P. M. Kim, X. Zhang and G. W. Brudvig, Photoelectrochemical Cells Utilizing Tunable Corroles, ACS Appl. Mater. Interfaces, 2015, 7, 16124–16130 CrossRef CAS PubMed.
- (a) J. O. Escobedo, O. Rusin, S. Lim and R. M. Strongin, NIR dyes for bioimaging applications, Curr. Opin. Chem. Biol., 2010, 14, 64–70 CrossRef CAS PubMed; (b) M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, The role of porphyrin chemistry in tumor imaging and photodynamic therapy, Chem. Soc. Rev., 2011, 40, 340–362 RSC; (c) M. K. Kumova, H. A. Collins, M. Balaz, E. Dahlsetdt, J. A. Levitt, N. Sergent, K. Suhling, M. Drobizhev, N. S. Markarov, A. Rebane, H. L. Anderson and D. Phillips, Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy, Org. Biomol. Chem., 2009, 7, 889–896 RSC.
- (a) B. Basumatary, A. R. Sekhar, R. V. R. Reddy and J. Sankar, Corrole-BODIPY Dyads: Synthesis, Structure, and Electrochemical and Photophysical Properties, Inorg. Chem., 2015, 54, 4257–4267 CrossRef CAS PubMed; (b) B. Brizet, N. Desbois, A. Bonnot, A. Langlois, A. Dubois, J.-M. Barbe, C. P. Gros, C. Goze, F. Denat and P. D. Harvey, Slow and Fast Singlet Energy Transfers in BODIPY-gallium(III)corrole Dyads Linked by Flexible Chains, Inorg. Chem., 2014, 53, 3392–3403 CrossRef CAS PubMed; (c) H. R. A. Golf, A. M. Oltmanns, D. H. Trieu, H.-U. Reissig and A. Wiehe, Synthesis of Functionalized BODIPYs, BODIPY-Corrole, and BODIPY-Porphyrin Arrays with 1,2,3-Triazole Linkers Using the 4-Azido(tetrafluorophenyl)-BODIPY Building Block, Eur. J. Org. Chem., 2015, 4224–4237 CrossRef CAS.
- (a) A. Mahammed and Z. Gross, Metallocorroles as Photocatalysts for Driving Endergonic Reactions, Exemplified by Bromide to Bromine Conversion, Angew. Chem., Int. Ed., 2015, 54, 12370–12373 CrossRef CAS PubMed; (b) N. Levy, A. Mahammed, M. Kosa, D. T. Major, Z. Gross and L. Elbaz, Metallocorroles as Nonprecious-Metal Catalysts for Oxygen Reduction, Angew. Chem., Int. Ed., 2015, 54, 14080–14084 CrossRef CAS PubMed; (c) C. Herrero, A. Qauaranta, R. Ricouz, A. Trehoux, A. Mahammed, Z. Gross, F. Banse and J.-P. Mahy, Oxidation catalysis via visible-light water activation of a [Ru(bpy)3]2+ chromophore BSA-metallocorrole couple, Dalton Trans., 2016, 45, 706–710 RSC.
-
(a) Z. Gross, N. Galili and I. Saltsman, The First Direct Synthesis of Corroles from Pyrrole, Angew. Chem. Int. Ed., 1999, 38, 1427–1429 CrossRef CAS;
(b) Z. Gross, N. Galili, L. Simkhovich, I. Saltsman, M. Botoshansky, D. Blaser, R. Boese and I. Goldberg, Solvent-Free Condensation of Pyrrole and Pentafluorobenzaldehyde:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Novel Synthetic Pathway to Corrole and Oligopyrromethenes, Org. Lett., 1999, 1, 599–602 CrossRef CAS.
A Novel Synthetic Pathway to Corrole and Oligopyrromethenes, Org. Lett., 1999, 1, 599–602 CrossRef CAS. - R. Paolesse, L. Jaquinod, D. J. Nurco, S. Mini, F. Sagoni, T. Boschi and K. M. Smith, 5,10,15-Triphenylcorrole: a product from a modified Rothemund reaction, Chem. Commun., 1999, 1307–1308 RSC.
- M. Goutermann, in The Porphyrins, ed. D. Dolphin, Academic Press, New York, 1978, vol. 3, ch. 1 Search PubMed.
- B. J. Littler, M. A. Miller, C.-H. Hung, R. W. Wagner, D. F. O'Shea, P. D. Boyle and J. S. Lindsey, Refined Synthesis of 5-Substituted Dipyrromethanes, J. Org. Chem., 1999, 64, 1391–1396 CrossRef CAS.
- A. Langlois, H.-J. Xu, P.-L. Karsenti, C. P. Gros and P. D. Harvey, Excited State N–H Tautomer Selectivity in the Singlet Energy Transfer of a Zinc(II)-Porphyrin–Truxene-Corrole Assembly, Chem.–Eur. J., 2017 DOI:10.1002/chem.201605909.
- (a) A. Ghosh and M. Ravikanth, Synthesis, Structure, Spectroscopic, and Electrochemical Properties of Highly Fluorescent Phosphorus(V)-meso-Triarylcorroles, Chem.–Eur. J., 2012, 18, 6386–6396 CrossRef CAS PubMed; (b) A. Ghosh, W.-Z. Lee and M. Ravikanth, Synthesis, Structure and Properties of a Five-Coordinate Oxophosphorus(V) meso-Triphenylcorrole, Eur. J. Inorg. Chem., 2012, 4231–4239 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c7ra02664a |
| This journal is © The Royal Society of Chemistry 2017 |