Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
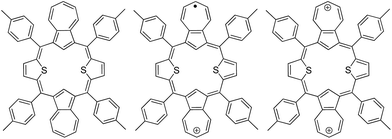
Retrieving aromaticity of dithiadiazuliporphyrin by oxidation: illustration by experimental and theoretical investigation†
Young Mo Sunga,
Natasza Spruttab,
Jun Oh Kima,
Yun Hee Kooa,
Lechosław Latos-Grażyński*b and
Dongho Kim *a
*a
aDepartment of Chemistry, Yonsei University, Seoul 120-749, Korea. E-mail: dongho@yonsei.ac.kr; Fax: +82-2-2123-2434; Tel: +82-2-2123-2652
bDepartment of Chemistry, University of Wrocław, 14 F. Joliot-Curie Street, 50-383, Wrocław, Poland. E-mail: lechoslaw.latos-grazynski@chem.uni.wroc.pl; Fax: +48-713-282-348; Tel: +48-71-3757256
First published on 3rd April 2017
Abstract
The photophysical properties of neutral, monocation radical, and dication of dithiadiazuliporphyrin have been examined with a particular focus on their aromaticity. Dithiadiazuliporphyrin exhibits significantly different characteristics depending on its oxidation state. From these features, we could conclude that the aromaticity of dithiadiazuliporphyrin is changed from nonaromatic to aromatic upon oxidation from neutral to monocation radical and dication by Br2.
Over the last several decades, porphyrinoids and porphyrin-related compounds have attracted considerable interest because of their fascinating properties, which can be used for diverse applications such as anion and cation sensing systems, nonlinear optical materials, magnetic materials, and near infrared (NIR) dyes.1 The stable aromatic and antiaromatic porphyrinoids have been easily prepared by a straightforward process, which can make them a good platform to realize versatile aromatic states including nonaromatic, Hückel aromatic and antiaromatic, and Möbius aromatic and antiaromatic states.1,2 Aromaticity is one of the most significant concepts in chemistry because it could determine the chemical and physical properties of molecules; aromatic species are more stable than nonaromatic compounds, while antiaromatic molecules are more unstable than nonaromatic compounds.3 In this regard, control of aromaticity in porphyrinoids has recently been in the limelight for chemists. To control aromaticity in porphyrinoids, various methods have been proposed including metalation, protonation, deprotonation, introduction of bridges, and the change in solvent polarity, temperature, and electronic state.4 Although there have been strenuous efforts to retrieve π-electron conjugation in porphyrinoids, only few studies have been conducted to find a direct effect of electronic oxidation in nonaromatic compounds.
Dithiadiazuliporphyrin5 synthesized by Sprutta et al. shows significantly different chemical properties from its dication species, which reflects closely their respective aromatic properties (Chart 1). Although the basic chemical properties of dithiadiazuliporphyrin depending on its oxidation state were characterized in previous study, the detailed analysis for aromaticity such as photophysical properties and theoretical calculations has rarely been studied. In this regard, dithiadiazuliporphyrin could be the best candidate to unravel the change of aromaticity depending on its charged species, which provides a clue to control the aromaticity by oxidation. Accordingly, we have investigated the aromaticity of neutral, dication and in particular monocation radical, of dithiadiazuliporphyrin by using spectroscopic measurements and theoretical calculations. Moreover, the aromaticity of monocation radical is of a special interest as, because of electronic structure, it could not be addressed by standard NMR approach.5
The absorption spectrum of dithiadiazuliporphyrin exhibits two broad bands at 443 and 514 nm with smeared and weak tail in near infrared (NIR) region, which is close to the absorption spectra of typical nonaromatic porphyrinoids (Fig. 1). The absorption spectrum of dithiadiazuliporphyrin is changed twice upon addition of oxidant (Br2). The oxidant shows weak absorption spectrum in 350–600 nm, which could be negligible from the absorption spectra of dithiadiazuliporphyrins.6 First, adding three equivalents of Br2 to dithiadiazuliporphyrin solution, distinct bands appear at longer than 800 nm region in the absorption spectra, assigned as monocation radical form. Furthermore, adding more 4.4 equivalents of Br2, the absorption spectra exhibit decreased band in 800–1000 nm region with red-shifted and intensified bands at 580 and 1311 nm, indicative of formation of dication. The previous study5 revealed that the dication species of dithiadiazuliporphyrin exhibit up-field shift of inner protons in 1H-NMR spectra, which indicate the retrieved aromatic features in oxidized states. Contemplating that the dication species exhibit aromatic features in 1H-NMR and the absorption spectra of monocation radical and dication of dithiadiazuliporphyrins exhibit similar trends, those absorption spectra exhibiting relatively intense band in visible region and weak bands in NIR region could be easily rationalized by Gouterman's four orbital model.7 In other words, the absorption spectra of oxidized dithiadiazuliporphyrins resembles those of typical aromatic porphyrinoids. In this regard, we could assume that the oxidized forms of dithiadiazuliporphyrin are aromatic, while its neutral form is nonaromatic.
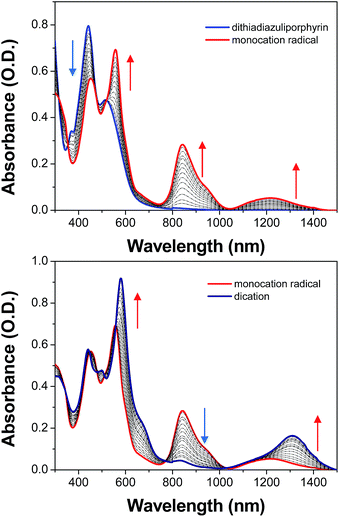 | ||
| Fig. 1 The absorption spectral change of dithiadiazuliporphyrin upon adding 0–3 equivalent (top) and 3–7.4 equivalent (bottom) of Br2. | ||
The characteristic aromatic features of oxidized dithiadiazuliporphyrins could also be observed in the excited state dynamics (Fig. 2). The femtosecond transient absorption (TA) spectra of neutral dithiadiazuliporphyrin show weak ground state bleaching with relatively strong excited state absorption. In addition, the decay profiles are fitted with double exponential function giving rise to two time constants of 0.6 and 27 ps. The excited state dynamics of neutral form is common features of nonaromatic porphyrinoids.8 Taking the origin of excited state dynamics in nonaromatic porphyrinoids into consideration, the fast decay process within 1 ps would be the internal conversion from high energy states to the lowest excited state, which is induced by the optically dark state in the low energy region. The relatively slow decay component (27 ps) could be assigned to the lowest singlet excited state lifetime. Contrary to this, the TA spectra of the oxidized species show strong ground state bleaching signal with relatively weak excited state absorption, which is the typical TA spectral features of aromatic porphyrinoids.8,9 Such macrocycles generally show weak excited state absorption with strong bleaching band due to the aromaticity reversal in the excited state.
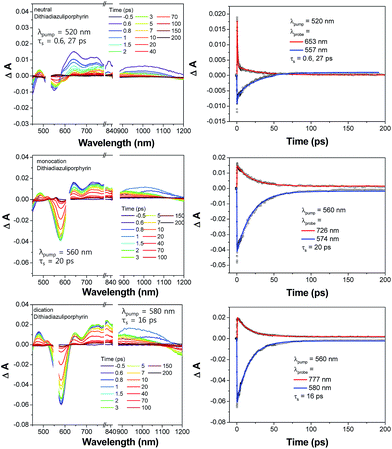 | ||
| Fig. 2 TA spectra of neutral (top), monocation radical (middle), and dication (bottom) of dithiadiazuliporphyrins. | ||
Aromaticity in the ground state of expanded porphyrins would be reversed in the lowest triplet or singlet excited state.9 Moreover, the absorption spectra of expanded porphyrins exhibit aromaticity-dependent behavior; the absorption spectra of aromatic expanded porphyrins show intense B-like bands with distinct Q-like bands, while those of antiaromatic expanded porphyrins present weak and broad bands in visible region with dark state in NIR region.8 In this regard, the transient absorption spectra of aromatic expanded porphyrins show strong ground state bleaching spectra originated from ground state aromatic behavior with weak excited state absorption caused by antiaromatic character in the excited state.
In addition, the single exponential decay process is typical in aromatic porphyrinoids due to their small energy gaps among the energy states which can induce fast internal conversion processes less than 100 fs. These results also represent that the aromatic features of dithiadiazuliporphyrins are generated by oxidation using Br2.
To understand the detailed photophysical properties observed in dithiadiazuliporphyrin upon oxidation with Br2, the vertical energy transitions were calculated (see the ESI†). In the case of neutral form, the lowest energy transition around 800 nm is a forbidden transition (HOMO–LUMO). Furthermore, the analysis of distribution of electron densities related to electron(s) placed on the frontier molecular orbitals (FMOs) reveals a preference for location on the selected sections but not on the entire ring, which is significantly different from aromatic porphyrinoids. These calculation results reflect the experimental features (broad absorption band with tail and double exponential decay process).8,9 On the contrary to neutral species, oxidized forms (mono- and dication) show different features. In the TD-DFT calculations, we couldn't observe the forbidden transition of oxidized dithiadiazuliporphyrins in the lowest energy region. Moreover, the frontier MOs of oxidized dithiadiazuliporphyrins exhibit porphyrin-like MO structures having fully localized electron densities in the entire ring, although the energy levels are not degenerate in HOMOs and LUMOs due to the expansion of π-conjugation to fused tropylium moieties in azulene. These results also support the retrieved aromaticity of oxidized dithiadiazuliporphyrins which induces the porphyrin-like absorption and single exponential decay process.
To get more detailed introduction on the aromaticity change in dithiadiazuliporphyin upon oxidation with Br2, several aromaticity indices were calculated (see the ESI†). The harmonic oscillator model of aromaticity (HOMA) values,10 the representative topological aromaticity indices, of a series of dithiadiazuliporphyrins were estimated. The estimated HOMA values increase in going from neutral dithiadiazuliporphyrin (0.301) to monocation radical (0.556) and dication forms (0.698), which implies that the aromaticity is enhanced in charged species. Thus, it could be deduced that the aromaticity is retrieved and enhanced upon oxidation from neutral form to monocation radical and dication species of dithiadiazuliporphyrin.
Furthermore, the magnetic aromaticity indices are consistent with topological aromaticity indices. The nucleus independent chemical shift (NICS)11–13 which is well-known as an absolute magnetic aromaticity index. In case of dithiadiazuliporphyrin and its oxidized forms the appropriate NICS values were calculated at the center of macrocyclic ring (see the ESI†). Although it is hard to observe the evidence for the aromaticity of monocation radical in 1H-NMR, NICS calculation provides clear features in aromaticity of radical species. The NICS(0) values of neutral, monocation radical, and dication of dithiadiazuliporphyrin were estimated to be 0.415, −2.881, and −6.592, respectively. More negative NICS(0) values in going from neutral to mono- and dication imply that the aromaticity of dithiadiazuliporphyrins changes from nonaromatic to aromatic, which are in line with the above experimental and theoretical results. In addition, the anisotropy of induced current density (ACID) plots,14 which illustrate the degree of aromaticity by the current flows of molecules in external magnetic fields, given for dithiadiazuliporphyrin and its cation show a similar tendency as shown by the calculated NICS values (Fig. 3). In the case of neutral species, the current flows are disconnected in the macrocyclic ring and localized on the local rings (azulene and thiophene). In the case of mono- and di-cation of dithiadiazuliporphyrins, however, both of the ACID plots display clockwise ring current flows in the macrocyclic ring, although thicker current densities which indicate stronger aromatic features are observed in dication form compared to those of monocation radical. In other words, the aromaticity of dithiadiazuliporphyrin is retrieved in monocation and enhanced in dication. Thus, all the calculation results would illustrate the retrieval of aromaticity in dithiadiazuliporphyrin upon oxidation by Br2.
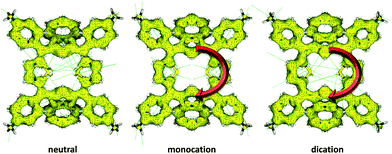 | ||
| Fig. 3 ACID plots of neutral (left), monocation radical (middle), and dication (right) of dithiadiazuliporphyrins. | ||
In summary, the photophysical properties and theoretical calculations of dithiadiazuliporphyrin, its monocation radical and dication have been investigated from a viewpoint of aromaticity. The changed absorption spectra and excited state dynamics in oxidized dithiadiazuliporphyrin accompanied by an alternation in theoretical aromaticity indices indicate the generation and enhancement of aromaticity in dithiadiazuliporphyrin resulting from oxidation. Furthermore, these results provide significant insight into rational redox control of an aromatic behavior.
Acknowledgements
The work at Yonsei University was supported by the Global Research Laboratory Program (GRL, 2013K1A1A2A0205183) funded by the Ministry of Science, ICT & Future, Korea. The quantum calculations were performed using the supercomputing resources of the Korea Institute of Science and Technology Information (KISTI). The work at the University of Wrocław was supported by National Science Centre (Grant Number: 2012/04/A/ST5/00593).Notes and references
- (a) J. L. Sessler and D. Seidel, Angew. Chem., Int. Ed., 2003, 42, 5134 CrossRef CAS PubMed; (b) M. Stępień, N. Sprutta and L. Latos-Grażyński, Angew. Chem., Int. Ed., 2011, 50, 4288 CrossRef PubMed; (c) S. Saito and A. Osuka, Angew. Chem., Int. Ed., 2011, 50, 4342 CrossRef CAS PubMed.
- (a) J.-Y. Shin, K. S. Kim, M.-C. Yoon, J. M. Lim, Z. S. Yoon, A. Osuka and D. Kim, Chem. Soc. Rev., 2010, 39, 2751 RSC; (b) S. Cho, Z. S. Yoon, K. S. Kim, M.-C. Yoon, D.-G. Cho, J. L. Sessler and D. Kim, J. Phys. Chem. Lett., 2010, 1, 895–900 CrossRef CAS.
- (a) V. I. Minkin, M. N. Glukhovtsev and B. Y. Simkin, Aromaticity and Antiaromaticity: Electronic and Structural Aspects, John Wiley & Sons, New York, 1994 Search PubMed; (b) M. K. Cyrański, Chem. Rev., 2005, 105, 3773 CrossRef PubMed.
- (a) M. Stepień, L. Latos-Grażyński, N. Sprutta, P. Chwalisz and L. Szterenberg, Angew. Chem., Int. Ed., 2007, 46, 7869 CrossRef PubMed; (b) J.-Y. Shin, L. J. Min, Z. S. Yoon, K. S. Kim, M.-C. Yoon, S. Hiroto, H. Shinokubo, H. Shimizu, A. Osuka and D. Kim, J. Phys. Chem. B, 2009, 113, 5794 CrossRef CAS PubMed; (c) A. Jana, M. Ishida, K. Cho, S. K. Ghosh, K. Kwak, K. Ohkubo, Y. M. Sung, C. M. Davis, V. M. Lynch, D. Lee, S. Fukuzumi, D. Kim and J. L. Sessler, Chem. Commun., 2013, 49, 8937 RSC; (d) Y. M. Sung, M.-C. Yoon, J. M. Lim, H. Rath, K. Naoda, A. Osuka and D. Kim, Nat. Chem., 2015, 7, 418 CrossRef CAS PubMed.
- N. Sprutta, M. Świderska and L. Latos-Grażyński, J. Am. Chem. Soc., 2005, 127, 13108 CrossRef CAS PubMed.
- S. Hubinger and J. B. Nee, J. Photochem. Photobiol., A, 1995, 86, 1–7 CrossRef CAS.
- M. Gouterman, G. H. Wagniere and L. C. Snyder, J. Mol. Spectrosc., 1963, 11, 108 CrossRef CAS.
- M.-C. Yoon, S. Cho, M. Suzuki, A. Osuka and D. Kim, J. Am. Chem. Soc., 2009, 131, 7360 CrossRef CAS PubMed.
- (a) Y. M. Sung, J. Oh, W. Kim, H. Mori, A. Osuka and D. Kim, J. Am. Chem. Soc., 2015, 137, 11856 CrossRef CAS PubMed; (b) J. Oh, Y. M. Sung, W. Kim, S. Mori, A. Osuka and D. Kim, Angew. Chem., Int. Ed., 2016, 55, 6487 CrossRef CAS PubMed; (c) Y. M. Sung, J. Oh, K. Naoda, T. Lee, W. Kim, M. Lim, A. Osuka and D. Kim, Angew. Chem., Int. Ed., 2016, 55, 11930 CrossRef CAS PubMed.
- (a) T. M. Krygowski and M. K. Cyrański, Tetrahedron, 1999, 55, 11143 CrossRef CAS; (b) T. M. Krygowski and M. K. Cyrański, Chem. Rev., 2001, 101, 1385 CrossRef CAS PubMed.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317 CrossRef CAS.
- V. Gogonea, P. v. R. Schleyer and P. R. Schreiner, Angew. Chem., Int. Ed., 1998, 37, 1945 CrossRef CAS.
- S. Suzuki, Y. Morita, K. Fukui, K. Sato, D. Shiomi, T. Takui and K. Nakasuji, J. Am. Chem. Soc., 2006, 128, 2530 CrossRef CAS PubMed.
- D. Geuenich, K. Hess, F. Köhler and R. Herges, Chem. Rev., 2005, 105, 3758 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02458d |
| This journal is © The Royal Society of Chemistry 2017 |