Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of ponasterone A by recombinant Escherichia coli harboring the glycosyltransferase GTBP1 with in situ product removal†
Bingfeng Liab,
Xuejun Heb,
Bo Fana,
Jianlin Chuac and
Bingfang He *ac
*ac
aCollege of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, China. E-mail: bingfanghe@njtech.edu.cn; Fax: +86 25 5813 9902; Tel: +86 25 5813 9902
bCollege of Biology and Environment, Nanjing Polytechnic Institute, China
cSchool of Pharmaceutical Sciences, Nanjing Tech University, China
First published on 26th April 2017
Abstract
A glycosyltransferase GTBP1 from Bacillus pumilus BF1 was isolated. Efficient production of ponasterone A was achieved by the recombinant E. coli/gtBP1 in a biphasic system with a molar yield of 92.7%. This in situ product removal provided the “driving force” for shifting the reaction equilibrium towards the synthesis of the product.
Ponasterone A, an insect-molting steroid hormone, was first isolated from the herb Podocarpus nakii HAY, a remedy for cancer.1 This compound was found to be an efficient inducer and to allow precise control of gene expression at specific times and with high tissue specificity.2,3 Ponasterone A-inducible wild-type p53 protein-expressing clones have been established for potential applications in cancer therapy.4 Recently, ponasterone A was found to be widely distributed in ferns5 such as Brainea insignis6 and Pteridiumaquilinum var. Latiusculum.7 However, the very low content (about 0.0096 mg g−1 in the rhizomes of Brainea insignis)6 and complex molecular structure of this effective component contained in medicinal plants have made its purification and synthesis particularly challenging. The chemical synthesis of ponasterone A from 20-hydroxyecdysone with multiple complicated reactions was investigated, with total yields of only 2.1%.8
Ponasteroside A (ponasterone A 3-β-D-glucopyranoside) is the glycoside of ponasterone A,9 which is abundant in Brainea insignis.6 Otaka reported that the stimulatory effects of ponasteroside A on protein expression in mice were lower than those of ponasterone A.10 A biocatalytic approach was shown to be an efficient strategy for steroid preparation from the steroidal glycoside owing to its high selectivity, mild reaction conditions, and environmental compatibility. Diosgenin could be produced through biotransformation of Dioscorea zingiberensis11 or through biotransformation of zingiberen newsaponin.12 Additionally, dioscin could be prepared through biotransformation of steroidal saponins, and progenin III (prosapogenin A of dioscin) could be prepared through biotransformation of dioscin.13 To the best of our knowledge, there are no reports on the enzymatic preparation of ponasterone A.
In this study, we reported the enzymatic preparation of ponasterone A from ponasteroside A for the first time. Bacillus pumilus BF1, a newly isolated bacterium, showed high activity in converting ponasteroside A to ponasterone A. The glycosyltransferase GTBP1 was cloned and expressed efficiently in E. coli BL21. With the strategy of in situ product removal (ISPR), efficient synthesis of ponasterone A catalyzed by recombinant E. coli/gtBP1 was successfully achieved. The successive production of ponasterone A was also discussed.
Ponasteroside A was reported to be abundant in the rhizomes of Brainea insignis6 and used as the main carbon source in screening medium. To obtain the target microbes, soil samples were collected from Brainea insignis gardens. Approximately 36 strains with the ability to biotransform ponasteroside A were screened from 200 samples. The strain BF1 was selected for further research because of its high transformation efficiency, and Bacillus pumilus was identified based on the 99% homology of 16S rDNA with that of B. pumilus MTCC B6033.
The effects of various inhibitors on the deglycosylation of ponasteroside A suggested that a glycosyltransferase (GTBP1) from Bacillus pumilus BF1 was responsible for the deglycosylation of ponasteroside A. The sequence of GTBP1 from Bacillus pumilus BF1 was cloned based on that of the glycosyltransferase in the typical strain Bacillus pumilus MTCC B6033. The open reading frame (ORF) of GTBP1 from Bacillus pumilus BF1 consisted of 1170 bp and encoded for 390 amino acid residues (accession number: KX523795.1). GTBP1 shared 99% homology with the putative glycosyltransferase (accession number: AHL72923.1) from Bacillus pumilus MTCC B6033 and showed 35.1%, 28.7%, 30.4% and 30.4% similarity with the glycosyltransferases CalG1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 (accession number: AAM70336), GtfD14 (accession number: WP_037311049), GtfE14 (accession number: AAB49299), and PcOGT15 (accession number: FJ854496) respectively.
14 (accession number: AAM70336), GtfD14 (accession number: WP_037311049), GtfE14 (accession number: AAB49299), and PcOGT15 (accession number: FJ854496) respectively.
The supernatant of recombinant E. coli BL21/pET-28a-gtBP1 (abbreviated as E. coli/gtBP1) lysate was analyzed by SDS-PAGE, resulting in a prominent band with an apparent molecular weight of about 47 kDa (Fig. S4, ESI†), consistent with the fusion of GTBP1 with a His-Tag at the N-terminus. The supernatant of the lysate exhibited the ability for deglycosylation of ponasteroside A into ponasterone A with the addition of UDP, whereas no bioconversion occurred using the supernatants of E. coli/pET-28alysates.
Glycosyltransferases (GTs), an essential class of ubiquitous enzymes, are generally perceived as unidirectional catalysts that drive the formation of glycosidic bonds from nucleotide diphosphate sugar (NDP-sugar) donors to aglycon acceptors.16 Some GTs, such as CalG1, GtfD, GtfE, and PcOGT have also been used to catalyze the deglycosylation of calicheamicin γ1, vancomycin, vancomycin 6-azidoglucose, and phlorizin, to produce their aglycons, respectively.14,15 Although ponasterone A could be chemically prepared through acidic hydrolysis of ponasteroside A, our preliminary experiments showed that a low yield of product was obtained owing to instability of the substrate and product (data not shown). The vicinal diols of ponasterone A and ponasteroside A would be feasible to facilitate pinacol rearrangement in such environment.17 The mild enzymatic deglycosylation of ponasteroside A would be a potent competitor for the preparation of ponasterone A.
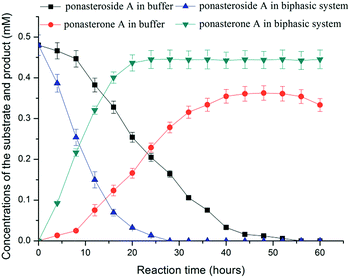
Ponasterone A was prepared from ponasteroside A by E. coli/gtBP1 in buffer. As shown in Fig. 1, the yield reached 47.5% at 24 h and 75.1% at about 48 h. However, the yield declined after 48 h, and no substrate was detected after 48 h. These results suggested that product and substrate were degraded by some enzymes from the recombinant cells.
The in situ product removal (ISPR) process has many advantages in thermodynamically controlled synthesis with extraction of products.18 The partitioning behavior of the substrate and the product between the two-phase media was considered the key element for ISPR.19 The partition coefficients of ponasterone A and ponasteroside A in the presence of various solvents with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ranging from 0.16 to 2.2 and the effect of solvents on the yield of ponasterone A were investigated (Table 1). The high yield (92.7%) of product was achieved in 24 h when ethyl acetate was used as the organic phase with a phase volumetric ratio ϕorganic/aqueous of 2
P ranging from 0.16 to 2.2 and the effect of solvents on the yield of ponasterone A were investigated (Table 1). The high yield (92.7%) of product was achieved in 24 h when ethyl acetate was used as the organic phase with a phase volumetric ratio ϕorganic/aqueous of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The high stability of E. coli/gtBP1 cells in the biphasic system was observed, although the free GTBP1 was relatively sensitive to the tested solvents (Table S1, ESI†). The solubility of ponasterone A and ponasteroside A in buffer were measured as 0.178 and 3.850 mg mL−1, respectively. The solubility of ponasterone A and ponasteroside A in ethyl acetate are 5.431 and 0.767 mg mL−1, respectively. The concentrations of product and substrate were detected in buffer and solvent layer respectively, and the results were calculated as total concentrations in buffer (Formula 1, ESI†). Fig. S5† shows the significant effect of phase volume ratios in the two-phase systemon the yield of product. The product yield was very low in a reaction system with the ϕ of 1
1. The high stability of E. coli/gtBP1 cells in the biphasic system was observed, although the free GTBP1 was relatively sensitive to the tested solvents (Table S1, ESI†). The solubility of ponasterone A and ponasteroside A in buffer were measured as 0.178 and 3.850 mg mL−1, respectively. The solubility of ponasterone A and ponasteroside A in ethyl acetate are 5.431 and 0.767 mg mL−1, respectively. The concentrations of product and substrate were detected in buffer and solvent layer respectively, and the results were calculated as total concentrations in buffer (Formula 1, ESI†). Fig. S5† shows the significant effect of phase volume ratios in the two-phase systemon the yield of product. The product yield was very low in a reaction system with the ϕ of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 due to severe emulsification under vigorous shaking. The highest yield was achieved with the ϕ of 2
1 due to severe emulsification under vigorous shaking. The highest yield was achieved with the ϕ of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ISPR system accelerated the deglycosylation of ponasteroside A and shortened the process of the reaction. The technique of ISPR contributed to reducing the degradation of the product and substrate,20 making it easy to recover the products.21
1. The ISPR system accelerated the deglycosylation of ponasteroside A and shortened the process of the reaction. The technique of ISPR contributed to reducing the degradation of the product and substrate,20 making it easy to recover the products.21
| Organic solvent | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Ks | Kp | Yield |
|---|---|---|---|---|
a Note: Ks and Kp: partition coefficients of ponasteroside A and ponastone A in the organic and aqueous phase, respectively. The reaction was catalyzed by the recombinant cell in the organic-aqueous system with a phase volume ratio (ϕorganic/aqueous) of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and an initial concentration of 0.48 mM ponasteroside A. 1 and an initial concentration of 0.48 mM ponasteroside A. |
||||
| Ethyl acetate | 0.68 | 0.14 | 7.39 | 92.73% |
| Menthyl acetate | 0.16 | 0.16 | 3.78 | 83.78% |
| Methyl propionate | 0.97 | 0.09 | 3.77 | 83.86% |
| n-Butyl acetate | 1.70 | 0.07 | 4.71 | 87.34% |
| n-Pentyl acetate | 2.20 | 0.08 | 4.63 | 86.25% |
| Isobutanol | 0.65 | 6.02 | 0 | 5.02% |
| Dichloromethane | 1.30 | 0 | 0 | 47.20% |
The time courses of ponasterone A preparation in buffer and the ethyl acetate-aqueous biphasic system are shown in Fig. 1. The yield of ponasterone A was significantly increased in the biphasic system; the molar yield was more than 50% at 10 h, whereas only 47.5% yield was observed at 24 h in buffer. The molar yield reached 92.7% in the biphasic system at 24 h, without an obvious decrease until 60 h. The preparation of ponasterone A was markedly improved when the product was extracted into ethyl acetate, which not only shifted the reaction equilibrium towards the synthesis of the product, but also shortened the reaction and minimized the degradation of substrate and product. Mass transfer (extraction of the product by ethyl acetate) was considered as a main “driving force” for the efficient synthesis of the products.
Successive production of ponasterone A was achieved using the fed-batch strategy. Since substrates inhibited the GTBP1 at concentrations higher than 0.5 mM, the reaction was carried out by keeping the concentration of substrates below 0.5 mM through fed-batch cultivation with the substrate. The preparation of ponasterone A using the fed-batch strategy was carried out. A high concentration of ponasterone A (2.80 mM, 1.30 mg mL−1) was produced after eight-batches loading with a molar yield of 89.8% (Fig. 2). The slight decrease of deglycosylation of ponasteroside A from the fifth to the eighth batches could be in the charge of the accumulation of ponasterone A in buffer layer, and the efficient production could be continued with the exchange of fresh solvent layer.
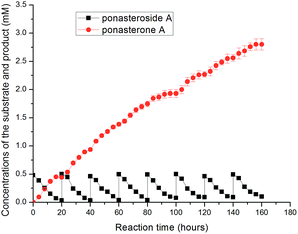 | ||
| Fig. 2 Successive production of ponasterone A from ponasteroside A using fed-batch by E. coli/gtBP1 in aqueous-organic biphasic medium. | ||
The product was directly concentrated in the solvent layer, and the purification was simplified. The collected solvent was evaporated, and the powders of product ponasterone A were obtained, resulting in a purity of 98.5%. The identity of the product was confirmed by mass spectrometry (MS; Fig. S2†) and nuclear magnetic resonance (NMR; Fig. S3†). The efficient preparation of ponasterone A with ISPR using a fed-batch strategy prevented substrate and product inhibition, promoted product accumulation, and simplified the downstream process, thereby making the synthesis more amenable to scale-up.
Conclusions
Ponasterone A is a potential inducer mediating p53 gene therapy in tumors. The bacterium Bacillus pumilus BF1, with considerable activity in the deglycosylation of ponasteroside A into ponasterone A, was isolated. The glycosyltransferase GTBP1 from strain BF1, which exhibited an apparent molecular weight of 47 kDa, was cloned and expressed in Escherichia coli BL21. Efficient production of ponasterone A by the recombinant E. coli/gtBP1 in a biphasic system was achieved with a molar yield of 92.7%. This in situ product removal provided “driving forces” for shifting the reaction equilibrium towards the synthesis of the product, markedly accelerated the production of ponasterone A, and minimized the degradation of the substrate and product. Successive production of ponasterone A was obtained, with a total yield of 89.8% using the fed-batch strategy. Through these strategies, ponasterone A was significantly concentrated and simply purified.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21376119, 81673321, 21506099), and Specialized Research Fund for the Doctoral Program of Higher Education (20123221130001).Notes and references
- K. Nakanishi, Steroids, 1992, 57, 649–657 CrossRef CAS PubMed
.
- C. Albanese, A. T. Reutens, B. Bouzahzah, M. Fu, M. D'Amico, T. Link, R. Nicholson, R. A. Depinho and R. G. Pestell, FASEB J., 2000, 14, 877–884 CAS
.
- Y. Y. Xiao, M. A. Beilstein, M. C. Wang, J. Purintrapiban and N. E. Forsberg, Int. J. Biochem. Cell Biol., 2003, 35, 79–85 CrossRef CAS PubMed
.
- M. Hori, K. Suzuki, M. U. Udono, M. Yamauchi, M. Mine, M. Watanabe, S. Kondo and Y. Hozumi, Arch. Dermatol. Res., 2009, 301, 631–646 CrossRef CAS PubMed
.
- U. A. Baltaev, Bioorg. Khim., 2000, 26, 892–926 CAS
.
- P. Wu, H. Xie, W. Tao, S. Miao and X. Wei, Phytochemistry, 2010, 71, 975–981 CrossRef CAS PubMed
.
- Y. J. Liu, W. Liu and L. Chun, Acta Soc. Bot. Pol., 2012, 81, 263–271 CrossRef
.
- V. N. Odinokov, R. G. Savchenko, S. R. Nazmeeva, I. V. Galyautdinov and L. M. Khalilov, Russ. J. Org. Chem., 2002, 33, 525–529 CrossRef
.
- H. Hikino, S. Arihara and T. Takemoto, Tetrahedron, 1969, 25, 3909–3917 CrossRef CAS PubMed
.
- T. Otaka, M. Uchiyama, T. Takemoto and H. Hikino, Chem. Pharm. Bull., 1969, 17, 1352–1355 CrossRef CAS PubMed
.
- Y. Dong, H. Teng, S. Qi, L. Liu, H. Wang, Y. Zhao and Z. Xiu, Biochem. Eng. J., 2010, 52, 123–130 CrossRef CAS
.
- H. Huang, M. Zhao, L. Lu, D. Tan, W. Zhou, C. Xiong, Y. Zhao, X. Song, L. Yu and B. Ma, J. Mol. Catal. B: Enzym., 2013, 98, 1–7 CrossRef CAS
.
- T. Liu, H. Yu, C. Liu, Y. Wang, M. Tang, X. Yuan, N. Luo, Q. Wang, X. Xu and F. Jin, Appl. Microbiol. Biotechnol., 2013, 97, 10035–10043 CrossRef CAS PubMed
.
- C. Zhang, B. R. Griffith, Q. Fu, C. Albermann, X. Fu, I. K. Lee, L. Li and J. S. Thorson, Science, 2006, 313, 1291–1294 CrossRef CAS PubMed
.
- A. C. L. B. Gutmann, Chem. Commun., 2014, 50, 5465–5469 RSC
.
- Y. Fukunaga, T. Miyata, N. Nakayasu, K. Mizutani, R. Kasai and O. Tanaka, Agric. Biol. Chem., 2014, 53, 1603–1607 Search PubMed
.
- Z.-L. Song, C.-A. Fan and Y.-Q. Tu, Chem. Rev., 2011, 111, 7523–7556 CrossRef CAS PubMed
.
- I. P. Ivanov, V. Kasche and D. D. Petkov, Biocatal. Biotransform., 1996, 14, 181–194 CrossRef
.
- H. B. J. B. P. K. Sun, Green Chem., 2011, 13, 1680–1686 RSC
.
- Y. S. Yang, T. Zhang, S. C. Yu, Y. Ding, L. Y. Zhang, C. Qiu and D. Jin, Molecules, 2011, 16, 4295–4304 CrossRef CAS PubMed
.
- J. Chang, Y. S. Lee, S. J. Fang, D. J. Park and Y. L. Choi, Int. J. Biol. Macromol., 2013, 61, 465–470 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02367g |
| This journal is © The Royal Society of Chemistry 2017 |