Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Involvement of ROS in nanosilver-caused suppression of aflatoxin production from Aspergillus flavus†
Jing Zhaoab,
Ling Wangab,
Dan Xu*cd and
Zhisong Lu *ab
*ab
aChongqing Key Laboratory for Advanced Materials & Technologies of Clean Energies, Southwest University, No. 1 Tiansheng Road, Chongqing 400715, P. R. China. E-mail: zslu@swu.edu.cn
bChongqing Engineering Research Center for Micro-Nano Biomedical Materials and Devices, Institute for Clean Energy & Advanced Materials, Faculty of Materials and Energy, Southwest University, No. 1 Tiansheng Road, Chongqing 400715, P. R. China
cDepartment of Gastroenterology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Shengli Street Jiang'an District No. 26, Wuhan 430014, P. R. China. E-mail: drxu0624@gmail.com
dKey Laboratory for Molecular Diagnosis of Hubei Province, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Shengli Street Jiang'an District No. 26, Wuhan 430014, P. R. China
First published on 26th April 2017
Abstract
Silver nanoparticles (AgNPs) have been extensively studied as antimicrobial materials, but their capability of suppressing aflatoxin production has not been investigated. In this work, AgNPs with an average size of 4.5 nm were synthesized to inhibit the growth of Aspergillus flavus (A. flavus). Based on the anti-fungal assay, the concentration of 5 μg mL−1 was chosen to study the direct inhibiting effects of AgNPs on aflatoxin production. Results show that AgNP treatment could significantly decrease secretion of aflatoxin B1 from A. flavus. Real-time measurements of O2− with an electrochemical sensor reveal that the AgNPs could trigger the release of O2− from fungal mycelia. A mechanism involving O2− release is proposed to explain AgNP-caused depression of aflatoxin production from A. flavus. This is the first attempt to study AgNP-induced inhibition on aflatoxin generation and its possible mechanisms.
Introduction
Aflatoxins, the secondary metabolites of Aspergillus flavus (A. flavus), Aspergillus parasiticus (A. parasiticus) and Aspergillus nonius (A. nonius),1 are a group of highly toxic substances that cause acute or chronic human liver diseases.2,3 Among them, aflatoxin B1 (AFB1) is recognized as the most toxic natural substance. At a very low level, AFB1 leads to significantly negative impacts on human and animal health.4 Since agricultural products such as peanut, corn, rice and wheat are easily contaminated by fungi,5 great efforts have been devoted to the development of efficient ways to suppress AFB1 production.Essential oils extracted from plants have been applied to inhibit fungal growth and mycotoxin production in recent years.6 Essential oils including aldehydes (cinnamaldehyde, citral, citronellal and neral), phenols (thymol, eugenol, phenol and thymol), alcohols (linalool and citronellol), as well as ketones (carvone and menthone) have been regarded as effective antifungal reagents.7–11 As advances of nanotechnology, nanomaterials have also been employed to inhibit fungal growth.12–16 Single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), graphene oxide nanosheets and reduce graphene oxide nanosheets have been proven to possess antifungal activities against Fusarium graminearum and Fusarium poae.12–16
As a nanomaterial with a broad bactericidal spectrum and long-lasting antimicrobial effects, nanosilver has also been applied to inhibit microbial growth.17–22 Nanosilver has been practically used as fresh-keeping packaging materials, food additives and food coating materials to control the growth of microbes in food and vegetables.23,24 A significant inhibitory effect on the growth of Candida albicans and Aspergillus could be achieved by using nanosilver as antifungal materials. The antifungal activity of silver nanoparticles (AgNPs) against Alternaria solani and Fusarium oxysporum has also been verified.25 Although nanosilver is a very promising material to restrain the growth of fungi including Aspergillus species, its capability of suppressing aflatoxin production in Aspergillus has not been systematically investigated so far.
Reactive oxygen species (ROS), which are closely related to a great deal of biological events such as aging, cancer development, and neurodegenerative diseases,26–32 may also participate in the generation of aflatoxins in A. flavus.33 Enhancement of oxidative stress leads to a higher level of aflatoxins production from toxigenic strains.34 Moreover, the application of antioxidants could reduce biosynthesis of aflatoxins from Aspergillus species.35,36 In our recent work, we demonstrated that the secretion of ROS upon citral stimulation might be the mechanism for citral-induced reduction of AFB1 production from A. flavus.30
In the present study, AgNPs synthesized via a wet-chemical approach were characterized with TEM and UV-vis spectrometry. The anti-fungal activity of the as-prepared AgNPs was evaluated by measuring their growth inhibition effects on A. flavus. A low concentration of AgNPs, which cannot cause a significant growth inhibition effect, was chosen to investigate direct effects of AgNPs on aflatoxin production. Release of O2− from A. flavus upon the stimulation of AgNPs was real-time monitored using a MWCNTs–Mn3O4 nanorods-based biosensor to reveal the possible mechanism for AgNPs-induced aflatoxin suppression.
Experimental
Reagents and materials
A. flavus GZ-6 was a gift from Institute of Agro-Products Processing Science and Technology, Chinese Academy of Agricultural Sciences, P. R. China. The MWCNTs, sucrose and yeast extract were purchased from Aladdin (Shanghai, China). Soybean peptone was bought from Qingdao Hope Bio-Technology Co. Ltd. (Qingdao, China). Nafion perfluorinated resin (Sigma-Aldrich, China) was dissolved in absolute ethanol to form a 5% Nafion solution. All other chemicals were of analytical grade and directly used in the present study without further purifications.Preparation of standard O2− solutions
The standard O2− solutions were prepared by adding KO2 powder into nitrogen gas-saturated phosphate-buffered saline (PBS). The concentration of O2− solution was determined spectrophotometrically using the ferrocytochrome C reduction assay, in which the absorbance change at 550 nm was recorded to calculate the amount of O2− with the extinction coefficient of 21.1 mM−1 cm−1.Synthesis of AgNPs and Mn3O4 nanorods
AgNPs were synthesized according to Shirtcliffe's route with minor modifications.36 Briefly, 1 mL AgNO3 solution (0.01 M) and 1 mL sodium citrate dihydrate solution (0.01 M) were mixed with 20 mL deionized water at 70 °C. Then, a NaBH4–NaOH solution, which was freshly prepared by dissolving 0.0189 g NaBH4 in 50 mL of 0.1 M NaOH solution, was added into the above mixture under stirring. The color of the solution changed to bright yellow instantly, showing the successful synthesis of AgNPs.Mn3O4 nanorods were fabricated with the following way: firstly, 0.2 g poly(vinylpyrrolidone) and 0.1 g stearic acid were mixed in 15 mL ethanol under stirring for 15 min. Then, 45 μL Mn(NO3)2 (50 wt%) and 400 μL H2O2 (30%) were added into the mixture successively, stirring for another 15 min. After heating at 160 °C for 8 h, the Mn3O4 nanorods were produced and harvested by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm min−1, 10 min).
000 rpm min−1, 10 min).
Fabrication of Mn3O4 nanorods-MWCNTs-modified electrodes
Glassy carbon electrodes (GCEs) were polished with 0.05 μm alumina slurry on a piece of chamois leather to achieve a smooth surface. 5 μL well-dispersed MWCNTs suspension (1 mg mL−1) and Mn3O4 nanorods (7.5 mg mL−1) were dropped onto the GCE surface successively to produce the modified electrode. A 5% Nafion solution was coated on the modified GCEs, storing at 4 °C for the electrochemical tests.Culture of A. flavus and AgNPs treatment
A. flavus was inoculated in a culture medium (450 g L−1 sucrose, 60 g L−1 yeast extract and 30 g L−1 soybean peptone in deionized water) and incubated at 30 °C under constant shaking (200 rpm) for 3 days. The A. flavus mycelia were harvested via filtration and washed with PBS for 3 times. The mycelia were re-suspended in a PBS solution and treated with 5 μg mL−1 AgNPs at 30 °C for 60 seconds. After washing for 3 times, the PBS- and AgNPs-treated mycelia were inoculated in fresh media, culturing at 30 °C under constant shaking. The media sampled at 0, 72 and 96 h were stored at 4 °C for the determination of the AFB1 concentrations.The collected media were filtered through a Waterman filter. The AFB1 was extracted from the filtrates with chloroform, followed by the dehydration with anhydrous sodium sulfate and the evaporation at 50 °C under vacuum. The amount of AFB1 was determined with a high-performance liquid chromatography (HPLC) containing an ultraviolet/visible spectrum Waters 2475 detection system (Waters Corporation, Milford, MA, USA).
Materials characterization and electrochemical measurements
Morphologies of the nanomaterials were characterized with SEM (JSM-7600, JEOL, Tokyo, Japan) and TEM (JEM-2100, JEOL, Tokyo, Japan). XRD spectra were examined using a Cu Kα-ray with tube conditions of 40 kV and 30 mA ranging from 10° to 80° (XRD-7000, Shimadzu, Japan). UV-vis spectrum of the AgNPs suspension was collected with a UV-2550 spectrophotometer (Shimadzu, Japan). All electrochemical measurements were carried out with the modified GCE as a working electrode, a Pt counter electrode and a Ag/AgCl reference electrode. The electrochemical data were obtained using a CHI 660E electrochemical workstation (Shanghai Chenhua Apparatus Corporation, China).Results and discussion
Since shape and size of AgNPs are critical parameters for the antimicrobial activity, transmission electron microscopy (TEM) was carried out to characterize the morphology of the as-prepared nanosilver. Nanoparticles with uniform shape and size can be observed in Fig. 1A. The TEM image with a higher magnification (Fig. 1B) clearly shows the spherical shape of a single nanoparticle. By measuring diameter of the nanoparticles in the TEM images, the size distribution of the AgNPs is exhibited in Fig. 1C. The AgNPs vary from 1.0 to 9.0 nm and possess an average value of 4.5 nm. Both shape and size of the as-prepared AgNPs are consistent with those reported in previous literatures.36 A well-defined peak located at 394 nm can be found in the UV-vis spectrum of the AgNPs suspension (Fig. 1D), attributing to the surface plasmon resonance absorption peak of AgNPs. The above results verify the successful synthesis of AgNPs with the spherical shape and an average size of ∼4.5 nm, the quality of which can meet the requirements of the following biological tests. Before its application in anti-fungal assays, the freshly prepared AgNPs have been stored at 4 °C for 4 days. In comparison to the data in Fig. 1D, there is no significant change on the UV-vis spectrum of the stored AgNPs suspension (Fig. S1†), suggesting the good stability of AgNPs during the storage process. The stability of AgNPs in the culture medium was also investigated with the dynamic laser scattering technique (Fig. S2†). After an incubation in a culture medium for 4 days, the hydrodynamic size of AgNPs increases to ∼7.5 nm. The results indicate that the AgNPs are quite stable in the culture medium and the surface adsorption of small peptides may cause the increment of the nanoparticle size.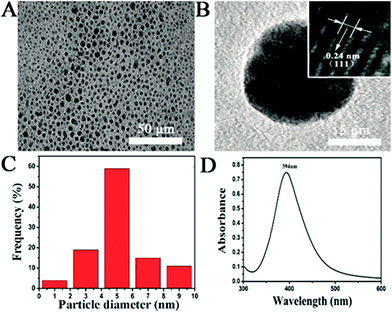 | ||
| Fig. 1 Characterization of the as-prepared AgNPs. (A) TEM image of the AgNPs; (B) TEM image of a single AgNP; (C) particle size distribution of the AgNPs; (D) UV-vis spectrum of a AgNPs suspension. | ||
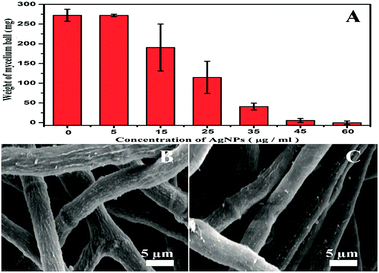
To investigate effects of the AgNPs exposure on fungal growth, dry weight of A. flavus mycelium balls before/after AgNPs treatments were measured. As the dose of AgNPs in the system increases, weight of the mycelium balls gradually decreases (Fig. 2A). When the AgNPs dose reaches to 60 μg mL−1, the weight is very close to zero (Table S1†), suggesting the complete inhibition of A. flavus growth by AgNPs. The results indicate that the AgNPs could effectively inhibit the A. flavus growth in a dose-dependent manner, agreeing well with the anti-fungal activity of AgNPs. Undoubtedly, the AgNPs-caused growth inhibition could significantly reduce the fungal amount in the system, further resulting in the decrease of aflatoxin production. At high doses (more than 15 μg mL−1), AgNPs-induced direct reduction of aflatoxins cannot be differentiated from the indirect one that was caused by the fungal amount decrease. As shown in Fig. 2A, AgNPs have no significant effect on the A. flavus growth at 5 μg mL−1. The growth of A. flavus exposed to 5 μg mL−1 AgNPs in a liquid culture medium was shown in Fig. S3 in ESI.† Moreover, morphologies of the mycelia treated with 5 μg mL−1 AgNPs are similar to those in control group (Fig. 2B and C). Therefore, the concentration of 5 μg mL−1 is selected in the following assays to explore the direct inhibition effects of AgNPs on the aflatoxin production from A. flavus. It has been reported that the antimicrobial activity of nanosilver may be caused by the penetration of the nanoparticles into the microbes.14 Since the size of AgNPs is less than 5 nm, their binding and penetration cannot be directly observed in the SEM images. TEM was also conducted to check the morphology of the filamentous fungi (Fig. S4†). However, the attachment and penetration of AgNPs were not observed in our investigation.
After exposure to 5 μg mL−1 AgNPs, the mycelia were harvested by centrifugation, further culturing in a fresh medium for 96 h. The media were collected at 0, 72 and 96 h for aflatoxin measurements (Table 1). At the beginning of incubation, amounts of AFB1 in the culture media are quite low (around 3–4 ng mL−1) in both PBS- and AgNPs-treated samples. Desorption of aflatoxin molecules from the mycelium may lead to the initial amount of AFB1. After incubation for 72 h, there is a significant enhancement on the AFB1 concentration. As the incubation time elongates to 96 h, the amount of AFB1 further increases. AFB1 has been bio-synthesized and gradually secreted into the culture media by A. flavus during the culture process in both control and AgNPs-exposed groups. By comparing both groups, it can be found that the pre-treatment of AgNPs could induce an obvious reduction on the AFB1 concentrations in the culture media. After a 96 h culture, the average AFB1 concentration in the AgNPs-exposed sample is around 28.96 ng mL−1, which is lesser than that in the PBS-treated one. The results strongly support that an instantaneous stimulation of A. flavus by AgNPs could effectively depress the generation of AFB1.
| Time (h) | PBS | AgNPs | ||
|---|---|---|---|---|
| AFB1 (ng mL−1) | ΔAFB1 (ng mL−1) | AFB1 (ng mL−1) | ΔAFB1 (ng mL−1) | |
| 0 | 3.22 ± 1.20 | — | 4.03 ± 2.16 | — |
| 72 | 24.44 ± 2.15 | 21.22 ± 3.35 | 17.17 ± 0.98 | 13.15 ± 3.14 |
| 96 | 66.23 ± 6.60 | 63.01 ± 7.80 | 28.96 ± 1.14 | 24.94 ± 3.30 |
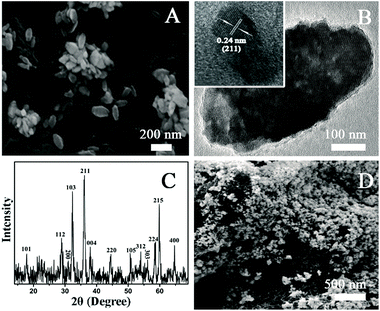
Electrochemical biosensor is a well-established method for in situ monitoring important biomolecules in biological systems.29,31,32,37 O2− is one of the ROS with high activity and short half-life. The measurement of O2− in a biological system is quite difficult. In the present work, O2− was chosen as a typical type of ROS to investigate the role of ROS in AgNPs-caused suppression of AFB1 production. In order to real-time study the possible role of O2− in the AgNPs-induced inhibition of AFB1 production, we synthesized Mn3O4 nanorod, a specific artificial nano-enzyme of O2−, for the fabrication of an O2−electrochemical biosensor. The as-prepared products are rod-shaped materials with the diameter of ∼100 nm and the length of ∼200 nm (Fig. 3A and B). The XRD pattern (Fig. 3C) displays several diffraction peaks, matching well with (101), (112), (200), (103), (211), (004), (220), (105), (312), (303), (224), (215) and (400) planes of tetragonal Mn3O4 (JCPDS card 24-0734). The scanning electron microscope (SEM) images and the X-ray diffraction (XRD) data verify the successful synthesis of Mn3O4 nanorods. After layer-by-layer deposition of MWCNTs and Mn3O4 nanorods, surface morphology of the modified electrode was imaged with SEM (Fig. 3D). A layer of nanorods covers on a network structure, clearly proving the existence of MWCNTs and Mn3O4 nanorods on the surface of the electrode.
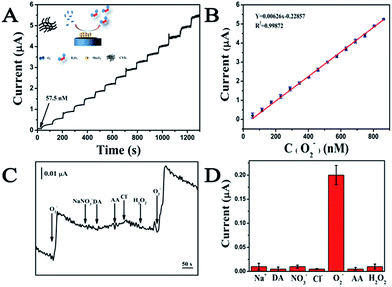
During the catalytic process, a O2− oxidizes the Mn2+ to produce MnO2+ and H2O2 while another O2− simultaneously reduces the MnO2+ to produce Mn2+ and O2. Performances of the electrochemical sensor including the sensitivity, dynamic range and specificity were investigated before its application in the system containing fungi. The amperometric responses of the sensor to successive addition of O2− is recorded at an applied potential of 700 mV (Fig. 4A). The response time is less than 5 s in response to a step injection of O2−. Well-defined steady-state currents can be obtained in a range of 57.5 to 862.5 nM, in which there is a linear relationship with a correlation coefficient of 0.999 between the current intensity and the O2− concentration (Fig. 4B). The detection limit of 19.87 nM (S/N = 3) and the sensitivity of 6.26 μA μM−1 can be calculated based on the calibration curve (Fig. 4B).38 The specificity of the O2− sensor is shown in Fig. 4C and D. The additions of 1 μM common compounds and ions existed in biological systems including H2O2, ascorbic acid (AA), Na+, NO3−, Cl− and dopamine (DA) do not lead to significant current responses. While, 57.5 nM O2− can trigger a dramatic increment of the current signal. The findings reveal that the MWCNTs–Mn3O4 nanorods-modified electrode with a low detection limit, a high sensitivity and excellent specificity for O2− detection could be utilized in real-time detection of O2− released from A. flavus.
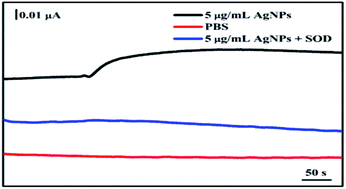
The secretion of O2− from A. flavus with/without AgNPs treatment was real-time monitored using the electrochemical sensor. As shown in Fig. 5, the addition of PBS in the A. flavus system does not cause any electrochemical response (red line). The data rule out the interference of the solvent in the electrochemical detection. Upon the injection of a 5 μg mL−1 AgNPs suspension, an obvious increase of the current occurs (black line). To validate that the current change is indeed caused by O2−, superoxide dismutase (SOD), a selective scavenger of O2−, was introduced in the detection system. SOD-catalyzed dismutation of O2− may generate hydrogen peroxide, which does not interfere the electrochemical monitoring of O2− as being illustrated in Fig. 4C and D. The existence of SOD could eliminate the current enhancement induced by AgNPs (blue line). The data indicate that the treatment of AgNPs could stimulate the fungal mycelia to release O2− rapidly. According to the calibration curve in Fig. 4B, the amount of the O2− released from AgNPs-exposed A. flavus is around 41.3 nM (the weight concentration of mycelia in the testing system is 1.15 g mL−1). In order to directly analyze the function of O2− in nanosilver-induced aflatoxin suppression, experiments need to be carried out to investigate influences of AgNPs on the expression of ROS-related genes in future works.
In the present study, AFB1 released into the culture medium was measured to represent the amount of aflatoxins synthesized by the A. flavus cells. Since ROS are closely related to the biosynthesis of aflatoxins, a possible mechanism involving quick release of O2− is proposed to explain the AgNPs-caused inhibition of AFB1 production from A. flavus on the basis of the above data (Fig. 6). The moderate accumulation of ROS in A. flavus mycelia could initiate a series of biochemical reactions for the aflatoxin biosynthesis. Upon the stimulation of AgNPs, O2− molecules are secreted from the mycelia immediately, ultimately resulting in the quick reduction of intracellular ROS level. The low intracellular oxidative level further influences the signalling pathway to the aflatoxin biosynthesis. Finally, the production of aflatoxins in A. flavus is significantly suppressed. In future works, effects of AgNPs on the secretion of other ROS such as hydroxyl radical and H2O2 in A. flavus should be investigated to further reveal the role of ROS in the mycotoxin production. It should also be noted that the inhibiting effects of AgNPs on the release process might also affect the reduction of aflatoxin secretion. As far as we known, there is no report on AgNPs-induced secreting inhibition of aflatoxin-containing vesicles. Thus, the mechanism for antifungal activity and aflatoxin reduction caused by AgNPs needs to be further investigated.
Conclusions
In summary, AgNPs-induced suppression of AFB1 production from A. flavus and its possible mechanism have been investigated in the present work. AgNPs synthesized with a wet-chemical approach were characterized using TEM and UV-vis spectrometry. Based on the anti-fungal assay, 5 μg mL−1 was selected as the concentration in the aflatoxin production and O2− release tests to avoid the interferences of indirect aflatoxin reduction due to the fungal growth inhibition. The exposure of A. flavus to AgNPs could significantly decrease the secretion of AFB1. The real-time measurements of O2− with a MWCNTs–Mn3O4 nanorods-based electrochemical sensor show that the AgNPs could trigger the release of O2− from fungal mycelia rapidly. A mechanism involving O2− release is proposed to explain the AgNPs-caused depression of aflatoxin production from A. flavus. This is the first attempt to study AgNPs-induced inhibition on aflatoxin generation. The electrochemical sensors fabricated in the present study could also be used to investigate other O2− related biological systems in future works.Acknowledgements
This work is financially supported by National Program on Key Basic Research Project of China (973 Program) under contract No. 2013CB127804, Natural Science Foundation of Hubei (2015CFB578), Funding for Health Bureau of Wuhan (2014WX14A04) and Fundamental Research Funds for the Central Universities under XDJK2015B016. Z. S. Lu would like to thank the supports by the Specialized Research Fund for the Doctoral Program of Higher Education (RFDP) (Grant No. 20130182120025) and Young Core Teacher Program of the Municipal Higher Educational Institution of Chongqing.Notes and references
- M. Thompson and R. Wood, Pure Appl. Chem., 1995, 67, 649–666 CrossRef CAS.
- M. Thompson and S. L. R. Ellison, Accredit. Qual. Assur., 2006, 11, 373–378 CrossRef CAS.
- M. Thompson, S. L. R. Ellison and R. Wood, Pure Appl. Chem., 2006, 78, 145–196 CrossRef CAS.
- D. L. Duewer, Accredit. Qual. Assur., 2008, 13, 193–216 CrossRef CAS.
- J. S. Wang and J. D. Groopman, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1999, 424, 167–181 CrossRef CAS PubMed.
- R. Bluma, M. R. Amaiden and M. Etcheverry, Int. J. Food Microbiol., 2008, 122, 114–125 CrossRef CAS PubMed.
- J. S. Dambolena, A. G. López, M. C. Cánepa, M. G. Theumer, J. A. Zygadlo and H. R. Rubinstein, Toxicon, 2008, 51, 37–44 CrossRef CAS PubMed.
- H. Hua, F. Xing, J. N. Selvaraj, Y. Wang, Y. Zhao, L. Zhou, X. Liu and Y. Liu, PLoS One, 2013, 9, e108285 Search PubMed.
- B. Jeršek, U. N. Poklar, M. Skrt, N. Gavarić, B. Božin and M. S. Smole, Arh. Hig. Rada Toksikol., 2014, 65, 199–208 CrossRef PubMed.
- E. Kim and I. K. Park, Molecules, 2012, 17, 10459–10469 CrossRef CAS PubMed.
- M. C. Manganyi, T. Regnier and E. I. Olivier, S. Afr. J. Bot., 2015, 99, 115–121 CrossRef CAS.
- S. Kang, M. Pinault and L. D. Pfefferle, Langmuir, 2007, 23, 8670–8673 CrossRef CAS PubMed.
- S. Liu, L. Wei and L. Hao, ACS Nano, 2016, 3, 3891–3902 CrossRef PubMed.
- S. Kang, M. S. Mauter and M. Elimelech, Environ. Sci. Technol., 2008, 42, 7528 CrossRef CAS PubMed.
- X. Wang, X. Liu and H. Han, Colloids Surf., B, 2013, 103, 136–142 CrossRef CAS PubMed.
- X. Wang, X. Liu and J. Chen, Carbon, 2014, 68, 798–806 CrossRef CAS.
- X. Cui, C. M. Li, H. Bao, X. Zheng and Z. Lu, J. Colloid Interface Sci., 2008, 327, 459–465 CrossRef CAS PubMed.
- L. Guo, W. Yuan, Z. Lu and C. M. Li, Colloids Surf., A, 2013, 439, 69–83 CrossRef CAS.
- Z. Lu, M. Meng, Y. Jiang and J. Xie, Colloids Surf., A, 2014, 447, 1–7 CrossRef CAS.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- J. Robidoux, T. L. Martin and S. Collins, Toxicol. Appl. Pharmacol., 2004, 44, 297–323 CAS.
- C. A. Campos, L. N. Gerschenson and S. K. Flores, Food Bioprocess Technol., 2011, 4, 849–875 CrossRef CAS.
- H. E. Qiang and L. V. Yuan-Ping, Trends Food Sci. Technol., 2008, 281–298 Search PubMed.
- B. B. N. Ames, M. K. Shigenaga and T. M. Hagen, Biochim. Biophys. Acta, 1995, 1271, 165–170 CrossRef.
- M. Akbarian, M. E. Olya, M. Ataeefard and M. Mahdavian, Prog. Org. Coat., 2012, 75, 344–348 CrossRef CAS.
- R. A. Floyd, FASEB J., 1990, 4, 2587–2597 CAS.
- E. D. Hall and J. M. Braughler, Free Radical Biol. Med., 1989, 6, 303–313 CrossRef CAS PubMed.
- A. Vanella, G. C. Di, V. Sorrenti, A. Russo, C. Castorina, A. Campisi, M. Renis and J. R. Perez-Polo, Neurochem. Res., 1993, 18, 1337–1340 CrossRef CAS PubMed.
- X. T. Zheng, W. Hu, H. Wang, H. Yang, W. Zhou and C. M. Li, Biosens. Bioelectron., 2011, 26, 4484–4490 CrossRef CAS PubMed.
- J. Li, J. Li, Z. Lu, Y. Liu and C. M. Li, Chem. Commun., 2015, 51, 17424 RSC.
- M. Reverberi, S. Zjalic, A. Ricelli, A. A. Fabbri and C. Fanelli, Mycotoxin Res., 2006, 22, 39–47 CrossRef CAS PubMed.
- T. Jayashree and C. Subramanyam, Free Radical Biol. Med., 2000, 29, 981–985 CrossRef CAS PubMed.
- J. H. Kim, B. C. Campbell, R. Molyneux, N. Mahoney, K. L. Chan, J. Yu, J. Wilkinson, J. Cary, D. Bhatnagar and T. E. Cleveland, Mycotoxin Res., 2006, 22, 3–8 CrossRef CAS PubMed.
- R. Rachmawati, H. Kinoshita and T. Nihira, Procedia Environ. Sci., 2013, 17, 142–149 CrossRef CAS.
- T. Jayashree, J. P. Rao and C. Subramanyam, FEMS Microbiol. Lett., 2000, 183, 215–219 CrossRef CAS PubMed.
- N. Shirtcliffe, U. Nickel and S. Schneider, J. Colloid Interface Sci., 1999, 211, 122–129 CrossRef CAS PubMed.
- Y. Luo, Y. Tian and Q. Rui, Chem. Commun., 2009, 21, 3014–3016 RSC.
- L. Yu, L. X. Gao, X. Q. Ma, F. X. Hu, C. M. Li and Z. Lu, Integr. Biol., 2014, 6, 1211–1217 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02312j |
| This journal is © The Royal Society of Chemistry 2017 |