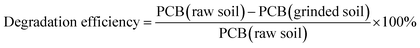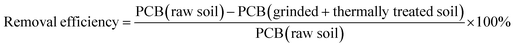Open Access Article
Open Access ArticleCombined mechanochemical and thermal treatment of PCBs contaminated soil
Zhonghua Zhao ,
Mingjiang Ni,
Xiaodong Li*,
Alfons Buekens and
Jianhua Yan
,
Mingjiang Ni,
Xiaodong Li*,
Alfons Buekens and
Jianhua Yan
State Key Laboratory of Clean Energy Utilization, Institute for Thermal Power Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: lixd@zju.edu.cn
First published on 13th April 2017
Abstract
This study combines a preliminary mechanochemical treatment and a subsequent thermal desorption for remediating soil, contaminated with polychlorinated biphenyls (PCBs). After 2 hours of grinding, assisted by addition of SiO2, the total concentration of PCBs and their TEQ-value decreased by 81.9% and 85.4%, respectively. The effect of thermal treatment at 400, 500 and 600 °C on the removal efficiency of PCBs from ground soil was then investigated. The residual amount of PCBs reduced with the rising temperature and dropped to 137 ng g−1 in the treated soil when the treatment temperature reached 600 °C, equivalent to a desorption efficiency of 99.85% and a removal efficiency of nearly 100%. The formation of polychlorinated dioxins and dibenzofurans (PCDD/Fs) was also monitored: PCDDs and PCDFs were generated, particularly at 400 °C, however their formation weakened at higher temperatures and hydrodechlorination dominated.
1. Introduction
All over the world, the reduction and control of persistent organic pollutants (POPs) is a significant problem that must be tackled, according to the Stockholm Convention. Polychlorinated biphenyls (PCBs) are one of these POPs and feature high thermal and chemical stability, as well as low volatility and flammability. Because they are produced industrially, PCBs have been applied in a wide variety of items, especially in transformer oil and capacitors.1 However, severe environmental consequences became apparent, due to bioaccumulation and their persistence in the environment. PCBs can cause cancer as well as produce serious effects on the immune system, reproductive system, nervous system and endocrine system.2Recently, much attention has been paid to soils contaminated with POPs and numerous methods have been proposed for their remediation. Mechanochemical (MC) hydrodechlorination and decomposition have attracted attention as a simple solution that enables the degradation of chlorinated, brominated and fluorinated POPs.3,4 Dehalogenation enhances this as grinding progresses and degradation of the POPs is also improved further by bringing in additives.5,6 Their effect on pentachlorophenol (PCP) hydrodechlorination was investigated, showing that the addition of CaO and quartz to the grinding mixture facilitated hydrodechlorination.7 Using a planetary ball mill, Saeki8 found that a mixture of CaO, SiO2, and Al2O3 was the most effective combination of additives for the hydrodechlorination of polyvinyl chloride (PVC). However, the catalytic performance depended heavily on the chosen substrate for hydrodechlorination. A mixture of metallic calcium and calcium oxide was found the most suitable degradation agent for degrading chlorinated contaminants. A simple preliminary washing under ultrasonication facilitated the following degradation.9 The preferred reactants are CaO and SiO2, because they are cheap and their reaction products can be dealt with safely. Debromination of hexabromobenzene proceeded smoothly with an increase in the molar ratio of CaO addition, and almost complete debromination was achieved after 6 hours of grinding with a molar ratio Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br kept constant at 2
Br kept constant at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.10 Iron powder and quartz sand were best for intensifying the destruction of mirex, an insecticide based on hexachlorocyclopentadiene, which was destroyed completely after 2 hours grinding at a charge ratio of 36
1.10 Iron powder and quartz sand were best for intensifying the destruction of mirex, an insecticide based on hexachlorocyclopentadiene, which was destroyed completely after 2 hours grinding at a charge ratio of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (reagent/mirex, m/m).11
1 (reagent/mirex, m/m).11
Due to PCBs' special physical and chemical properties, Nah12 assessed the potential of mechanochemical methods of removal, using fine metal powder for removing PCBs from waste insulating oil. After treatment for 4 hours at room temperature with a dosage of 1.53 mol metal per kg oil, only 70% PCBs removal was achieved when zinc was added. Hydrodechlorination of pure 3-chlorobiphenyl (3-MCP) was conducted by Zhang.13 The residual 3-MCP in the sample decreased rapidly within 20 minutes and diminished more gradually with further grinding. After 6 hours of grinding, over 99.5% 3-MCP was decomposed, but the weight ratio of 3-MCP to the inorganic powder was merely 5%. When the method was applied to soil contaminated with PCBs, 20 hours of grinding were required to attain a PCBs removal efficiency of 98%.14
Thermal desorption was therefore considered as a subsequent treatment process in order to save MC-treatment time and improve removal efficiency. Because highly chlorinated PCBs were broken down into lower chlorinated ones during the mechanochemical treatment, which had lower boiling points, the thermal treatment could more easily eliminate them. Also, the risk of a de novo synthesis of high amounts of PCBs and dioxins was lowered.
Thermal desorption is essentially a thermally induced physical separation process. Organic pollutants are vaporised from a solid matrix and then transferred into a carrier gas stream. In contrast with treatment by incineration, the decomposition of organic contaminants is not the immediate result desired. The contaminants in the gas stream may eventually be condensed, burned in an afterburner or cleaned by carbon adsorption.15 However, depending on operating temperature and strength of desorption, these liberated contaminants may still need to be degraded or converted during treatment.16 During thermal desorption, synergetic treatment with the addition of zero-valent iron nano-powder or base-catalysed additives effectively enhanced desorption and decomposition.17,18 However, the processes developed for chlorinated compounds caused the additional formation of polychlorinated dioxins and dibenzofurans (PCDD/Fs), which are often accompanied by higher toxic equivalency,19 especially in the presence of oxygen.20
The combined use of mechanochemical hydrodechlorination and thermal desorption was studied. Highly chlorinated contaminants were degraded by grinding and treatment time was reduced by the subsequent use of thermal desorption. The combined decontamination effects of these two methods were investigated. All 209 PCBs congeners and 12 toxic dl-PCBs as well as their TEQ were presented for analysis. Additionally, PCDD/Fs were analysed to evaluate the evolution of the dioxins during treatment. This has allowed differentiation between the original PCBs and their PCDD/Fs content, those that remained after milling, and the final residual PCBs and PCDD/Fs.
2. Materials and methods
2.1. Materials
Soil from a PCBs storage site in Zhejiang province, China, was used in this study. It was contaminated due to leakage from discarded transformers and capacitors. Its initial PCBs concentration was 505 μg g−1. Tri-(TrCBs) and tetra-chlorobiphenyls (TeCBs) were the predominant homologues groups, since their sum accounted for 90 wt% of total PCBs concentration. Their composition was similar to Aroclor 1248 and Kanechlor KC 400, as presented by Huang.21Calcium oxide and quartz used as additives during grinding were purchased from Sinopharm Chemical Reagent Co., Ltd. China, and used without treatment.
2.2. Apparatus and methods
A planetary ball mill (XQM-0.4L, Kexi, China) was used for grinding a mixture of soil and additives: 5 g of contaminated soil was mixed together with 5 g CaO and 10 g SiO2. The total 20 g of this mixture was added to a stainless steel pot containing 200 g of stainless steel balls. The planetary ball mill operated under atmospheric conditions for 2 hours at a speed of 400 rpm. To prevent over-heating, the milling paused for 30 minutes after every 30 minutes of grinding.After 2 hours of effective milling, the sample was taken out and then thermally treated. The thermal desorption system contained three major parts (Fig. 1): a carrier gas flow system, a horizontal tubular reactor and electric furnace, and a trap for collecting PCBs and PCDD/Fs from the exhaust gas. The trap was composed of a XAD-2 absorption tube and two toluene absorption bottles in series. During each test, 2 g of grinded soil was heated for 40 minutes at 400, 500 and 600 °C in a flow of 400 mL min−1 high purity nitrogen (≥99.99%). Evaporated PCBs and PCDD/Fs were carried by the N2 flow and then captured by the trap system. After thermal treatment, both the soil and the gas phase were collected and their PCBs and PCDD/Fs were detected and analysed. The thermal desorption procedures and apparatus have been described in full detail in former studies.18
2.3. PCBs, PCDD/Fs analysis
The grinded soil and the thermally treated soil, as along with the XAD-2 that had been collected after treatment, were extracted with toluene for 24 hours. Thereafter, the PCBs samples were cleaned up serially by percolation through a multi-silica gel column and a Florisil column. All sample clean-up procedures were conducted according to the EPA 1668 method. The PCDD/Fs samples were cleaned up using a multi-silica gel column and an alkaline alumina column in succession on the basis of the EPA method 1613. PCBs and PCDD/Fs were separated, identified and quantified by HRGC/HRMS (JEOL JMS-800D, Japan), equipped with a DB-5MS column (60 m × 0.25 mm × 0.25 μm). All 209 PCBs congeners and 136 PCDD/Fs congeners were detected.2.4. QA & QC
For the quantification of PCBs and PCDD/Fs, three distinct 13C-labeled standard solutions were added before Soxhlet extraction, before purification and then before analysis. All recovery rates of each internal standard were in accordance with the usual analytical recovery requirements.Some repeated experimental conditions were performed. The results reported for the gas phase were expressed based on the initial weight of contaminated soil.
2.5. Degradation, desorption and removal efficiency
Degradation efficiency was calculated to assess the degree of PCBs decomposition after the mechanochemical treatment:The desorption efficiency for assessing the performance of the thermal treatment was calculated by:
To evaluate the combined decontamination of PCBs, removal efficiency was defined and calculated as:
2.6. Weight average chlorination degree
The weight average chlorination degree reflects the average extent and depth of chlorination of all PCBs or PCDD/Fs congeners. It is calculated bywhere Cj stands for the concentration of each PCBs or PCDD/Fs isomer group and nj for the number of chlorine atoms of each PCBs or PCDD/Fs homologue group; C corresponds to the total concentration of PCBs or PCDD/Fs.
3. Results and discussion
3.1. Degradation effect of ball milling
Numerous studies have demonstrated the feasibility of the mechanochemical method as a potential and promising technique for degrading POPs.3,9,22PCBs samples were decomposed mechanochemically through hydrodechlorination by grinding jointly with CaO.23,24 In this study, the PCBs-contaminated soil was first grinded for 2 hours at 400 rpm together with CaO and SiO2. The residual PCBs, dl-PCBs and TEQ-values found in the soil after grinding are listed in Table 1.
After 2 hours of grinding, the total amount of PCBs in the soil decreased from 505 μg g−1 to 91.2 μg g−1, attaining a degradation efficiency of 81.9%. The residual dl-PCBs concentration in grinded soil was 2.4 μg g−1, corresponding with a degradation efficiency of 87.5%, while the degradation efficiency of their WHO-TEQ reached 85.4%. Compared with the soil that included only the CaO, the addition of SiO2 improved the degradation efficiency of all samples. These results accord with the study of Zhang,13 who found that the addition of quartz to the grinding mix facilitated hydrodechlorination of 3-MCP, especially in cases with a higher weight ratio than 10% of 3-MCP to CaO.
After 2 hours of grinding, a degradation efficiency of 81.9% was attained, but the treatment times required are too long if the destruction should be augmented much further. After 5 hours of grinding, about 0.75% of the original PCPs were left in the treated soil, but during the next 5 hour treatment, the degradation efficiency of PCPs was only up by 0.66%.7 So, for the purpose of reducing the treatment time, thermal desorption was introduced for further remediation.
3.2. PCBs removal during thermal treatment
Table 2 shows the amount of PCBs in both soil and gas phases, as determined after thermal desorption. The total residual amount of PCBs remaining in the treated soil lessened with the rising treatment temperature. After treatment at 600 °C, the residual PCBs concentration dropped to 137 ng g−1 and the desorption efficiency rose up to 99.85%, to be compared with 99.8% at a furnace temperature of 500 °C and with 96.45% at 400 °C. All desorption efficiency values are much higher than the earlier efficiency values, obtained in our previous studies, yet without preliminary grinding.29
There are three reasons that could possibly account for the difference: (a) after grinding, the larger particles (420–841 μm) were converted into finer ones (<150 μm) and decontaminated more deeply than the initial coarser particles;29 (b) PCBs molecules were gradually degraded during grinding, producing lower chlorinated species with lower boiling points, and therefore desorbed more easily from the solid matrix when heated; (c) compared with the soil being heated directly, moisture in the soil after grinding was absorbed by CaO, converting it into Ca(OH)2 and both CaO and Ca(OH)2 facilitated the hydrodechlorination and destruction of PCBs.16,30
The removal efficiency based on the initial concentration of PCBs present in the raw soil is also represented in Table 2. Considerable removal efficiency was achieved by suitably combining the mechanochemical treatment with thermal desorption: a removal efficiency of 99.36% was obtained after heating for 40 minutes at only 400 °C. When increasing the thermal treatment temperature to 600 °C, removal efficiency even approached 100%. These results indicate that combining the two techniques is feasible for remediating PCBs-contaminated soil, a proposition that considerably improves the PCBs removal efficiency reached by thermal desorption, after a relatively short time of mechanochemical pretreatment.
The proportion of low chlorinated PCBs homologues increased after thermal treatment, while the highly chlorinated PCBs homologues showed the opposite trend. As the temperature rises, this variation becomes even more pronounced, consistent with the findings from Qi.29 After heating for 40 minutes at 600 °C, the fraction of MCBs in the grinded soil rose from 0.09% to 4.57%, DiCBs from 1.8% to 12.97%, and TrCBs also increased from 32.9% to 50.84%. In contrast, the proportion of TeCBs decreased from 58.5% to 28.70% and that of PeCBs reduced from 6.2% to 2.55%.
At the same time, the weight average chlorination degree of PCBs in the treated soil lessened gradually as the temperature rose, as observed in Fig. 2a. Initially, it was still 3.71 in the grinded soil and decreased to 3.13 after heating for 40 minutes at 600 °C, demonstrating strong hydrodechlorination during thermal desorption. Possible decomposition pathways were presented by He.31
The weight average chlorination degree of PCBs in the gas varied rather little, from 3.35 at 400 °C to 3.49 at 600 °C, in contrast with the tendency seen in the soil. This may be due to soil minerals promoting contaminant decomposition.
Still, large amounts of PCBs are transferred from the soil matrix to the off-gas, though destruction, decomposition and hydrodechlorination all occur. Further treatments such as dust extraction, vapour condensation and adsorption of POPs are therefore required to decrease the concentration of contaminants in the off-gas to meet the emissions requirements prior to discharge.33,34
| Raw soil | Grinded soil | 400 °C | 500 °C | 600 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Soil | Gas | Soil | Gas | Soil | Gas | |||
| a Unit: ng g−1.b Unit: %.c Unit: pg WHO-TEQ per g. | ||||||||
| Suma | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2370 | 39.8 | 39 | 1.2 | 53 | 0.71 | 47 |
| Desorption efficiencyb | — | — | 98.32 | — | 99.95 | — | 99.97 | — |
| Removal efficiencyb | — | 87.49 | 99.79 | — | 99.99 | — | 100 | — |
| TEQc | 5760 | 839 | 23.5 | 29 | 2 | 21.5 | 0.77 | 20 |
| Desorption efficiencyb | — | — | 97.20 | — | 99.79 | — | 99.91 | — |
| Removal efficiencyb | — | 85.44 | 99.59 | — | 99.97 | — | 99.99 | — |
Both the desorption efficiency and removal efficiency of dl-PCBs improve within a temperature range of 400 to 600 °C. The desorption efficiency of dl-PCBs reaches 99.97% at 600 °C, clearly higher than the desorption efficiency of all PCBs at the same temperature. The removal efficiency of dl-PCBs approaches 100% after grinding for 2 hours and heated at 600 °C for 40 minutes. The dl-PCBs consist of relatively high chlorinated PCBs, which explains the higher desorption efficiency value.
The World Health Organization (WHO) toxic equivalence factors (TEFs) are used to calculate the WHO-TEQ contributions for PCBs,36 and this was 5760 pg WHO-TEQ per g in the raw soil. After ball milling, the TEQ dropped to 839 pg WHO-TEQ per g (degradation efficiency of 85.4%), and then further decreased to 0.77 pg WHO-TEQ per g after thermal treatment at 600 °C for 40 minutes, attaining a removal efficiency of 99.99%.
3.3. Formation of PCDD/Fs during treatment
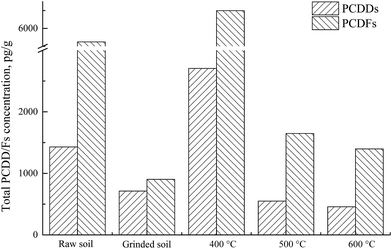
As represented in Fig. 3, the concentration of PCDDs and PCDFs in the raw soil were 1430 pg g−1 and 5250 pg g−1, respectively, and decreased to 713 pg g−1 and 903 pg g−1 after 2 hours of grinding treatment. After thermal treatment at 400 °C, both levels increased, especially PCDFs, to 6990 pg g−1, nearly 7 times higher than that in grinded soil. The results indicate that PCDDs and PCDFs were generated, in particular at 400 °C. When the treatment temperature rises to 500 or 600 °C, the concentration again drops, since 500 °C is higher than the optimum PCDD/Fs generation temperature,38 and higher temperatures facilitate the degradation and decomposition of PCDD/Fs.
Table 4 lists the concentration of PCDDs and PCDFs and their weight average chlorination degree in soil and gas. After thermal treatment, the levels of PCDDs in the soil continuously decrease as the temperature rises. PCDFs, however, show a distinct tendency. At 400 °C, their concentration peaks at 1260 pg g−1, 353 pg g−1 higher than in the grinded soil. But at higher temperatures, the amount of PCDFs quickly declined again and maintained comparable values for temperatures reaching 500 or 600 °C.
| Raw soil | Grinded soil | 400 °C | 500 °C | 600 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Soil | Gas | Soil | Gas | Soil | Gas | |||
| ∑PCDDs | 1430 | 713 | 498 | 2210 | 225 ± 16 | 325 | 203 | 255 |
| ∑PCDFs | 5250 | 903 | 1260 | 5740 | 598 ± 33 | 1050 | 550 | 847 |
| ∑PCDD/Fs | 6680 | 1620 | 1750 | 7950 | 823 | 1370 | 753 | 1100 |
| Chlorination degree | 4.36 | 4.74 | 5.25 | 5.08 | 5.24 | 4.87 | 5.12 | 4.79 |
The weight average chlorination degree of PCDD/Fs in grinded soil is 4.74 and this always rises after thermal treatment. With the rising temperature, the value slightly reduces. This observation can be explained by the fact that the formation of PCDD/Fs weakens at temperatures that are above the optimum temperature range for their generation.39 Conversely, the hydrodechlorination reaction is stronger at these higher temperatures.
In the flue gas a maximum concentration of PCDDs and PCDFs also occurs at 400 °C (Table 4), attaining 2210 pg g−1 and 5740 pg g−1, respectively. When the treatment temperature rises to 500 or 600 °C, decomposition becomes stronger than formation.
The trend of the chlorination degree in the gas phase resembles that in the soil. As treatment temperature increases, the value reduces slightly. All chlorination levels are a little lower in the soil than in the gas phase: formation occurs before the contaminant desorbs from the soil surface and decomposition dominates once the contaminant transfer into the gas phase.
| Grinded soil | 400 °C | 500 °C | 600 °C | ||||
|---|---|---|---|---|---|---|---|
| Soil | Soil | Gas | Soil | Gas | Soil | Gas | |
| a Unit: pg g−1.b Unit: pg WHO-TEQ per g. | |||||||
| ∑PCDDsa | 31.1 | 111 | 389 | 47.8 | 28.5 | 33.4 | 22.2 |
| ∑PCDFsa | 101 | 365 | 1390 | 178 | 204 | 129 | 160 |
| ∑PCDD/Fsa | 132 | 476 | 1780 | 226 | 233 | 162 | 182 |
| TEQ-∑PCDDsb | 8.63 | 10.91 | 72.87 | 6.38 | 4.87 | 4.16 | 0.55 |
| TEQ-∑PCDFsb | 10.31 | 20.05 | 86.31 | 10.78 | 15.93 | 9.11 | 13.40 |
| TEQ-∑PCDD/Fsb | 18.94 | 30.95 | 159.18 | 17.17 | 20.80 | 13.27 | 13.95 |
PCDD/Fs formation was inevitable during the thermal processing of chlorinated contaminants. In practical thermal remediation processes, higher temperatures were recommended. Hydrodechlorination and the destruction reactions played an important part in the removal of PCDD/Fs when the temperature was higher than 350 °C.40 Alternatively, some appropriate chemical inhibitors could be introduced to suppress the formation of PCDD/Fs during thermal desorption.41
4. Conclusions
A combination of the mechanochemical pre-treatment and thermal desorption is a feasible alternative for remediating PCBs-contaminated soil, as it reduces remediation time and also achieves a considerable removal efficiency. After ball milling for 2 hours, the total concentration, and the TEQ, of PCBs in the soil decreases by 81.9% and 85.4%, respectively, and degradation is facilitated by the addition of SiO2.The temperature of the thermal treatment significantly influences the desorption efficiency of PCBs in the grinded soil. The residual total amount of PCBs in the thermally treated soil decreases as the temperature rises. When the temperature increased to 600 °C, the residual PCBs concentration was 137 ng g−1 and the desorption efficiency reached 99.85%. The removal efficiency, based on the raw soil, reached almost 100% after the combined treatment. Hydrodechlorination and decomposition occur during desorption, especially at high temperatures.
PCDDs and PCDFs are generated during the thermal treatment process, especially at 400 °C, while formation becomes weaker at higher temperatures. Conversely, the process of hydrodechlorination and decomposition strengthen at these higher temperatures, thereby reducing the residual content of PCDD/Fs.
References
- Y. Xing, Y. Lu, R. W. Dawson, Y. Shi, H. Zhang, T. Wang, W. Liu and H. Ren, Chemosphere, 2005, 60, 731–739 CrossRef CAS PubMed.
- Z. Qi, A. Buekens, J. Liu, T. Chen, S. Lu, X. Li and K. Cen, Environ. Sci. Pollut. Res., 2014, 21, 6448–6462 CrossRef CAS PubMed.
- S. Lu, J. Huang, P. Zheng, X. Li and J. Yan, Chem. Eng. J., 2012, 195–196, 62–68 CrossRef CAS.
- K. Zhang, J. Huang, Z. Wang, Y. Yu, S. Deng and G. Yu, J. Hazard. Mater., 2012, 243, 278–285 CrossRef CAS PubMed.
- Z. Wang, J. Huang, F. Xu, S. Deng, W. Zhu and G. Yu, J. Hazard. Mater., 2011, 198, 275–281 CrossRef PubMed.
- K. Zhang, J. Huang, H. Wang, K. Liu, G. Yu, S. Deng and B. Wang, Chemosphere, 2014, 116, 40–45 CrossRef CAS PubMed.
- Y. Wei, J. Yan, S. Lu and X. Li, J. Environ. Sci., 2009, 21, 1761–1768 CrossRef CAS.
- S. Saeki, J. Kano, F. Saito, K. Shimme, S. Masuda and T. Inoue, J. Mater. Cycles Waste Manage., 2001, 3, 20–23 CAS.
- Y. Mitoma, H. Miyata, N. Egashira, A. M. Simion, M. Kakeda and C. Simion, Chemosphere, 2011, 83, 1326–1330 CrossRef CAS PubMed.
- Q. Zhang, H. Matsumoto, F. Saito and M. Baron, Chemosphere, 2002, 48, 787–793 CrossRef CAS PubMed.
- Y. F. Yu, J. Huang, W. Zhang, K. L. Zhang, S. B. Deng and G. Yu, Chemosphere, 2013, 90, 1729–1735 CrossRef CAS PubMed.
- I. W. Nah, K. Hwang and Y. Shul, Chemosphere, 2008, 73, 138–141 CrossRef CAS PubMed.
- Q. Zhang, F. Saito, T. Ikoma, S. Tero-Kubota and K. Hatakeda, Environ. Sci. Technol., 2001, 35, 4933–4935 CrossRef CAS PubMed.
- Y. Wei, PhD Dissertation, Zhejiang Univ, 2010.
- Z. Zhao, M. Ni, X. Li and A. Buekens, Int. J. Environ. Pollut., 2016, 60, 171–189 CrossRef.
- R. Weber, T. Takasuga, K. Nagai, H. Shiraishi, T. Sakurai, T. Matuda and M. Hiraoka, Chemosphere, 2002, 46, 1255–1262 CrossRef CAS PubMed.
- P. Varanasi, A. Fullana and S. Sidhu, Chemosphere, 2007, 66, 1031–1038 CrossRef CAS PubMed.
- J. Liu, Z. Qi, Z. Zhao, X. Li, A. Buekens, J. Yan and M. Ni, Environ. Sci. Pollut. Res., 2015, 22, 19538–19545 CrossRef CAS PubMed.
- T. Sato, T. Todoroki, K. Shimoda, A. Terada and M. Hosomi, Chemosphere, 2010, 80, 184–189 CrossRef CAS PubMed.
- J. Liu, Z. Qi, X. Li, T. Chen, A. Buekens, J. Yan and M. Ni, Environ. Sci. Pollut. Res., 2015, 22, 12289–12297 CrossRef CAS PubMed.
- J. Huang, T. Matsumura, G. Yu, S. Deng, M. Yamauchi, N. Yamazaki and R. Weber, Chemosphere, 2011, 85, 239–246 CrossRef CAS PubMed.
- J. H. Yan, Z. Peng, S. Y. Lu, X. D. Li, M. J. Ni, K. F. Cen and H. F. Dai, J. Hazard. Mater., 2007, 147, 652–657 CrossRef CAS PubMed.
- Y. Tanaka, Q. Zhang, K. Mizukami and F. Saito, Bull. Chem. Soc. Jpn., 2003, 76, 1919–1925 CrossRef CAS.
- Y. Tanaka, Q. Zhang and F. Saito, J. Phys. Chem. B, 2003, 107, 11091–11097 CrossRef CAS.
- J. Merino and V. Bucala, J. Hazard. Mater., 2007, 143, 455–461 CrossRef CAS PubMed.
- P. P. Falciglia, M. G. Giustra and F. Vagliasindi, J. Hazard. Mater., 2011, 185, 392–400 CrossRef CAS PubMed.
- L. Lundin, J. Aurell and S. Marklund, Chemosphere, 2011, 84, 305–310 CrossRef CAS PubMed.
- Z. Qi, PhD Dissertation, Zhejiang Univ, 2014.
- Z. Qi, T. Chen, S. Bai, M. Yan, S. Lu, A. Buekens, J. Yan, C. Bulmău and X. Li, Environ. Sci. Pollut. Res., 2014, 21, 4697–4704 CrossRef CAS PubMed.
- Y. Mitoma, S. R. Mallampati, H. Miyata and M. Kakeda, Arch. Environ. Contam. Toxicol., 2013, 64, 180–186 CrossRef CAS PubMed.
- N. He, P. Li, Y. Zhou, W. Ren, S. Fan and V. A. Verkhozina, J. Hazard. Mater., 2009, 164, 126–132 CrossRef CAS PubMed.
- H. I. Gomes, C. Dias-Ferreira and A. B. Ribeiro, Sci. Total Environ., 2013, 445, 237–260 CrossRef PubMed.
- S. Kim, K. Lee, K. Kim, M. Kwon and G. Song, J. Waste Manage., 2007, 27, 1593–1602 CrossRef CAS PubMed.
- P. C. Hung, W. C. Lo, K. H. Chi, S. H. Chang and M. B. Chang, Chemosphere, 2011, 82, 72–77 CrossRef CAS PubMed.
- J. Liu, Z. Cui and H. Xu, The review of the environmental behaviors of polychlorinated biphenyls (PCBs) in soils and sediments, J. Shandong Univ., 2006, 36, 94–98 Search PubMed.
- M. Van den Berg, L. S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg and L. Haws, Toxicol. Sci., 2006, 93, 223–241 CrossRef CAS PubMed.
- K. Olie, P. L. Vermeulen and O. Hutzinger, Chemosphere, 1977, 6, 455–459 CrossRef CAS.
- M. Yan, X. Li, T. Chen, S. Lu, J. Yan and K. Cen, J. Environ. Sci., 2010, 22, 1637–1642 CrossRef CAS.
- M. B. Chang and T. F. Huang, Chemosphere, 2000, 40, 159–164 CrossRef CAS PubMed.
- H. Wu, S. Lu, J. Yan, X. Li and T. Chen, Chemosphere, 2011, 84, 361–367 CrossRef CAS PubMed.
- Z. Zhao, M. Ni, X. Li, A. Buekens and J. Yan, Environ. Sci. Pollut. Res., 2016, 23, 25335–25342 CAS.
| This journal is © The Royal Society of Chemistry 2017 |