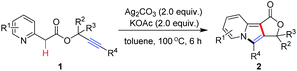Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of fused tricyclic indolizines by intramolecular silver-mediated double cyclization of 2-(pyridin-2-yl)acetic acid propargyl esters†
Hai-Yuan Zhao‡
a,
Ying-chun Wang‡b,
Xiao-Lin Caoa,
Qiu-Fang Panga,
Heng-shan Wang *a and
Ying-ming Pan
*a and
Ying-ming Pan *a
*a
aState Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences of Guangxi Normal University, Guilin 541004, People's Republic of China. E-mail: whengshan@163.com; panym2013@hotmail.com
bCollege of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, People's Republic of China
First published on 3rd May 2017
Abstract
The treatment of a toluene solution of easily accessible 2-(pyridin-2-yl)acetic acid propargyl esters with 2.0 equiv. of Ag2CO3 in the presence of potassium acetate (2.0 equiv.) at 100 °C afforded fused tricyclic indolizines in good to excellent yields. The reaction proceeded through a domino silver-mediated double cyclization sequence involving a 5-exo-dig cyclization and 1,3-hydrogen shift followed by an intramolecular cycloisomerization.
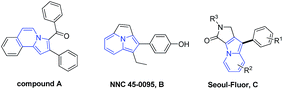
The indolizine ring system is prevalent in a wide range of natural and synthetic compounds and possesses different biological and pharmacological activities,1 such as anti-inflammatory,1e antimicrobial,1g antioxidant,1h–i and 5-HT3 receptor antagonist1j activities, to name a few. It is also used as a building block in the syntheses of many bioactive and heterocyclic compounds.2 Among such compounds, fused polycyclic indolizines are particularly attractive since their analogues have been used as biologically interesting compounds1a and fluorescent molecules.3 For example, compound A exhibited dual antifungal and antibacterial activity with MIC values in the range of 500–1000 μg mL−1 against fungal strains A. niger, C. albicans and C. tropicalis, while for bacterial strains MIC values were in the range of 32–500 μg mL−1.4 Compound B (NNC 45-0095) possesses comparable estrogen agonist activity with IC50 = 9.5 nM when compared to standard drug moxestrol (IC50 = 2.5 nM).5 Polycyclic indolizine C (Seoul-Fluor) is a novel full-color-tunable fluorescent core skeleton developed by Park and co-workers.6 Based on their structural and biological importance, the development of more direct and economical methods for their preparation is highly desirable.
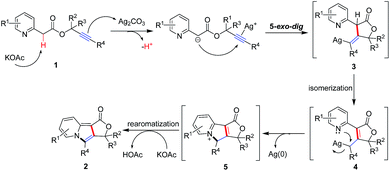
Intramolecular cascade reactions are one of the most ideal processes in organic synthesis from an atom- and step-economical point of view, which can allow for the straightforward and selective construction of complex cyclic molecular structures in a one-pot manner.7 In our former study, we developed a silver-mediated sequential oxidative C–H functionalization and 5-endo-dig cyclization of 2-alkylpyridines with terminal and internal alkynes. This reaction provides a straightforward route to access biologically important 1,3-disubstituted and 1,2,3-trisubstituted indolizines.8 Inspired by this perspective and for the purpose of constructing the fused polycyclic indolizines skeleton, we designed substrate 1, 2-(pyridin-2-yl)acetic acid propargyl esters, and anticipated that in the presence of Ag2CO3 and KOAc, compound 1 underwent deprotonation and 5-exo-dig cyclization9 to produce intermediate 3, which thus yielded intermediate 4 followed by isomerization. Subsequent intramolecular aromatization of 4 would afford fused tricyclic indolizine product 2 as shown in Scheme 1. This silver-mediated double cyclization of 2-(pyridin-2-yl)acetic acid propargyl esters would provide a rapid, straightforward and atom-economic route to access biologically important fused tricyclic indolizines. Although extensive works have generated a significant number of approaches for the synthesis of indolizines,10 the silver-mediated intramolecular cascade annulations of 2-(pyridin-2-yl)acetic acid propargylesters have not been reported, and it offers an attractive alternative method for the synthesis of fused polycyclic indolizines (Fig. 1).
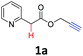
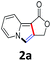
We began our studies using the easily accessible prop-2-ynyl 2-(pyridin-2-yl)acetate (1a)11 as a model substrate for the survey of reaction conditions. As shown in Table 1, the double cyclization of 1a proceeded efficiently in the presence of 2.0 equiv. of Ag2CO3 and 2.0 equiv. of KOAc in toluene at 100 °C to afford furo[3,4-a]indolizin-1(3H)-one (2a) in 90% yield (Table 1, entry 1). Without any metal salts, most of the starting material 1a was recovered (Table 1, entry 2), other metal salts, such as Cu(OAc)2, CdCO3, Pd(OAc)2, Pd[P(C6H5)3]4 or AgNO3, were totally ineffective for this conversion (Table 1, entries 3–7). Whereas AgOTf could also provide the fused tricyclic indolizine product 2a in 66% yield (Table 1, entry 8). The screening of bases revealed that the base played an important role in this transformation, and KOAc provided the best yield of 90% (Table 1, entry 1 vs. entries 10–12). In the absence of base, no reaction occurred (Table 1, entry 9). Solvent screening studies showed that none of the other solvents used, namely, PhCl, DMF, DMSO and CH3CN gave a higher yield than toluene (Table 1, entries 13–16). Additionally, 100 °C was found to be optimal reaction temperature. Although the reaction proceeded much more cleanly when the temperature was lowered to 60 °C, this resulted in a much lower yield of the product (Table 1, entry 17), and increasing the reaction temperature to 140 °C, the yield of 2a dramatically decreased to 10% (Table 1, entry 18). Unfortunately, the mediator and base loading (2.0 equiv.) could not be decreased. Running the reaction at a lower loading of Ag2CO3 (1.0 equiv.) or KOAc (1.0 equiv.) hampered the reaction efficiency (Table 1, entries 19 and 20).
| Entry | Catalyst (equiv.) | Base (equiv.) | Solvent | t (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions conditions: 1a (0.5 mmol), catalyst, base, solvent (2.0 mL), 100 °C (except for entry 17 and entry 18) for 6 h.b Isolated yield of pure product based on 1a. Entry in bold highlights optimized reaction conditions. | |||||
| 1 | Ag2CO3 (2) | KOAc (2) | Toluene | 100 | 90 |
| 2 | None | KOAc (2) | Toluene | 100 | 0 |
| 3 | Cu(OAc)2 (2) | KOAc (2) | Toluene | 100 | 0 |
| 4 | CdCO3 (2) | KOAc (2) | Toluene | 100 | 0 |
| 5 | Pd(OAc)2 (2) | KOAc (2) | Toluene | 100 | 0 |
| 6 | Pd[P(C6H5)3]4 (2) | KOAc (2) | Toluene | 100 | 0 |
| 7 | AgNO3 (2) | KOAc (2) | Toluene | 100 | 0 |
| 8 | AgOAc (2) | KOAc (2) | Toluene | 100 | 66 |
| 9 | Ag2CO3 (2) | None | Toluene | 100 | 0 |
| 10 | Ag2CO3 (2) | NaOH (2) | Toluene | 100 | Trace |
| 11 | Ag2CO3 (2) | t-BuOK (2) | Toluene | 100 | Trace |
| 12 | Ag2CO3 (2) | K2CO3 (2) | Toluene | 100 | Trace |
| 13 | Ag2CO3 (2) | KOAc (2) | PhCl | 100 | 82 |
| 14 | Ag2CO3 (2) | KOAc (2) | DMF | 100 | 75 |
| 15 | Ag2CO3 (2) | KOAc (2) | DMSO | 100 | 28 |
| 16 | Ag2CO3 (2) | KOAc (2) | CH3CN | 100 | 41 |
| 17 | Ag2CO3 (2) | KOAc (2) | Toluene | 60 | 53 |
| 18 | Ag2CO3 (2) | KOAc (2) | Toluene | 140 | 10 |
| 19 | Ag2CO3 (1) | KOAc (2) | Toluene | 100 | 42 |
| 20 | Ag2CO3 (2) | KOAc (1) | Toluene | 100 | 45 |
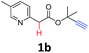
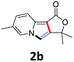
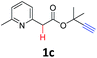
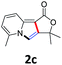
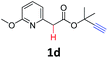
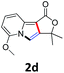
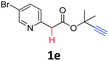
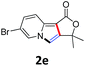
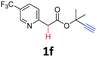
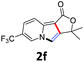
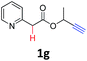
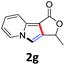
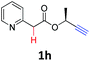
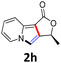
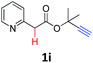
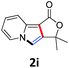
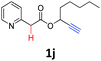
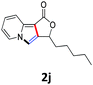
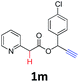
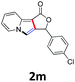
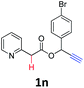
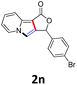
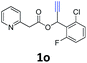
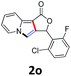
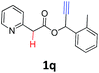
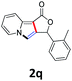
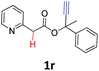
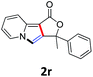
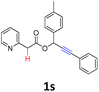
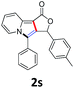
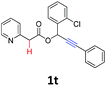
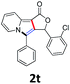
A series of substrates 1 were prepared (see the ESI† for details) to investigate the scope of the double cyclization reaction under the optimized conditions (Table 2). The R1 in the pyridine ring has been substituted with 5-methyl, 6-methyl, 6-methoxy, 5-bromo and 5-trifluoromethyl groups whereas R2 and R3 in the propargyl group included alkyl and aryl moieties. The R4 in the alkyne has been substituted by a phenyl group. As shown in Table 2, all the reactions proceeded smoothly to afford the corresponding fused tricyclic indolizines in good to excellent yields (63–90%). It was found that the electronic properties of the substituent on the pyridine ring had a negligible effect on the yields of the final compounds (2b–2f). While replacing R2 or R3 with an aromatic ring (2m–2r) resulted in somewhat lower yields (63–78%) than an aliphatic moiety (2g–2k, 86–89%). Owing to the steric hindrance of the o-F and o-Cl (Table 2, entry 15), the substrate 1o gave the desired product 2o in a lower yield (63%) compared to the p-Cl-substituted 1m (71%) and p-Br-substituted 1n (75%). When optically active (S)-but-3-yn-2-yl 2-(pyridin-2-yl)acetate 1h was examined as a substrate, to our delight, (S)-3-methylfuro[3,4-a]indolizin-1(3H)-one 2h was formed in 86% yield (Table 2, entry 8). Additionally, substituting R4 with a phenyl group furnished 2s and 2t in reduced yields of 77 and 66% respectively (Table 2, entries 19 and 20). The crystallization of compound 2i from chloroform and ethanol gave a single crystal suitable for X-ray analysis. Fig. 2 illustrates the molecular structure of the fused tricyclic indolizine 2i.12
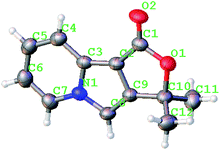 | ||
| Fig. 2 X-ray crystal structure of fused tricyclic indolizine 2i (CCDC 1501568). | ||
To further support the proposed reaction pathway, additional control experiments were carried out. It was observed that the presence of 2 equiv. of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) did not suppress the double cyclization of substrate 1a under optimized conditions, suggesting that a radical mechanism was not likely involved. ESI/MS experiments were performed to gain evidence for the possible intermediates in the proposed mechanism. A mixture of 1a (0.5 mmol), KOAc (1.0 mmol) and Ag2CO3 (1.0 mmol) in toluene (2.0 mL) was reacted at 100 °C for 30 min and 50 μL of the mixture was used for the ESI analysis in CH3CN. The ESI/MS analyses showed a peak at m/z 174.0548, which was identified as intermediate 5a (see the ESI†).
In conclusion, we have developed a rapid, simple and efficient double cyclization reaction for the synthesis of fused tricyclic indolizines from easily available starting materials in good to excellent yields. The salient feature of this method involves a silver-mediated 5-exo-dig cyclization and 1,3-hydrogen shift followed by an intramolecular cycloisomerization in one pot. Molecular biology studies involving derivatives of this scaffold are currently in progress.
Acknowledgements
We would like to thank the National Natural Science Foundation of China (21362002 and 81260472), the Ministry of Education of China (IRT_16R15), Guangxi Natural Science Foundation of China (2014GXNSFDA118007, 2016GXNSFGA380005 and 2016GXNSFEA380001), the State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2014-A02 and CMEMR2012-A20), Hunan Province Natural Science Foundation of China (2016JJ4075), and Science Research Project of Hunan Provincial Department of Education (16B211).Notes and references
- (a) G. S. Singh and E. E. Mmatli, Eur. J. Org. Chem., 2011, 5237 CAS; (b) H. Li, Z. Xia, S. Chen, K. Koya, M. Ono and L. Sun, Org. Process Res. Dev., 2007, 11, 246 CrossRef CAS; (c) J. P. Michael, Nat. Prod. Rep., 2007, 24, 191 RSC; (d) J. P. Michael, Nat. Prod. Rep., 2008, 25, 139 RSC; (e) S. Hagishita, M. Yamada, K. Shirahase, T. Okada, Y. Murakami, Y. Ito, T. Matsuura, M. Wada, T. Kato, M. Ueno, Y. Chikazawa, K. Yamada, T. Ono, I. Teshirogi and I.-M. Ohtani, J. Med. Chem., 1996, 39, 3636 CrossRef CAS PubMed; (f) R. C. Oslund, N. Cermark and M. H. Gelb, J. Med. Chem., 2008, 51, 4708 CrossRef CAS PubMed; (g) L.-L. Gundersen, C. Charnock, A. H. Negussie and F. Rise, Eur. J. Pharm. Sci., 2007, 30, 26 CrossRef CAS PubMed; (h) O. B. Østby, B. Dalhus, L.-L. Gundersen, F. Rise, A. Bast and G. R. M. M. Haenen, Eur. J. Org. Chem., 2000, 3763 CrossRef; (i) S. Teklu, L. L. Gundersen, T. Larsen, K. E. Malterud and F. Rise, Bioorg. Med. Chem., 2005, 13, 3127 CrossRef CAS PubMed; (j) J. Bermudez, C.-S. Fake, G.-F. Joiner, K.-A. Joiner, F.-D. King, W.-D. Miner and G.-J. Sanger, J. Med. Chem., 1990, 33, 1919 CrossRef.
- (a) K. M. Elattar, I. Youssef and A. A. Fadda, Synth. Commun., 2016, 46, 719 CrossRef CAS; (b) J.-B. Xia and S.-L. You, Org. Lett., 2009, 11, 1187 CrossRef CAS PubMed; (c) H. Hu, Y. Liu, H. Zhong, Y. Zhu, C. Wang and M. Ji, Chem.–Asian J., 2012, 7, 884 CrossRef CAS PubMed; (d) Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC.
- (a) Y.-M. Shen, G. Grampp, N. Leesakul, H.-W. Hu and J.-H. Xu, Eur. J. Org. Chem., 2007, 3718 CrossRef CAS; (b) B. Liu, Z. Wang, N. Wu, M. Li, J. You and J. Lan, Chem.–Eur. J., 2012, 18, 1599 CrossRef CAS PubMed; (c) A. Vlahovici, M. Andrei and I. Druta, J. Lumin., 2002, 96, 279 CrossRef CAS; (d) A. Vlahovici, I. Druta, M. Andrei, M. Cotlet and R. Dinica, J. Lumin., 1999, 82, 155 CrossRef CAS; (e) T. Mitsumori, M. Bendikov, O. Dautel, F. Wudl, T. Shioya, H. Sato and Y. Sato, J. Am. Chem. Soc., 2004, 126, 16793 CrossRef CAS PubMed.
- A. Hazra, S. Mondal, A. Maity, S. Naskar, P. Saha, R. Paira, K. B. Sahu, P. Paira, S. Ghosh, C. Sinha, A. Samanta, S. Banerjee and N. B. Mondal, Eur. J. Med. Chem., 2011, 46, 2132 CrossRef CAS PubMed.
- A. S. Jorgensen, P. Jacobsen, L. B. Christiansen, P. S. Bury, A. Kanstrup, S. M. Thorpe, S. Bain, L. Naerum and K. Wassermann, Bioorg. Med. Chem. Lett., 2000, 10, 399 CrossRef CAS PubMed.
- (a) E. Kim, M. Koh, J. Ryu and S. B. Park, J. Am. Chem. Soc., 2008, 130, 12206 CrossRef CAS PubMed; (b) E. Kim, M. Koh, B. J. Lim and S. B. Park, J. Am. Chem. Soc., 2011, 133, 6642 CrossRef CAS PubMed; (c) E. Kim, Y. Lee, S. Lee and S. B. Park, Acc. Chem. Res., 2015, 48, 538 CrossRef CAS PubMed.
- For some reviews, see: (a) M. Malacria, Chem. Rev., 1996, 96, 289 CrossRef CAS PubMed; (b) K. A. Millera and R. M. Williams, Chem. Soc. Rev., 2009, 38, 3160 RSC; (c) L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Acc. Chem. Res., 2012, 45, 1278 CrossRef CAS PubMed; (d) K. C. Majumdar, RSC Adv., 2011, 1, 1152 RSC.
- X. Tan, Y. Liang, F. Bao, H. Wang and Y. Pan, Tetrahedron, 2014, 70, 6717 CrossRef CAS.
- (a) L. Wang, S.-Y. Cai, X.-Y. Xing, Y. Gao, T. Wang and D. Z.-G. Wang, Org. Lett., 2013, 15, 2362 CrossRef CAS PubMed; (b) K.-H. Kwon, C. M. Serrano, M. K. L. R. Barrows and R. E. Looper, Org. Lett., 2014, 16, 6048 CrossRef CAS PubMed; (c) C. Gronnier, P. F. D. Bel, G. Henrion, S. Kramer and F. Gagosz, Org. Lett., 2014, 16, 2092 CrossRef CAS PubMed; (d) D. Hack, P. Chauhan, K. Deckers, G. N. Hermann, L. Mertens, G. Raabe and D. Enders, Org. Lett., 2014, 16, 5188 CrossRef CAS PubMed; (e) T. Godet, C. Vaxelaire, C. Michel, A. Milet and P. Belmont, Chem.–Eur. J., 2007, 13, 5632 CrossRef CAS PubMed; (f) E. Parker, N. Leconte, T. Godet and P. Belmont, Chem. Commun., 2011, 47, 343 RSC.
- For representative examples, see: (a) Y. Shi and V. Gevorgyan, Chem. Commun., 2015, 51, 17166 RSC; (b) S. Tang, K. Liu, Y. Long, X. Qi, Y. Lan and A. Lei, Chem. Commun., 2015, 51, 8769 RSC; (c) X. Wang, S. Li, Y. Pan, H. Wang, H. Liang, Z. Chen and X. Qin, Org. Lett., 2014, 16, 580 CrossRef CAS PubMed; (d) A. N. Pandya, J. T. Fletcher, E. M. Villa and D. K. Agrawal, Tetrahedron Lett., 2014, 55, 6922 CrossRef CAS PubMed; (e) H. Hu, J. Feng, Y. Zhu, N. Gu and Y. Kan, RSC Adv., 2012, 2, 8637 RSC; (f) Y. Liu, H.-Y. Hu, Q.-J. Liu, H.-W. Hu and J.-H. Xu, Tetrahedron, 2007, 63, 2024 CrossRef CAS; (g) R. Azpíroz, A. D. Giuseppe, R. Castarlenas, J. J. Pérez-Torrente and L. A. Oro, Chem.–Eur. J., 2013, 19, 3812 CrossRef PubMed.
- B. Sahoo, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 15545 CrossRef CAS PubMed.
- CCDC 1501568 (2i) contains the supplementary crystallographic data for this paper.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental procedures, and spectral data, NMR spectra, high resolution mass spectra for all compounds, and X-ray crystallographic files (CIF) for 2g. CCDC 1501568. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra00892a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |