Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective water-based oxychlorination of phenol with hydrogen peroxide catalyzed by manganous sulfate†
Hongchuan Xina,
Shilei Yangb,
Baigang An*b and
Zengjian An *a
*a
aKey Laboratory of Biobased Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, 189 Songling Road, Qingdao 266101, China. E-mail: anzj@qibebt.ac.cn
bSchool of Chemical Engineering, University of Science and Technology Liaoning, Anshan 114051, China. E-mail: bgan@ustl.edu.cn
First published on 28th February 2017
Abstract
An efficient method for the selective oxychlorination of phenol to 2,4-dichlorophenol catalyzed by manganous(II) sulfate is developed using hydrogen chloride as a chlorinating source, hydrogen peroxide as an oxidant and water as a solvent. The catalyst has high activity and selectivity under mild conditions. The products are automatically isolated from aqueous solution, which also contains the catalyst at the end of the reaction, and hence product separation and catalyst recycling are both simple in this system. The performance of manganous(II) sulfate with the oxidative chlorinating system HCl/H2O2 indicates that this is a promising synthetic method for the manufacture of various 2,4-dichlorophenol derivatives.
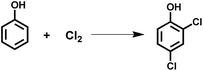
Chlorination of phenols is a key synthetic method because chloro-substituted phenols are essential materials in the synthesis of herbicides, pharmaceuticals, insecticides, dyes, etc.1,2 Among the various chloro-substituted phenols, main products include p-chlorophenol, o-chlorophenol, 2,4-dichlorophenol, 2,6-dichlorophenol and 2,4,6-trichlorophenol, which belong to a group named “light chlorophenols”.3 In this group, 2,4-dichlorophenol is the most important material since it is an intermediate for 2,4-dichlorophenoxyacetic acid, a herbicide that is widely used in crops, rice and other massive cultivations. Traditional methods for the synthesis of 2,4-dichlorophenol usually involve electrophilic aromatic chlorination of phenol with chlorine gas (Scheme 1).3–5 However, the utilization of chlorine atoms in these processes is quite low, nearly half of the chlorine is released as waste gas, which results in a waste of material and environment hazards.6,7
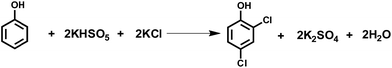
An alternative solution is oxidative chlorination, i.e. oxychlorination, which uses chloride anions as a chlorine source in the presence of an oxidant (Scheme 2).7–12 In these systems, chloride anions are oxidized to chlorine by the oxidant and subsequently incorporated into the products. In general, chlorinating agents, such as sulfuryl chloride,4,13 N-chlorosuccinimide,11,12 copper chloride,14–16 titanium tetrachloride17 and p-toluene sulfonyl chloride,18 are used as chlorine sources, and reagents like sulfuric acid,19 perchloric acid,12,20 lithium diisopropylamide,18 dimethylsulfoxide,21 etc.22 are employed as oxidants. While the utilization of chlorine atoms in oxychlorination is remarkably increased compared to only half in traditional methods, further research is still required to use cheaper and environmentally friendly reagents.
Compared with most studies focused on the synthesis of monochloro-substituted phenols,4,23–28 the oxychlorination of phenols to 2,4-dichlorophenols is rather limited so far.3,20,29 For example, Gusevskaya et al. reported a method for aerobic oxychlorination of phenols over a CuCl2 catalyst, in which metal chlorides were used as chlorinating agents.30,31 Feng et al. found a microwave method for aerobic oxychlorination of phenols catalyzed by CuCl2 with hydrochloric acid as a chlorine source.23 However, these methods are exploited for the synthesis of p-chlorophenols. Notably, Ratnasamy et al. developed a promising method for the oxychlorination/oxybromination of aromatics including phenols over copper phthalocyanines encapsulated in zeolites with HCl and alkali chlorides/bromides as halogen sources and dioxygen and hydrogen peroxide as oxidants,32 but the selectivity for 2,4-dichloroaromatics was low. Moreover, VOCs (volatile organic compounds) are usually present in these systems (Scheme 3).
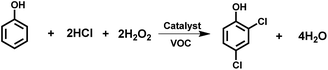 | ||
| Scheme 3 Oxychlorination for the synthesis of 2,4-dichlorophenol with environmentally friendly oxidants. | ||
As for oxychlorination, we consider a desirable route for the chlorination of phenols with HCl using environmentally friendly oxidants, such as dioxygen or hydrogen peroxide,23,32,33 in which chlorine anions are incorporated into the product via oxidation and water is the only by-product from the oxidants. On the other hand, the use of water as a solvent is particularly attractive because: (1) water is cheaper than VOC solvents; (2) the risk of explosions using a water-based system is much lower than that for systems containing VOCs; (3) 2,4-dichlorophenol is almost insoluble in water, and hence the product can be obtained by simple phase separation, which is convenient in application. However, this is a challenging topic, especially since catalytic methods for 2,4-dichlorophenol with high activity and selectivity have not been reported previously.
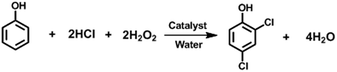
Herein, we present an efficient manganous(II) sulfate-catalyzed oxychlorination of phenol in water using the oxidative chlorinating system HCl/H2O2 (Scheme 4). Complete conversion of phenol and high selectivity for 2,4-dichlorophenol are both achieved under mild conditions. To the best of our knowledge, this is the first report on selective water-based oxychlorination of phenol into 2,4-dichlorophenol in the liquid phase under VOC-free conditions.
In a typical reaction, phenol (1a) and a catalyst were added into water in a glass flask, and gaseous HCl was introduced to form a homogeneous solution. Then, 30% of an aqueous solution of H2O2 was added to start the reaction. In the initial study, the molar ratio of HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a at 7.1
1a at 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used to screen the catalysts, and the results are shown in Table 1. In the absence of H2O2, no reaction occurred even with a prolonged time (Table 1, run 1). When H2O2 was added in the absence of a catalyst, conversion of 1a reached 69% with a total yield of 67%, the products were composed of <1% of 2,4-dichlorophenol (1b), 45% of p-chlorophenol (p-) and 21% of o-chlorophenol (o-) (Table 1, run 2). In the presence of H2O2, metal salts showed different activities. Among the tested metal salts, NaCl, CaCl2, MgCl2, ZnCl2, FeSO4, FeCl3, NiSO4, Co(OAc)2, Co(acac)2, LiCl, Cu(NO3)2, Cu(OAc)2 and CuBr2 had a poor effect (Table 1, run 3–15), as both the conversions and total yields were lower than those using H2O2 alone. Notably, CuCl2 and MnSO4 indicated high activities; the product distribution showed a dependence on the catalysts. A conversion of 85% for 1a was obtained over CuCl2, the total yield was 83%, containing <1% of 1b, 54% of p- and 29% of o- (Table 1, run 16). Conversion of 1a over MnSO4 reached as high as 93% with a total yield of 91%, and the products were 16% of 1b, 49% of p-, 26% of o- and trace 2,6-dichlorophenol (1c) (Table 1, run 17). Obviously, MnSO4 is more effective in the formation of 1b. Meanwhile, various manganese compounds were used, and the effect of anions on the reactions was tested (Table 1, run 18–20). Conversions and yields over MnCl2 and Mn(NO3)2 were comparable with those of MnSO4. Because the solubility of MnO2 in the solution was not good and some solid precipitate was always observed during the reaction, its activity was poor. These results indicated that anions had no effect on the reactions, and Mn2+ was key in this system.
1 was used to screen the catalysts, and the results are shown in Table 1. In the absence of H2O2, no reaction occurred even with a prolonged time (Table 1, run 1). When H2O2 was added in the absence of a catalyst, conversion of 1a reached 69% with a total yield of 67%, the products were composed of <1% of 2,4-dichlorophenol (1b), 45% of p-chlorophenol (p-) and 21% of o-chlorophenol (o-) (Table 1, run 2). In the presence of H2O2, metal salts showed different activities. Among the tested metal salts, NaCl, CaCl2, MgCl2, ZnCl2, FeSO4, FeCl3, NiSO4, Co(OAc)2, Co(acac)2, LiCl, Cu(NO3)2, Cu(OAc)2 and CuBr2 had a poor effect (Table 1, run 3–15), as both the conversions and total yields were lower than those using H2O2 alone. Notably, CuCl2 and MnSO4 indicated high activities; the product distribution showed a dependence on the catalysts. A conversion of 85% for 1a was obtained over CuCl2, the total yield was 83%, containing <1% of 1b, 54% of p- and 29% of o- (Table 1, run 16). Conversion of 1a over MnSO4 reached as high as 93% with a total yield of 91%, and the products were 16% of 1b, 49% of p-, 26% of o- and trace 2,6-dichlorophenol (1c) (Table 1, run 17). Obviously, MnSO4 is more effective in the formation of 1b. Meanwhile, various manganese compounds were used, and the effect of anions on the reactions was tested (Table 1, run 18–20). Conversions and yields over MnCl2 and Mn(NO3)2 were comparable with those of MnSO4. Because the solubility of MnO2 in the solution was not good and some solid precipitate was always observed during the reaction, its activity was poor. These results indicated that anions had no effect on the reactions, and Mn2+ was key in this system.
| Run | Catalyst | Conversionb (%) | Total yieldc (%) | Yieldd (%) | |||
|---|---|---|---|---|---|---|---|
| 1b | p- | o- | 1c | ||||
| a Reaction conditions: 1a: 21.3 mmol, catalyst: 1 mol%, HCl: 151.3 mmol, H2O2 (30% aq. solution): 4.05 ml, 39.7 mmol, H2O: 9.8 ml, rt., 3 h.b Conversion (%) = [the converted 1a (mol)/initial 1a (mol)] × 100.c Total yield (%) = [all products (mol)/initial 1a (mol)] × 100.d Yield (%) = [target product (mol)/initial 1a (mol)] × 100.e No H2O2 was added. | |||||||
| 1e | — | <1 | — | — | — | — | — |
| 2 | — | 69 | 67 | <1 | 45 | 21 | — |
| 3 | NaCl | 38 | 34 | <1 | 22 | 12 | — |
| 4 | CaCl2 | 45 | 41 | <1 | 26 | 14 | — |
| 5 | MgCl2 | 40 | 38 | <1 | 22 | 16 | — |
| 6 | ZnCl2 | 36 | 33 | <1 | 19 | 14 | — |
| 7 | FeSO4 | 54 | 51 | <1 | 41 | 10 | — |
| 8 | FeCl3 | 61 | 55 | <1 | 33 | 22 | — |
| 9 | NiSO4 | 56 | 40 | <1 | 25 | 15 | — |
| 10 | Co(OAc)2 | 21 | 20 | <1 | 15 | 5 | — |
| 11 | Co(acac)2 | 28 | 27 | <1 | 19 | 8 | — |
| 12 | LiCl | 48 | 28 | <1 | 18 | 10 | — |
| 13 | Cu(NO3)2 | 18 | 16 | <1 | 12 | 4 | — |
| 14 | Cu(OAc)2 | 27 | 23 | <1 | 16 | 7 | — |
| 15 | CuBr2 | 56 | 51 | <1 | 34 | 17 | — |
| 16 | CuCl2 | 85 | 83 | <1 | 54 | 29 | — |
| 17 | MnSO4 | 93 | 91 | 16 | 49 | 26 | Trace |
| 18 | MnCl2 | 91 | 89 | 14 | 47 | 28 | Trace |
| 19 | Mn(NO3)2 | 94 | 90 | 19 | 45 | 26 | Trace |
| 20 | MnO2 | 67 | 65 | 8 | 38 | 19 | — |
Reactions over MnSO4 were optimized, and the results are listed in Table 2. The effect of the catalyst was examined at a ratio of HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a = 7.1
1a = 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, run 1–3). As MnSO4 was varied from 1 mol% to 10 mol%, 1a was completely converted at room temperature, the total yield decreased from 91% to 80%, the yield of 1b increased from 16% to 57%, but the yield for p-decreased from 49% to 15% and o- was from 26% to 8%. In these processes, trace 1c was found. Obviously, over 5 mol% of MnSO4 resulted in lower total yields due to over-oxidation. When the amount of HCl was reduced and HCl
1 (Table 2, run 1–3). As MnSO4 was varied from 1 mol% to 10 mol%, 1a was completely converted at room temperature, the total yield decreased from 91% to 80%, the yield of 1b increased from 16% to 57%, but the yield for p-decreased from 49% to 15% and o- was from 26% to 8%. In these processes, trace 1c was found. Obviously, over 5 mol% of MnSO4 resulted in lower total yields due to over-oxidation. When the amount of HCl was reduced and HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was varied to 2.4
1a was varied to 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion of 1a was 76% with a total yield of 74%, containing 9% of 1b, 39% of p-, 25% of o- and trace 1c (Table 2, run 4). A large excess amount of HCl promotes the formation of 1b. The effect of temperature was tested at a HCl
1, the conversion of 1a was 76% with a total yield of 74%, containing 9% of 1b, 39% of p-, 25% of o- and trace 1c (Table 2, run 4). A large excess amount of HCl promotes the formation of 1b. The effect of temperature was tested at a HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a ratio of 2.4
1a ratio of 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, run 5–8). As the temperature was increased from 45 °C to 90 °C, 1a was completely converted at 80 °C and the total yield was 95%; the yield for 1b reached a maximum of 72% with 13% of p-, 9% of o- and 3% of 1c. Further increasing the temperature to 90 °C resulted in over-oxidation and the total yield decreased to 83%. Thus, 80 °C is desirable for the reactions.
1 (Table 2, run 5–8). As the temperature was increased from 45 °C to 90 °C, 1a was completely converted at 80 °C and the total yield was 95%; the yield for 1b reached a maximum of 72% with 13% of p-, 9% of o- and 3% of 1c. Further increasing the temperature to 90 °C resulted in over-oxidation and the total yield decreased to 83%. Thus, 80 °C is desirable for the reactions.
| Run | MnSO4 (mol%) | T (°C) | HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (molar ratio) 1a (molar ratio) |
Conversionb (%) | Total yieldc (%) | Yieldd (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1b | p- | o- | 1c | ||||||
| a Reaction conditions: 1a: 21.3 mmol, HCl: 151.3 mmol, H2O2 (30% aq. solution): 4.05 ml, 39.7 mmol, H2O: 9.8 ml, 3 h.b Conversion (%) = [the converted 1a (mol)/initial 1a (mol)] × 100.c Total yield (%) = [all products (mol)/initial 1a (mol)] × 100.d Yield (%) = [target product (mol)/initial 1a (mol)] × 100.e HCl: 50.4 mmol.f HCl: 50.4 mmol, H2O2 (30% aq. solution): 6.08 ml, 58.8 mmol.g HCl: 44.7 mmol, H2O2 (30% aq. solution): 6.08 ml, 58.8 mmol.h HCl: 42.6 mmol, H2O2 (30% aq. solution): 4.35 ml, 42.6 mmol.i Under Ar atmosphere.j HCl: 42.6 mmol, H2O2 (30% aq. solution): 6.08 ml, 58.8 mmol.k HCl: 44.7 mmol, H2O2 (30% aq. solution): 8.10 ml, 79.4 mmol. | |||||||||
| 1 | 1 | 25 | 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 | 91 | 16 | 49 | 26 | Trace |
| 2 | 5 | 25 | 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 82 | 55 | 16 | 9 | Trace |
| 3 | 10 | 25 | 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 80 | 57 | 15 | 8 | Trace |
| 4 | 1 | 25 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76 | 74 | 9 | 39 | 25 | Trace |
| 5e | 1 | 45 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 | 86 | 25 | 38 | 22 | 1 |
| 6e | 1 | 60 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 | 92 | 41 | 31 | 18 | Trace |
| 7e | 1 | 80 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 95 | 72 | 13 | 9 | 3 |
| 8e | 1 | 90 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 83 | 65 | 11 | 5 | 2 |
| 9f | 1 | 80 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 97 | 93 | 1 | Trace | 3 |
| 10g | 1 | 80 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 95 | 91 | 1 | Trace | 3 |
| 11g | — | 80 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 96 | 34 | 39 | 23 | Trace |
| 12h | — | 80 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 | 91 | 31 | 35 | 25 | Trace |
| 13h | 1 | 80 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 | 83 | 58 | 15 | 10 | 1 |
| 14g,i | 1 | 80 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 93 | 89 | 2 | Trace | 2 |
| 15j | 1 | 80 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 92 | 85 | 3 | 2 | 2 |
| 16k | 1 | 80 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 88 | 73 | 4 | 2 | 9 |
When we increased the amount of H2O2, i.e. the ratio of HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was varied from 2.4
1a was varied from 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2.4
1 to 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of 1b reached as high as 93% with 1% of p-, trace o- and 3% of 1c (Table 2, run 9). If HCl was further decreased and HCl
1, the yield of 1b reached as high as 93% with 1% of p-, trace o- and 3% of 1c (Table 2, run 9). If HCl was further decreased and HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was tuned to 2.1
1a was tuned to 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the total yield was 95%, and the yield for 1b was 91% with 1% of p-, trace o- and 3% of 1c (Table 2, run 10). In the absence of MnSO4, the full conversion of 1a with a total yield of 96% was achieved, and the yield for 1b was 34% with 39% of p-, 23% of o- and trace 1c (Table 2, run 11). When the reaction was carried out based on the theoretical equation (Scheme 4), i.e. HCl
1, the total yield was 95%, and the yield for 1b was 91% with 1% of p-, trace o- and 3% of 1c (Table 2, run 10). In the absence of MnSO4, the full conversion of 1a with a total yield of 96% was achieved, and the yield for 1b was 34% with 39% of p-, 23% of o- and trace 1c (Table 2, run 11). When the reaction was carried out based on the theoretical equation (Scheme 4), i.e. HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was 2.0
1a was 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0
2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 85% of 1a was converted with a total yield of 83%, and the products were 58% of 1b, 15% of p-, 10% of o- and 1% of 1c (Table 2, run 13). In the absence of MnSO4, 94% of 1a was converted with a total yield of 91%, containing 31% of 1b, 35% of p-, 25% of o- and trace 1c (Table 2, run 12). Obviously, the presence of MnSO4 significantly increased the yield of 1b, and hence MnSO4 is key for the selective chlorination of 1a to 1b. When the reaction was performed under an Ar atmosphere (Table 2, run 14), the results were comparable with those in air (Table 2, run 10), so oxygen has no effect on the reactions. If 1a was chlorinated based on a ratio of HCl
1, 85% of 1a was converted with a total yield of 83%, and the products were 58% of 1b, 15% of p-, 10% of o- and 1% of 1c (Table 2, run 13). In the absence of MnSO4, 94% of 1a was converted with a total yield of 91%, containing 31% of 1b, 35% of p-, 25% of o- and trace 1c (Table 2, run 12). Obviously, the presence of MnSO4 significantly increased the yield of 1b, and hence MnSO4 is key for the selective chlorination of 1a to 1b. When the reaction was performed under an Ar atmosphere (Table 2, run 14), the results were comparable with those in air (Table 2, run 10), so oxygen has no effect on the reactions. If 1a was chlorinated based on a ratio of HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a at 2.0
1a at 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of 1b was 85% with 3% of p-, 2% of o- and 2% of 1c (Table 2, run 15). As a large excess amount of H2O2 was used (HCl
1, the yield of 1b was 85% with 3% of p-, 2% of o- and 2% of 1c (Table 2, run 15). As a large excess amount of H2O2 was used (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a = 2.1
1a = 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7
3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), over-oxidation occurred and the total yield decreased to 88% (Table 2, run 16). Because the decomposition of H2O2 is inevitable, the used amount of H2O2 in the reaction is higher than the theoretical value. The utilization of H2O2 and HCl based on the optimized conditions (Table 2, run 10) is 66% and 87%, respectively.
1), over-oxidation occurred and the total yield decreased to 88% (Table 2, run 16). Because the decomposition of H2O2 is inevitable, the used amount of H2O2 in the reaction is higher than the theoretical value. The utilization of H2O2 and HCl based on the optimized conditions (Table 2, run 10) is 66% and 87%, respectively.
When HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was 3.1
1a was 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2
4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, full conversion of 1a was achieved at 80 °C for 3 h and the total yield was 91%, the yield for 2,4,6-trichlorophenol was 73% with 11% of 1b, 7% of 1c and trace p- and o-. If HCl
1, full conversion of 1a was achieved at 80 °C for 3 h and the total yield was 91%, the yield for 2,4,6-trichlorophenol was 73% with 11% of 1b, 7% of 1c and trace p- and o-. If HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was increased to 3.5
1a was increased to 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2
4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, full conversion of 1a with a total yield of 94% was obtained, and 80% of 2,4,6-trichlorophenol with 6% of 1b and 8% of 1c were found as the products. Thus, while the performance of MnSO4 is better in the synthesis of 1b, it is also active in the synthesis of 2,4,6-trichlorophenol.
1, full conversion of 1a with a total yield of 94% was obtained, and 80% of 2,4,6-trichlorophenol with 6% of 1b and 8% of 1c were found as the products. Thus, while the performance of MnSO4 is better in the synthesis of 1b, it is also active in the synthesis of 2,4,6-trichlorophenol.
In our experiments, we found that the reagents formed a homogeneous solution before reaction, and two discrete phases were observed at the end of the reaction. The top phase is an aqueous solution containing the catalyst, and the lower is an organic phase mainly composed of 1b, p-, o- and 1c. This means that the products automatically come out from the aqueous solution. This property is really convenient for product collection via phase separation. Moreover, since MnSO4 remains in the aqueous solution, recycling of the catalyst is simple.
In fact, commercial hydrochloric acid can be directly used rather than gaseous HCl in the reactions, as similar results were also obtained under the optimized conditions. However, we think that the use of gaseous HCl is more economical, particularly for large scale production. If hydrochloric acid is used, the products can be obtained by phase separation, but the collection of the catalyst requires the removal the whole aqueous solution via evaporation. Then, the collected catalyst, 1a and hydrochloric acid are mixed for the next run. As gaseous HCl is used, the products were isolated by phase separation, the remaining aqueous solution was concentrated by removing excess water from the solution of H2O2 under reduced pressure, and gaseous HCl was introduced into the concentrated solution. Then, 1a is added for the next run. Compared with gaseous HCl, it is clear that more water needs to be evaporated when using hydrochloric acid.
Based on the optimized conditions, the recycle test results of the catalyst are shown in Table 3. After each run, the same procedure was performed until the catalyst was used 6 times. These results indicate that the catalyst was recyclable, and no significant decrease in activity was observed even after 6 runs.
| Run time | Conversionb (%) | Total yieldc (%) | Yieldd (%) | |||
|---|---|---|---|---|---|---|
| 1b | p- | o- | 1c | |||
| a Reaction conditions: 1a: 21.3 mmol, MnSO4: 1 mol%, HCl: 44.7 mmol, H2O2 (30% aq. solution): 6.08 ml, 58.8 mmol, H2O: 9.8 ml, 80 °C, 3 h.b Conversion (%) = [the converted 1a (mol)/initial 1a (mol)] × 100.c Total yield (%) = [all products (mol)/initial 1a (mol)] × 100.d Yield (%) = [target product (mol)/initial 1a (mol)] × 100. | ||||||
| 1 | 100 | 95 | 91 | 1 | Trace | 3 |
| 3 | 100 | 94 | 90 | 1 | Trace | 2 |
| 6 | 98 | 92 | 88 | 2 | Trace | 1 |
Since 2,4-dichloro-substituted phenol derivatives present a series of valuable materials for fine chemicals, this method was further used in the chlorination of various phenol derivatives (see ESI, Table 1S†). Although monochloro-substituted phenols, such as p- and o-, are undesirable products in our reactions, these compounds can be efficiently converted into 1b in our method. Complete conversion of p- was obtained in a high yield of 96% for 1b, and the yield of 1b was 94% for the total conversion of o-. Thus, monochloro-substituted phenols can be effectively utilized, and the by-product that remains in our system is only 1c. As for bromo- or iodo-substituted phenols, side-reactions of debromination/deiodination occurred and resulted in a complex mixture. This method is effective for alkylphenols, which were successfully chlorinated as various 2,4-dichlorophenols. A yield of 75% was achieved for 3b from o-cresol (3a), and an 81% yield for 4b was obtained from 2-tert-butylphenol (4a). Complete conversion of 5a (3,5-dimethylphenol) was achieved, and the yield for 5b was 86%. The chlorination of ethers of phenol can also be easily performed under similar conditions. Complete conversion of anisole (6a) led to a yield of 82% for 6b, and a high yield of 81% for 7b was achieved from 3,5-dimethylanisole (7a). Thus, we consider that our study offers a versatile synthetic method in the manufacture of various 2,4-dichlorophenol derivatives (Scheme 5).
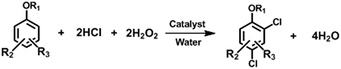 | ||
| Scheme 5 Oxychlorination for the synthesis of 2,4-dichloro-substituted phenol derivatives with H2O2 under VOC-free conditions. | ||
The molar ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a was 2
1a was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the theoretical equation (Scheme 4), but it was 2.8
1 according to the theoretical equation (Scheme 4), but it was 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
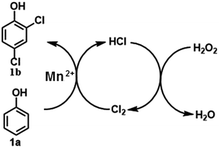
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under the optimized conditions (Table 2, run 10). The used amount of H2O2 is higher than the theoretical value. It is well known that the catalytic decomposition of H2O2 over metal ions is inevitable, and the interactions of Mn2+ with H2O2 lead to numerous reactive species, such as oxygen atoms, active oxygen species or OH˙/OOH˙ radicals, which are possible active species for reactions. In our experiments, when a free radical scavenger, 2,6-di-tert-butyl-4-methyl phenol, was added into the reactions, the yield of 1b significantly decreased to 30%. Although this result indicates that a free radical pathway is possibly involved in the reactions, our attempts to find out the free radicals or active species failed because of the complexity of this system. In controlled experiments, we found that the addition of H2O2 into an aqueous solution of HCl immediately resulted in light yellow gas, which was Cl2 based on GC-MS analysis. Thus, while the exact free radicals or active species are not yet clear, the main pathway can be presented as shown in Scheme 6: HCl is oxidized by H2O2 to Cl2; the generated Cl2 reacts with 1a to form 1b with the release of HCl, which is re-oxidized and re-used until 1a is exhausted. Details of the reaction mechanism are under investigation, and we will report the results in future.
1 under the optimized conditions (Table 2, run 10). The used amount of H2O2 is higher than the theoretical value. It is well known that the catalytic decomposition of H2O2 over metal ions is inevitable, and the interactions of Mn2+ with H2O2 lead to numerous reactive species, such as oxygen atoms, active oxygen species or OH˙/OOH˙ radicals, which are possible active species for reactions. In our experiments, when a free radical scavenger, 2,6-di-tert-butyl-4-methyl phenol, was added into the reactions, the yield of 1b significantly decreased to 30%. Although this result indicates that a free radical pathway is possibly involved in the reactions, our attempts to find out the free radicals or active species failed because of the complexity of this system. In controlled experiments, we found that the addition of H2O2 into an aqueous solution of HCl immediately resulted in light yellow gas, which was Cl2 based on GC-MS analysis. Thus, while the exact free radicals or active species are not yet clear, the main pathway can be presented as shown in Scheme 6: HCl is oxidized by H2O2 to Cl2; the generated Cl2 reacts with 1a to form 1b with the release of HCl, which is re-oxidized and re-used until 1a is exhausted. Details of the reaction mechanism are under investigation, and we will report the results in future.
Conclusions
In summary, we have developed a simple, mild and efficient method for oxychlorination of phenol to 2,4-dichlorophenol catalyzed by manganous(II) sulfate in the liquid phase. In this system, hydrogen chloride was used as a chlorinating agent, hydrogen peroxide as an oxidant and water as a solvent. We envisage that our method will be effective in the manufacture of various 2,4-dichlorophenol derivatives based on the following reasons: (1) high activity and selectivity; (2) VOC free; (3) simple product separation and recyclable catalyst.Experimental section
Typical procedure for oxychlorination of phenol
Phenol and a catalyst were added to water in a three-neck flask equipped with a gas inlet, a liquid inlet and a reflux condenser (open to air). Gaseous HCl was introduced and dissolved as an aqueous solution. The flask was immersed in a preheated oil bath and vigorously stirred with a magnetic stirrer. Then, H2O2 (30% aq. solution) was added dropwise by a channel pump during the reaction. At the end of the reaction, the mixtures were left to stand for 1.5 h, and an isolated organic phase from the aqueous solution formed at the bottom. The organic phase was collected and diluted with acetonitrile to prepare the sample for quantitative analysis. The conversions and yields were determined by gas chromatography. Each experiment was reproduced at least three times. The experimental error in the determination of the conversions and yields normally did not exceed 4%. Pure products were obtained by column chromatography using silica gel (petroleum ether) and confirmed by GC-MS, 1H and 13C NMR.1H NMR and 13C NMR analytical data of products
Acknowledgements
We are thankful for the funding support from the Innovative Research Team in College and Universities of Liaoning Province (No. LT2014007).Notes and references
- Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, New York, 6th edn, 1993, vol. 5 Search PubMed.
- Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 6th edn, 1998 Search PubMed.
- A. Białek and W. Moszczyński, Pol. J. Chem. Technol., 2009, 11(2), 21–30 CrossRef.
- W. D. Watson, J. Org. Chem., 1985, 50(12), 2145–2148 CrossRef CAS.
- S. Ratton and J.-C. Leblanc, European Patent, 0196260, 1986.
- P. V. Vyas, A. K. Bhatt, G. Ramachandraiah and A. V. Bedekar, Tetrahedron Lett., 2003, 44(21), 4085–4088 CrossRef CAS.
- C. Chiappe, E. Leandri and M. Tebano, Green Chem., 2006, 8, 742–745 RSC.
- J. G. P. Born, H. W. A. V. D. Wart, P. Mulder and R. Louw, Recl. Trav. Chim. Pays-Bas, 1993, 112, 262–270 CrossRef CAS.
- B. S. Samant, Y. P. Saraf and S. S. Bhagwat, J. Colloid Interface Sci., 2006, 302(1), 207–213 CrossRef CAS PubMed.
- L. Yang, L. Zhan and S. S. Stahl, Chem. Commun., 2009, 6460–6462 RSC.
- X. Sun, G. Shan, Y. Sun and Y. Rao, Angew. Chem., Int. Ed., 2013, 52(16), 4440–4444 CrossRef CAS PubMed.
- Y. Goldberg and H. Alper, J. Org. Chem., 1993, 58(11), 3072–3075 CrossRef CAS.
- L. Delaude and P. Laszlo, J. Org. Chem., 1990, 55(18), 5260–5269 CrossRef CAS.
- S. R. Bansal, D. C. Nonhebel and J. M. Mancilla, Tetrahedron, 1973, 29(7), 993–999 CrossRef CAS.
- X. Wan, Z. Ma, B. Li, K. Zhang, S. Cao, S. Zhang and Z. Shi, J. Am. Chem. Soc., 2006, 128(23), 7416–7417 CrossRef CAS PubMed.
- D. Niu, T. Wang, B. P. Woods and T. R. Hoye, Org. Lett., 2014, 16(1), 254–257 CrossRef CAS PubMed.
- J. S. Grossert and G. K. Chip, Tetrahedron Lett., 1970, 11(30), 2611 CrossRef.
- K. M. Brummond and K. D. Gesenberg, Tetrahedron Lett., 1999, 40(12), 2231–2234 CrossRef CAS.
- J. R. L. Smith, L. C. Mckeer and J. M. Taylor, J. Chem. Soc., Perkin Trans. 2, 1988, 10, 1533–1537 Search PubMed.
- B. S. Moon, Y. C. Han, H. Y. Koh and D. Y. Chi, Bull. Korean Chem. Soc., 2011, 32(2), 472–476 CrossRef CAS.
- G. Majetich, R. Hicks and S. Reister, J. Org. Chem., 1997, 62(13), 4321–4326 CrossRef CAS PubMed.
- R. Prebil, K. K. Laali and S. Stavber, Org. Lett., 2013, 15(9), 2108–2111 CrossRef CAS PubMed.
- Y. Xiong, H. Duan, X. Meng, Z. Ding and W. Feng, J. Chem., 2016, 2016, 1–5 CrossRef.
- C. U. Dinesh, R. Kumar, B. Pandey and P. Kumar, J. Chem. Soc., Chem. Commun., 1995, 6, 611–612 RSC.
- N. B. Barhate, A. S. Gajare, R. D. Wakharkar and A. V. Bedekar, Tetrahedron Lett., 1998, 39(35), 6349–6350 CrossRef CAS.
- N. B. Barhate, A. S. Gajare, R. D. Wakharkar and A. V. Bedekar, Tetrahedron, 1999, 55(36), 11127–11142 CrossRef CAS.
- N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, Synth. Commun., 2002, 33, 279–286 CrossRef.
- B. S. Bhatkhande, M. V. Adhikari and S. D. Samant, Ultrason. Sonochem., 2002, 9(1), 31–35 CrossRef CAS PubMed.
- L. K. Liu and C. S. Lin, J. Chin. Chem. Soc., 1996, 43(1), 61–66 CrossRef CAS.
- L. Menini and E. V. Gusevskaya, Appl. Catal., A, 2006, 309(1), 122–128 CrossRef CAS.
- L. Menini and E. V. Gusevskaya, Chem. Commun., 2006, 209–211 RSC.
- R. Raja and P. Ratnasamy, J. Catal., 1997, 170(2), 244–253 CrossRef CAS.
- A. Podgorsek, M. Zupan and J. Iskra, Angew. Chem., Int. Ed., 2009, 48(45), 8424–8450 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00319f |
| This journal is © The Royal Society of Chemistry 2017 |