Open Access Article
Open Access ArticleCharacterization of an inorganic polymer coagulant and coagulation behavior for humic acid/algae-polluted water treatment: polymeric zinc–ferric–silicate–sulfate coagulant†
Yong Liaoab,
Xiaomin Tang *ac,
Qingqing Yangab,
Wei Chenab,
Bingzhi Liuab,
Chuanliang Zhaoab,
Jun Zhaiab and
Huaili Zheng*ab
*ac,
Qingqing Yangab,
Wei Chenab,
Bingzhi Liuab,
Chuanliang Zhaoab,
Jun Zhaiab and
Huaili Zheng*ab
aKey Laboratory of the Three Gorges Reservoir Region's Eco-Environment, State Ministry of Education, Chongqing University, Chongqing 400045, P. R. China. E-mail: txmno1@126.com; zhl@cqu.edu.cn; Fax: +86 023 65120827; Tel: +86 23 65120827
bNational Centre for International Research of Low-carbon and Green Buildings, Chongqing University, Chongqing 400045, P. R. China
cChongqing Key Laboratory of Catalysis and Environmental New Materials, College of Environment and Resources, Chongqing Technology and Business University, Chongqing 400067, P. R. China
First published on 4th April 2017
Abstract
Algae and algae organic matter (AOM) are not the sole pollutants in algae-polluted water. Other pollutants such as colloidal particles and natural organic matter should be simultaneously removed and might influence the treatment of algae and AOM. A new polymeric zinc–ferric–silicate–sulfate (PZFSiS) coagulant was prepared, and the relationship between its structure and performance in the treatment of humic acid (HA)/algae-polluted water was discussed. PZFSiS coagulants prepared under different conditions had different distributions of Fe(III) species. The coagulant possessing the highest Feb content was able to treat turbidity and HA well. As a copolymer of Fe(III), Zn(II) and Si(IV), PZFSiS had a positive charge in water and thus neutralized the negative surface charges of pollutants. The adsorption of hydroxyl polymer formed by Fe/Zn during the hydrolysis process contributed to the removal of organic matter. The dosage of PZFSiS and pH significantly influenced pollutant removal. Colloidal particles in the water competed with the organic matter, markedly decreasing the removal efficiency of organic matter by coagulation.
1 Introduction
The excessive reproduction of algae has become an urgent environmental problem.1 The treatment of algae and the organic pollutants generated by algae has attracted a lot of attention in recent years.2–4 However, other pollutants in algae-polluted water, such as colloidal particles and natural organic matter (NOM), should also be treated. These pollutants might also influence algae removal and thus should be further studied. Coagulation is an important step in the traditional drinking water treatment process.5 It is mainly used to treat the suspended solids and colloidal particles in the water.5,6 Although coagulation can efficiently remove algae or NOM from water in some cases,3,5 algae organic matter and the hydrophilic NOM are usually difficult to treat.7,8 For instance, traditional aluminum (Al)-based and iron (Fe)-based coagulants have been widely applied in water treatment.3,5,9 Their coagulation performances are sometimes unacceptable for the removal of algae organic matter in the treatment of algae-polluted water.4,7,10 In addition, the residual metal ions in the treated water might change the color of the water or cause Alzheimer's disease.6,11 The stability of Fe-based coagulants is poor.12 Thus, multiple-metal composite coagulants such as Al–Fe composite coagulants have been prepared to overcome these weaknesses.13,14 It has been reported that polyaluminium ferric silicate chloride coagulant performed more efficiently than polyaluminium in removing turbidity, organic matter and total phosphate, and the stability of polyaluminium ferric silicate chloride coagulant was better.14 Zinc (Zn) chloride and zinc composite coagulants have been used in wastewater treatment for their better coagulation performance and non-toxicity compared to traditional coagulants.6,15,16 However, there is no information about the preparation of Zn composite coagulants with Fe and Si. Little attention has been paid to the treatment of humic acid (HA)/algae-polluted water by Zn–Fe–Si composite coagulants.In this paper, a new inorganic composite coagulant, polymeric zinc–ferric–silicate–sulfate (PZFSiS), was prepared. The distribution of Fe(III) species was measured to evaluate the coagulation efficiency. The structure and morphology of flocculants were characterized by X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), ultraviolet (UV) spectroscopy and scanning electron microscopy (SEM) to analyze the preparation mechanism and the relationship between the characteristics of PZFSiS and its coagulation performance. In the treatment of HA/algae-polluted water, the effects of PZFSiS dosage on the removal rates of pollutants were evaluated to represent the coagulation efficiency of PZFSiS. Other coagulation conditions such as water pH, temperature and turbidity were also considered in the treatment. In addition, the performance of PZFSiS was compared with the performances of some commercial coagulants, and their coagulation mechanisms were discussed.
2 Materials and methods
2.1 Materials
All chemical reagents used in this study were analytical-grade chemicals. Deionized water was used to make all the reagent solutions. The glassware and other labware were washed and dried prior to use.2.2 Preparation of PZFSiS coagulant
First, a certain amount of sodium silicate (Na2SiO3·9H2O) was dissolved in water, and sulfuric acid (H2SO4) was added to adjust the pH to 1.5. A polysilicic acid (PSA) solution was prepared after heating at 25 °C for 60 min, and its concentration was 0.1024 mol L−1 as SiO2.17 Second, a certain amount of ferrous sulfate (FeSO4·7H2O) was acidified by H2SO4. After mixing for 20 min, sodium chlorate (NaClO3) was added into the reaction vessel. Then, zinc sulfate (ZnSO4) with a pre-determined Zn/Fe molar ratio was added and mixed with the other chemical reagents. Third, PSA with a pre-determined Si/Fe molar ratio was added into the liquid mixture containing Zn and Fe. The mixture were heated at 70 °C for 1 h and stirred until a homogeneous liquid mixture was obtained. Then, sodium bicarbonate (NaHCO3) powder was added to the above homogeneous liquid mixture to adjust the r value (r = OH/Fe molar ratio in the range of 0.2–0.7). Finally, PZFSiS was prepared after aging for 24 h at room temperature (25 °C).2.3 Characterization methods
2.4 Water sample
A stock solution of HA was prepared by dissolving 0.5 g of HA in 0.01 mol L−1 NaOH solution under continuous stirring for 24 h.19 After that, the solution was filtered through a 0.45 μm membrane and stored at 4 °C.Kaolin powder was first sieved through a 200-mesh sifter and then mixed with deionized water under continuous stirring for 24 h to make the stock suspension of kaolin.20
The algae (Microcystis aeruginosa) were cultivated in BG11 medium at 25 °C and algae-polluted water was treated by coagulation at the stationary phase of algae.
The water sample was prepared by mixing a certain amount of HA stock solution, the stock suspension of kaolin and algae-polluted water. The pH of the water sample was adjusted to 7.2 ± 0.2 with HCl (0.1 mol L−1) and NaOH (0.1 mol L−1). The water quality indices of the water sample are shown in Table 1.
| pH | Temperature (°C) | Turbidity (NTU) | UV254 | Chlorophyll a (μg L−1) | Zeta potential (mV) |
|---|---|---|---|---|---|
| 7.2 ± 0.2 | 25 ± 2 | 16.6 ± 0.5 | 0.62 ± 0.03 | 111 ± 10 | −14.3 ± 0.6 |
2.5 Coagulation tests
Coagulation tests were carried out using a ZR4-6 six-paddle gang stirrer (Shenzhen Zhongrun Water Industry Technology and Development Co., Ltd, China). A certain dosage of coagulant (in Fe mass, mg L−1) was added into the water sample. The water was then mixed at a high speed of 300 rpm for 1 min and then at a low speed of 40 rpm for 10 min. After that, the water was allowed to settle for 30 min. The turbidity and UV254 values of the treated water were measured using a 2100P turbidity meter (HACH, USA) and a TU-1900 UV/vis spectrophotometer. The chlorophyll a concentration was measured based on existing protocols.21 The removal rates of turbidity, organic matter and algae were calculated using eqn (1):| R = (1 − Tf/Ti) × 100%, | (1) |
3 Results and discussion
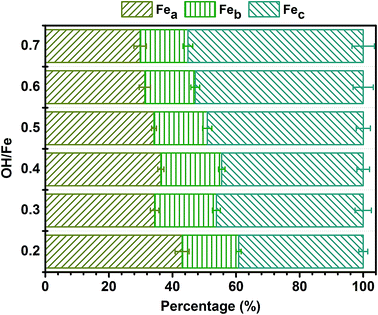
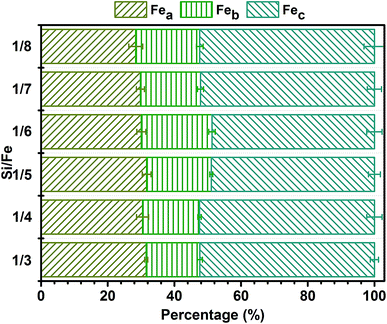
3.1 Distribution of Fe(III) species in PZFSiS
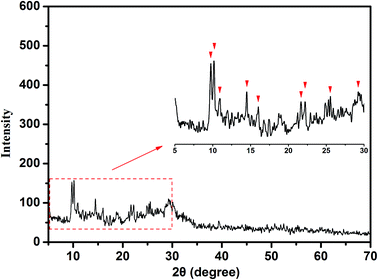
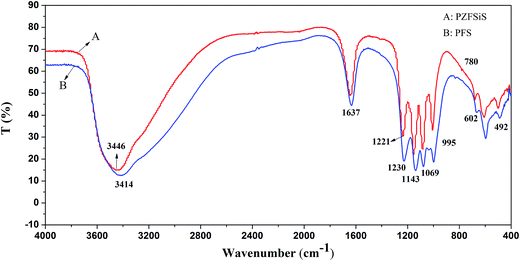
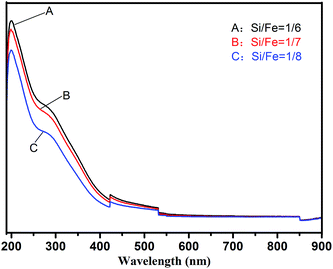
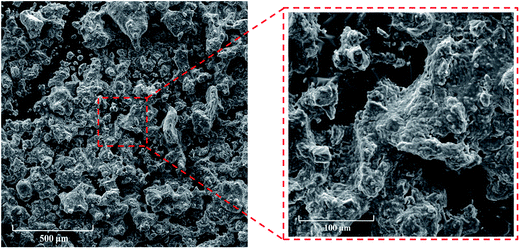
3.2 Characterization
PZFSiS used to analysis its structure and characteristics was prepared at the OH/Fe molar ratio of 0.3, the Si/Fe molar ratio of 1/6 and Zn/Fe molar ratio of 1/9.3.3 Application of PZFSiS in the treatment of humic acid/algae-polluted water
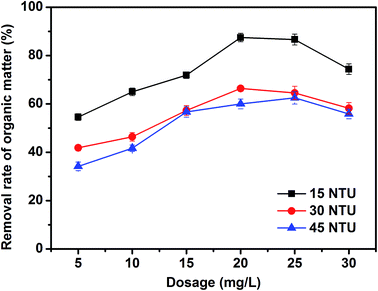
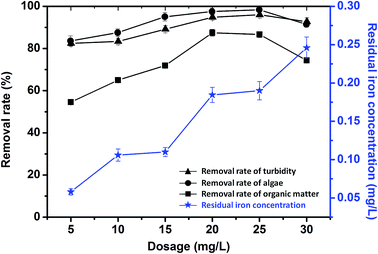 | ||
| Fig. 7 Effects of PZFSiS dosage on the removal rates of pollutants from HA/algae-polluted water and the residual iron concentrations in the treated water. | ||
For comparison, PFS and polyacrylamide (PAM) were applied in the treatment of HA/algae-polluted water under the same coagulation conditions. Their removal efficiencies of pollutants were obviously lower than those of PZFSiS, especially for PAM, which had a removal rate of organic matter below 60% (Fig. S5†). It is speculated that charge neutralization and entrapment might play important roles in the treatment of HA/algae-polluted water. PAM might interact with some AOMs, impeding flocculation.
3.4 Coagulation mechanisms in the treatment of humic-acid/algae-polluted water by coagulation
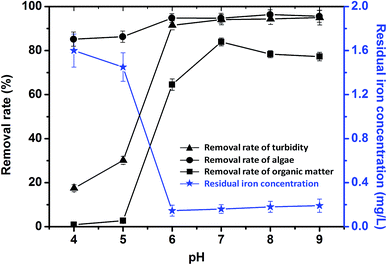
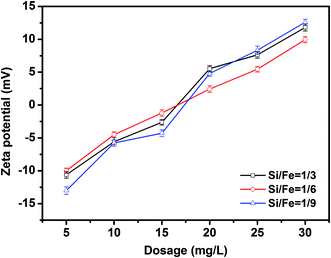
Zeta potential is an important indicator of the stability of colloidal dispersions. Colloids with high zeta potentials are electrically stabilized, while colloids with low zeta potentials tend to coagulate in water due to Brownian motion. Zeta potential slowly increased with increasing dosage of PZFSiS (Fig. 11). More PZFSiS is required to make the zeta potential of the solution approach zero. It has been argued that the best coagulation performance is achieved near at a zeta potential near zero, when charge neutralization is the main coagulation mechanism.20 However, in this study, the highest removal efficiencies were obtained at potentials below zero, indicating that charge neutralization was not the only coagulation mechanism. The bridging of polymeric iron/zinc, the adsorption of hydroxyl polymer/metal precipitation and the entrapment of flocs also contributed to pollutant removal.4 Conclusions
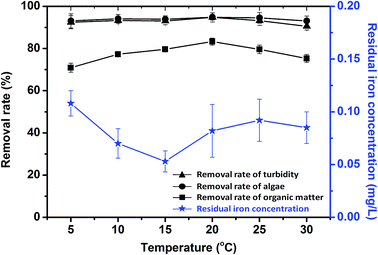
In this study, a novel composite coagulant, PZFSiS, was prepared. Different distributions of Fe(III) species in PZFSiS were found at different OH/Fe and Si/Fe molar ratios. The highest Feb content obtained at r = 0.3 and Si/Fe = 1/6 resulted in the best performance for the removal of turbidity and HA. The XRD, FT-IR and UV results confirmed that PZFSiS was prepared via the copolymerization of Fe(III), Zn(II) and Si(IV). The composite coagulant possessed the structure of Fe–O–Fe, Zn–O–Zn, Si–O–Si, Si–O–Fe, Si–O–Zn, Si–OH–Fe and Si–OH–Zn. The structures of PZFSiS contributed to its acceptable performance in the treatment of HA/algae-polluted water. The type and dosage of coagulant obviously influenced pollutant removal, especially organic matter removal. In water treatment, the coagulation efficiency of PZFSiS was higher than those of PFS and PAM. Moderate PZFSiS dosage increased the coagulation efficiency. Neutral or slightly alkaline pH was benefit for pollutant removal. PZFSiS was able to be potentially applied in water treatment at low temperature. The colloidal particles in the water competed with organic matter for coagulation, and increasing the amount of colloidal particles significantly reduced the removal efficiency of organic matter. Charge neutralization was not the only coagulation mechanism in the water treatment. The bridging of polymeric iron/zinc, the adsorption of hydroxyl polymer/metal precipitation and the entrapment of flocs played important roles in pollutant removal.Abbreviations
| PZFSiS | Polymeric zinc–ferric–silicate–sulfate |
| AOM | Algae organic matter |
| NOM | Natural organic matter |
| HA | Humic acid |
| XRD | X-ray diffraction |
| FT-IR | Fourier transform-infrared spectroscopy |
| UV | Ultraviolet |
| SEM | Scanning electron microscopy |
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Project No. 21677020 and 51478062), the Science and Technology Research Project of Chongqing Municipal Education Commission of China (Project No. KJ1600601) and the Natural Science Foundation Project of CQ CSTC (Project No. cstc2016jcyjA0197).References
- S. B. Watson, C. Miller, G. Arhonditsis, G. L. Boyer, W. Carmichael, M. N. Charlton, R. Confesor, D. C. Depew, T. O. Höök, S. A. Ludsin, G. Matisoff, S. P. McElmurry, M. W. Murray, R. Peter Richards, Y. R. Rao, M. M. Steffen and S. W. Wilhelm, Harmful Algae, 2016, 56, 44–66 CrossRef CAS PubMed.
- Q. Shen, J. Zhu, L. Cheng, J. Zhang, Z. Zhang and X. Xu, Desalination, 2011, 271, 236–240 CrossRef CAS.
- X. Tang, H. Zheng, Y. Wang, W. Chen, J. Guo, Y. Zhou and X. Li, J. Taiwan Inst. Chem. Eng., 2016, 63, 195–201 CrossRef CAS.
- X. Tang, H. Zheng, C. Zhao, J. Zhai, B. Liu, W. Chen, Z. Zhang and F. Li, Desalin. Water Treat., 2016, 1–8 Search PubMed.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Adv. Colloid Interface Sci., 2010, 159, 189–197 CrossRef CAS PubMed.
- G. Zhu, Q. Wang, J. Yin, Z. Li, P. Zhang, B. Ren, G. Fan and P. Wan, Water Res., 2016, 100, 201–210 CrossRef CAS PubMed.
- T. Takaara, D. Sano, H. Konno and T. Omura, Water Res., 2007, 41, 1653–1658 CrossRef CAS PubMed.
- A. J. Garzonsanabria, S. S. Ramirezcaballero, F. E. Moss and Z. L. Nikolov, Bioresour. Technol., 2013, 143, 231–237 CrossRef CAS PubMed.
- J. T. Alexander, F. I. Hai and T. M. Al-aboud, J. Environ. Manage., 2012, 111, 195–207 CrossRef CAS PubMed.
- X. Tang, H. Zheng, B. Gao, C. Zhao, B. Liu, W. Chen and J. Guo, J. Hazard. Mater., 2017, 332, 1–9 CrossRef CAS PubMed.
- R. Jiao, H. Xu, W. Xu, X. Yang and D. Wang, J. Hazard. Mater., 2015, 290, 16–25 CrossRef CAS PubMed.
- D. Jia, M. Li, G. Liu, P. Wu, J. Yang, Y. Li, S. Zhong and W. Xu, Colloids Surf., A, 2017, 512, 111–117 CrossRef CAS.
- G. Zhu, H. Zheng, W. Chen, W. Fan, P. Zhang and T. Tshukudu, Desalination, 2012, 285, 315–323 CrossRef CAS.
- X. Niu, X. Li, J. Zhao, Y. Ren and Y. Yang, J. Environ. Sci., 2011, 23, 1122–1128 CrossRef CAS.
- Y. Zeng and J. Park, Colloids Surf., A, 2009, 334, 147–154 CrossRef CAS.
- Y. Wei, X. Dong, A. Ding and D. Xie, J. Taiwan Inst. Chem. Eng., 2015, 58, 351–356 CrossRef.
- X. Huang, B. Gao, Q. Yue, Y. Wang and Q. Li, RSC Adv., 2016, 6, 24898–24905 RSC.
- H. Dong, B. Gao, Q. Yue, H. Rong, S. Sun and S. Zhao, Desalination, 2014, 335, 102–107 CrossRef CAS.
- X. Hui, R. Jiao, F. Xiao and D. Wang, Colloids Surf., A, 2016, 490, 189–199 CrossRef CAS.
- X. Tang, H. Zheng, H. Teng, C. Zhao, Y. Wang, W. Xie, W. Chen and C. Yang, Chem. Eng. Res. Des., 2015, 104, 208–217 CrossRef CAS.
- M. A. Borowitzka and N. R. Moheimani, Algae for Biofuels and Energy, 2013 Search PubMed.
- J. Shi, Y. Zhang, K. Zou and F. Xiao, J. Environ. Sci., 2011, 23, 749–756 CrossRef CAS.
- R. Li, C. He and Y. He, Chem. Eng. J., 2013, 223, 869–874 CrossRef CAS.
- Y. Fu and S. L. Yu, Desalination, 2009, 247, 442–455 CrossRef CAS.
- P. A. Moussas and A. I. Zouboulis, Sep. Purif. Technol., 2008, 63, 475–483 CrossRef CAS.
- Y. Fu, S.-l. Yu, Y.-Z. Yu, L.-P. Qiu and B. Hui, J. Environ. Sci., 2007, 19, 678–688 CrossRef CAS.
- S. Mirlohi, A. M. Dietrich and S. E. Duncan, Environ. Sci. Technol., 2011, 45, 6575–6583 CrossRef CAS PubMed.
- J. E. Gregor, C. J. Nokes and E. Fenton, Water Res., 1997, 31, 2949–2958 CrossRef.
- Y. Fu, Y.-Z. Wang and M.-M. Su, J. Water Process Eng., 2014, 4, 58–66 CrossRef.
- F. Ying, Z. Jichao, W. Yanzheng and Y. Yanzhen, Chem. Eng. J., 2012, 203, 301–308 CrossRef.
- F. Qu, H. Liang, J. He, J. Ma, Z. Wang, H. Yu and G. Li, Water Res., 2012, 46, 2881–2890 CrossRef CAS PubMed.
- F. Xiao, J. Ma, P. Yi and J.-C. H. Huang, Water Res., 2008, 42, 2983–2992 CrossRef CAS PubMed.
- Z. Zhou, Y. Yang, X. Li, W. Wang, Y. Wu, C. Wang and J. Luo, Sep. Purif. Technol., 2014, 123, 1–8 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00232g |
| This journal is © The Royal Society of Chemistry 2017 |