Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
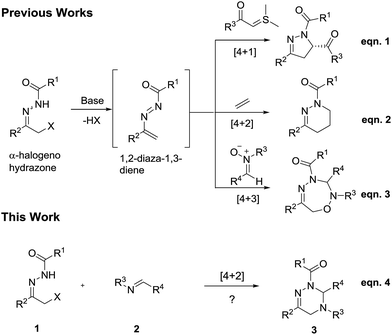
Construction of 2,3,4,5-tetrahydro-1,2,4-triazines via [4 + 2] cycloaddition of α-halogeno hydrazones to imines†
Hong-Wu Zhao *,
Hai-Liang Pang
*,
Hai-Liang Pang ,
Yu-Di Zhao
,
Yu-Di Zhao ,
Yue-Yang Liu
,
Yue-Yang Liu ,
Li-Jiao Zhao*,
Xiao-Qin Chen
,
Li-Jiao Zhao*,
Xiao-Qin Chen ,
Xiu-Qing Song,
Ning-Ning Feng
,
Xiu-Qing Song,
Ning-Ning Feng and
Juan Du
and
Juan Du
College of Life Science and Bio-engineering, Beijing University of Technology, No. 100 Pingleyuan, Chaoyang District, Beijing 100124, P. R. China. E-mail: hwzhao@bjut.edu.cn; zhaolijiao@bjut.edu.cn
First published on 30th January 2017
Abstract
In the presence of sodium carbonate, the [4 + 2] cycloaddition of α-halogeno hydrazones to imines proceeded readily, and furnished 2,3,4,5-tetrahydro-1,2,4-triazines in moderate to high chemical yields.
1. Introduction
α-Halogeno hydrazones represent a class of versatile and robust building blocks, which have been widely applied in the construction of structurally diverse and complex N-containing heterocycles possessing varying ring sizes. Normally, α-halogeno hydrazones can undergo [4 + 1],1 [4 + 2]2 or [4 + 3]3 cycloadditions with structurally different dienophiles via the in situ formed 1,2-diaza-1,3-diene intermediates under basic reaction conditions. For example, in 2012, Bolm and co-workers realized the enantioselective synthesis of dihydropyrazoles by means of the [4 + 1] cycloaddition of α-halogeno hydrazones to sulphur ylides (Scheme 1, eqn. (1)).4 In 2015, the Luo research group reported the [4 + 2] cycloaddition of α-halogeno hydrazones to simple olefins for the preparation of tetrahydropyridazines (Scheme 1, eqn (2)).5 Recently, our research group successfully designed the [4 + 3] cycloaddition of α-halogeno hydrazones to nitrones for the preparation of 1,2,4,5-oxatriazepines (Scheme 1, eqn (3)).6 In particular, most of the previously reported [4 + 2] cycloadditions of α-halogeno hydrazones mainly focused on the use of the differently functionalized olefins as dienophiles.7 In addition, only three other pioneering works respectively dealt with the use of arylacetic acids,8 methoxyallene9 or azodicarboxylates10 as dienophiles in the [4 + 2] cycloadditions of α-halogeno hydrazones. It is well known that the employment of imines as dienophiles in [4 + 2] cycloaddition of α-halogeno hydrazones has been fully unexplored to date. So, the development of novel [4 + 2] cycloadditions of α-halogeno hydrazones with imines is highly demanded for the synthesis of potentially bioactive heterocycles.On the basis of the previously published elegant examples, in this work, we first attempted the novel [4 + 2] cycloaddition of α-halogeno hydrazones with synthetically useful and important imines11 for the construction of 2,3,4,5-tetrahydro-1,2,4-triazines bearing potential biological activities12 (Scheme 1, eqn (4)). To our delight, under the mild reaction conditions, the [4 + 2] cycloaddition of α-halogeno hydrazones with imines underwent readily, and furnished the target molecules in moderate to high chemical yields. To the best of our knowledge, such a work has not been reported in the literature so far.
2. Results and discussion
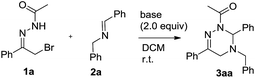
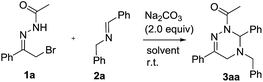
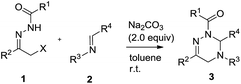
At the outset, we explored the base effects on the chemical yield of the [4 + 2] cycloaddition of α-halogeno hydrazone 1a with imine 2a in DCM solvent at room temperature as summarized in Table 1. Indeed, the used base affected the chemical yield of the [4 + 2] cycloaddition significantly. Using DBU as a base gave product 3aa in a trace amount after 48 h (entry 8). The choice of NaHCO3 and Et3N as bases did not enhance the chemical yield of 3aa dramatically (entries 4 & 7). By comparison with the former cases, the chemical yield of [4 + 2] cycloaddition increased differently by using KOH and MeONa as bases (entries 5–6). Moreover, when Na2CO3, K2CO3 and Cs2CO3 were examined as bases, the chemical yield of the [4 + 2] cycloaddition ranged from 33% to 44% (entries 1–3). Obviously, among all the bases screened, Na2CO3 performed best and delivered product 3aa in highest chemical yield (entry 1).Simultaneously, by using Na2CO3 (2.0 equiv.) as base, we investigated the solvent effects on the chemical yield of the [4 + 2] cycloaddition of α-halogeno hydrazone 1a with imine 2a as shown in Table 2. Remarkably, the chemical yield of the [4 + 2] cycloaddition was largely influenced by the attempted solvents. In MeOH solvent, the [4 + 2] cycloaddition furnished product 3aa in 20% chemical yield in 36 h (entry 7). In contrast with the former case, the chemical yield of the [4 + 2] cycloaddition increased to 35% by choosing MeCN as solvent (entries 6 vs. 7). As for solvents DCE, THF, Et2O and DME, they afforded product 3aa in similar chemical yields (entries 1–4). Finally, with the use of toluene, PhCl and benzene as solvents, the chemical yield of the [4 + 2] cycloaddition changed from 68% to 75% (entries 5 and 8–9). Therefore, Na2CO3 behaved most efficiently in toluene solvent, thus providing 3aa in the highest chemical yield (entry 5). In addition, we examined other equivalent amounts of Na2CO3 in the [4 + 2] cycloaddition by using toluene as solvent, and found that use of 2.0 equiv. of Na2CO3 furnished product 3aa in the highest chemical yield (Table 2, entries 5 vs. 10–11).
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise noted, reactions were carried out with 1a (0.2 mmol), 2a (0.3 mmol), Na2CO3 (0.4 mmol) in the solvent (1.0 mL) at room temperature.b Isolated yield.c 0.5 equiv. of Na2CO3.d 1.0 equiv. of Na2CO3. | |||
| 1 | DCE | 24 | 54 |
| 2 | THF | 24 | 52 |
| 3 | Et2O | 24 | 52 |
| 4 | DME | 24 | 51 |
| 5 | Toluene | 24 | 75 |
| 6 | MeCN | 24 | 35 |
| 7 | MeOH | 36 | 20 |
| 8 | PhCl | 24 | 68 |
| 9 | Benzene | 24 | 71 |
| 10c | Toluene | 24 | 16 |
| 11d | Toluene | 24 | 46 |
Subsequently, under the optimal reaction conditions, we broadened the reaction scope of the [4 + 2] cycloaddition by diversifying α-halogeno hydrazones 1 and imines 2 as outlined in Table 3. Noticeably, the chemical yield of the [4 + 2] cycloaddition highly depended on the structural nature of the used α-halogeno hydrazones 1 and imines 2. Regarding the [4 + 2] cycloaddition with α-halogeno hydrazone 1a, most imine substrates 2 well tolerated the structural variation of R3 and R4 groups, thus delivering products 3 in 62–88% chemical yields (entries 1–8 & 10–12). In contrast with the former cases, the imines 2i and 2m individually bearing a para-methoxy-substituted benzyl group or a phenyl group at R3 position furnished products 3ai and 3am in the dramatically decreased chemical yields in the [4 + 2] cycloaddition with 1a (entries 1 vs. 9, 1 vs. 13). Meanwhile, it was noted that in the [4 + 2] cycloaddition with 1a, the regioisomers 2c–2e, which derived from the different substitution pattern of nitro group at R4 moiety, provided products 3ac–3ae in the quite different chemical yields (entries 3–5).
| Entry | 1 (X, R1, R2) | 2 (R3, R4) | 3 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were carried out with 1 (0.2 mmol), 2 (0.3 mmol), Na2CO3 (0.4 mmol) in toluene (1.0 mL) at room temperature.b Isolated yield. | |||||
| 1 | 1a (Br, Me, Ph) | 2a (Bn, Ph) | 3aa | 24 | 75 |
| 2 | 1a (Br, Me, Ph) | 2b (Bn, 4-MeOC6H4) | 3ab | 30 | 85 |
| 3 | 1a (Br, Me, Ph) | 2c (Bn, 4-NO2C6H4) | 3ac | 30 | 67 |
| 4 | 1a (Br, Me, Ph) | 2d (Bn, 3-NO2C6H4) | 3ad | 30 | 62 |
| 5 | 1a (Br, Me, Ph) | 2e (Bn, 2-NO2C6H4) | 3ae | 30 | 75 |
| 6 | 1a (Br, Me, Ph) | 2f (Bn, 4-BrC6H4) | 3af | 24 | 88 |
| 7 | 1a (Br, Me, Ph) | 2g (Bn, 2-naphthyl) | 3ag | 30 | 66 |
| 8 | 1a (Br, Me, Ph) | 2h (Bn, 2-furyl) | 3ah | 30 | 80 |
| 9 | 1a (Br, Me, Ph) | 2i (4-MeO C6H4CH2, Ph) | 3ai | 24 | 29 |
| 10 | 1a (Br, Me, Ph) | 2j (4-FC6H4CH2, Ph) | 3aj | 24 | 77 |
| 11 | 1a (Br, Me, Ph) | 2k (4-ClC6H4CH2, Ph) | 3ak | 24 | 77 |
| 12 | 1a (Br, Me, Ph) | 2l (Me, Ph) | 3al | 24 | 75 |
| 13 | 1a (Br, Me, Ph) | 2m (Ph, Ph) | 3am | 36 | 33 |
| 14 | 1b (Cl, Me, Ph) | 2a (Bn, Ph) | 3aa | 24 | 61 |
| 15 | 1c (Br, Me, 4-MeOC6H4) | 2a (Bn, Ph) | 3ca | 24 | 57 |
| 16 | 1d (Br, Me, 4-MeC6H4) | 2a (Bn, Ph) | 3da | 24 | 71 |
| 17 | 1e (Br, Me, 4-BrC6H4) | 2a (Bn, Ph) | 3ea | 24 | 80 |
| 18 | 1f (Br, Me, 4-ClC6H4) | 2a (Bn, Ph) | 3fa | 24 | 86 |
| 19 | 1g (Br, Me, 4-FC6H4) | 2a (Bn, Ph) | 3ga | 24 | 74 |
| 20 | 1h (Br, Me, 3-ClC6H4) | 2a (Bn, Ph) | 3ha | 24 | 80 |
| 21 | 1i (Br, Me, 4-NO2C6H4) | 2a (Bn, Ph) | 3ia | 30 | 71 |
| 22 | 1j (Br, Me, t-Bu) | 2a (Bn, Ph) | 3ja | 36 | 21 |
| 23 | 1k (Cl, Ph, Ph) | 2a (Bn, Ph) | 3ka | 36 | 34 |
| 24 | 1l (Cl, MeO, Ph) | 2a (Bn, Ph) | 3la | 30 | 59 |
| 25 | 1m (Br,MeO, CO2Et) | 2a (Bn, Ph) | 3ma | 30 | 70 |
| 26 |  |
2a (Bn, Ph) | 3na | 30 | 64 |
| 27 | 1f (Br, Me, 4-Cl C6H4) | 2b (Bn, 4-MeOC6H4) | 3fb | 30 | 81 |
| 28 | 1f (Br, Me, 4-ClC6H4) | 2f (Bn, 4-BrC6H4) | 3ff | 24 | 71 |
| 29 | 1h (Br, Me, 3-ClC6H4) | 2f (Bn, 4-BrC6H4) | 3hf | 24 | 81 |
| 30 | 1d (Br, Me, 4-MeC6H4) | 2f (Bn, 4-BrC6H4) | 3df | 24 | 73 |
In case of the [4 + 2] cycloaddition with 2a, most α-halogeno hydrazones 1 could better endure the wide variation in R1 and R2 groups, and led to the formation of products 3 in 57–86% chemical yields (entries 14–21 & 24–26). With respect to the imines 1j with a bulky tert-butyl as R2 group and 1k with a phenyl as R1 group, they preferred to afford products 3 in the relatively lowered chemical yields in the [4 + 2] cycloaddition with 2a (entries 1 vs. 22, 14 vs. 23). Generally, in the [4 + 2] cycloaddition with 2a, the imines 1 including an electron-poor phenyl group at R2 position usually behaved better than the imines 1 containing an electron-rich phenyl group at R2 position, and produced products 3 in higher chemical yields (entries 15–16 vs. 17–20). Simultaneously, it should be addressed that α-halogeno hydrazones 1a and 1b, which differ from each other in X group, gave rise to the same product 3aa in the tremendously different chemical yield in the [4 + 2] cycloaddition with 2a (entries 1 vs. 14). Moreover, the [4 + 2] cycloaddition of 1f with 2b gave product 3fb in 81% chemical yield (entry 27). At last, we further performed the extension of the reaction scope of the [4 + 2] cycloaddition by treating α-halogeno hydrazone 2f with imines 1d, 1f and 1h, and the chemical yield of the [4 + 2] cycloaddition changed from 71–83%. Of course, the α-halogeno hydrazones 1f and 1d, where R2 group individually has an electron-withdrawing or -donating group attached to the phenyl moiety, did not behaved quite differently in the [4 + 2] cycloaddition with 2f, and furnished products 3ff and 3df in the similar chemical yields (entries 28 vs. 30).
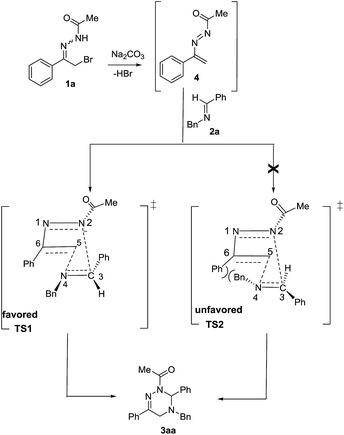
Moreover, the chemical structure of 3aa was firmly confirmed by single crystal X-ray analysis as depicted in Fig. 1.13 The conformational analysis showed that the 2,3,4,5-tetrahydro-1,2,4-triazine ring of 3aa adopts a highly twisted conformation. By virtue of the non-planar structure of the 2,3,4,5-tetrahydro-1,2,4-triazine ring of 3aa, as a result, the two protons at C-5 become chemically non-equivalent: one proton occupies the pseudo-oxial position; the other one resides in the pseudo-equatorial position. This fact was clearly identified by the 1H NMR performance of the two protons at C-5: one proton resonates at 3.48 ppm; the other one signals at 3.55 ppm (see details in ESI†). These observations proved that the inversion barrier of 2,3,4,5-tetrahydro-1,2,4-triazine ring is big enough at room temperature, and as a consequence, the two protons exchange pretty slowly at 1H NMR timescale. Meanwhile, we proposed the reaction mechanism for the formation of 3aa (Scheme 2). In the presence of Na2CO3, the elimination reaction of 1a takes place to give 1,2-diaza-1,3-diene 4. Then, two possible transition states TS1 and TS2 will be produced for the [4 + 2] cycloaddition between 4 and 2a. With the aid of the molecular model, it was found that in TS2 phenyl group at C-6 sterically repulse benzyl group at N-4 severely; whereas, this strong destabilizing interaction does not exist in TS1 at all. Therefore, the transition state TS1 is more stable than the transition state TS2, and mainly accounts for the formation of the desired cycloadduct 3aa.
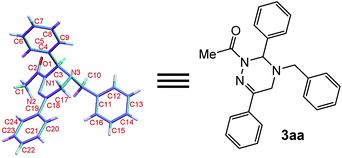 | ||
| Fig. 1 X-ray single crystal structure of 3aa (with thermal ellipsoid shown at the 50% probability level). | ||
3. Conclusions
In conclusion, the [4 + 2] cycloaddition of α-halogeno hydrazones with imines underwent efficiently, and provided the easy access to the novel potentially bioactive 2,3,4,5-tetrahydro-1,2,4-triazines in the reasonable chemical yields. Furthermore, the exploration of other novel cycloadditions of α-halogeno hydrazones with various 1,3-, 1,4- and 1,5-dipoles is ongoing in our laboratory, and will be reported in due course.4. Experimental section
4.1 General information
Proton (1H) and carbon (13C) NMR spectra were recorded on 400 MHz instrument (400 MHz for 1H NMR, 100 MHz for 13C NMR) and calibrated using tetramethylsilane (TMS) as internal reference. High resolution mass spectra (HRMS) were recorded under electrospray ionization (ESI) conditions. Flash column chromatography was performed on silica gel (0.035–0.070 mm) using compressed air. Thin layer chromatography (TLC) was carried out on 0.25 mm SDS silica gel coated glass plates (60F254). Eluted plates were visualized using a 254 nm UV lamp. Unless otherwise indicated, all reagents were commercially available and used without further purification. All solvents were distilled from the appropriate drying agents immediately before using. α-Chloro- or α-bromo hydrazones (1a–1n) were prepared according to literature procedures.3c,4,7b Imines (2a–2m) were synthesized according to known procedures.144.2 Procedure for the synthesis of products 3
Na2CO3 (2.0 equiv., 0.4 mmol) was added to a solution of α-chloro- or α-bromo hydrazone 1 (1.0 equiv., 0.2 mmol) and imine 2 (1.5 equiv., 0.3 mmol) in toluene (1.0 mL). The mixture was monitored by TLC plate and stirred for 24–36 h at room temperature. The crude products were purified by flash column chromatography on silica gel using EtOAc–petroleum as eluent to give products 3 (21–88% yield).Acknowledgements
We thank Beijing Municipal Commission of Education (No. JC015001200902), Beijing Municipal Natural Science Foundation (No. 7102010, No. 2122008), Basic Research Foundation of Beijing University of Technology (X4015001201101), Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (No. PHR201008025), Doctoral Scientific Research Start-up Foundation of Beijing University of Technology (No. 52015001200701) for financial supports.References
- For selected examples, see: (a) O. A. Attanasi, L. De Crescentini, G. Favi, F. Mantellini, S. Mantenuto and S. Nicolini, J. Org. Chem., 2014, 79, 8331 CrossRef CAS PubMed; (b) O. A. Attanasi, P. Filippone, B. Guidi, T. Hippe, F. Mantellini and L. F. Tietze, Tetrahedron Lett., 1999, 40, 9277 CrossRef CAS.
- For selected examples, see: (a) A. M. Shelke and G. Suryavanshi, Org. Lett., 2016, 18, 3968 CrossRef CAS PubMed; (b) R. Huang, X. Chang, J. Li and C. J. Wang, J. Am. Chem. Soc., 2016, 138, 3998 CrossRef CAS PubMed; (c) S. M. M. Lopes, M. S. C. Henriques, J. A. Paixao and T. M. V. D. P. E. Melo, Eur. J. Org. Chem., 2015, 2015, 6146 CrossRef CAS; (d) J. Li, R. Huang, Y. K. Xing, G. Qiu, H. Y. Tao and C. J. Wang, J. Am. Chem. Soc., 2015, 137, 10124 CrossRef CAS PubMed; (e) M. C. Tong, X. Chen, J. Li, R. Huang, H. Tao and C. J. Wang, Angew. Chem., Int. Ed. Engl., 2014, 53, 4680 CrossRef CAS PubMed; (f) S. M. M. Lopes, A. Lemos and T. M. V. D. P. E. Melo, Eur. J. Org. Chem., 2014, 2014, 7039 CrossRef CAS; (g) F. Palacios, D. Aparicio, Y. Lopez, J. M. D. Santos and C. Alonso, Eur. J. Org. Chem., 2005, 2005, 1142 CrossRef.
- For selected examples, see: (a) W. Yang, C. Yuan, Y. Liu, B. Mao, Z. Sun and H. Guo, J. Org. Chem., 2016, 81, 7597 CrossRef CAS PubMed; (b) C. Guo, B. Sahoo, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2014, 136, 17402 CrossRef CAS PubMed; (c) X. Q. Hu, J. R. Chen, S. Gao, B. Feng, L. Q. Lu and W. J. Xiao, Chem. Commun., 2013, 49, 7905 RSC.
- J. R. Chen, W. R. Dong, M. Candy, F. F. Pan, M. Jorres and C. Bolm, J. Am. Chem. Soc., 2012, 134, 6924 CrossRef CAS PubMed.
- X. Zhong, J. Lv and S. Luo, Org. Lett., 2015, 17, 1561 CrossRef CAS PubMed.
- H.-W. Zhao, H.-L. Pang, T. Tian, B. Li, X.-Q. Chen, X.-Q. Song, W. Meng, Z. Yang, Y.-Y. Liu and Y.-D. Zhao, Adv. Synth. Catal., 2016, 358, 1826 CrossRef CAS.
- For selected examples, see: (a) L. Wei and C. J. Wang, Chem. Commun., 2015, 51, 15374 RSC; (b) S. Gao, J. R. Chen, X. Q. Hu, H. G. Cheng, L. Q. Lu and W. J. Xiao, Adv. Synth. Catal., 2013, 355, 3539 CrossRef CAS; (c) S. M. M. Lopes, A. F. Brigas, F. Palacios, A. Lemos and T. M. V. D. P. E. Melo, Eur. J. Org. Chem., 2012, 2012, 2152 CrossRef CAS; (d) A. Lemos and J. P. Lourenco, Tetrahedron Lett., 2009, 50, 1311 CrossRef CAS; (e) H. Salaheddine, S. L. Titouani, M. Soufiaoui and A. Tahdi, Tetrahedron Lett., 2002, 43, 4351 CrossRef CAS.
- X. Li, K. Gai, Z. Yuan, J. Wu, A. Lin and H. Yao, Adv. Synth. Catal., 2015, 357, 3479 CrossRef CAS.
- O. A. Attanasi, G. Favi, F. Mantellini, S. Mantenuto, G. Moscatelli and S. Nicolini, Synlett, 2015, 26, 193 CAS.
- H.-W. Zhao, H.-L. Pang, B. Li, T. Tian, X.-Q. Chen, X.-Q. Song, W. Meng, Z. Yang, Y.-Y. Liu and Y.-D. Zhao, RSC Adv., 2016, 6, 25562 RSC.
- For selected reviews, see: (a) J. Vesely and R. Rios, Chem. Soc. Rev., 2014, 43, 611 RSCM. González-López and J. T. Shaw, Chem. Rev., 2009, 109, 164 CrossRef CAS PubMed; S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef PubMed For selected examples, see: (b) H. W. Zhao, B. Li, H. L. Pang, T. Tian, X. Q. Chen, X. Q. Song, W. Meng, Z. Yang, Y. D. Zhao and Y. Y. Liu, Org. Lett., 2016, 18, 848 Search PubMed; (c) J. Xu, S. Yuan and M. Miao, Org. Lett., 2016, 18, 3822 Search PubMed; (d) L. Cai, K. Zhang and O. Kwon, J. Am. Chem. Soc., 2016, 138, 3298 Search PubMed; (e) D. Xie, L. Yang, Y. Lin, Z. Zhang, D. Chen, X. Zeng and G. Zhong, Org. Lett., 2015, 17, 2318 Search PubMed; (f) B. M. Trost and S. M. Silverman, J. Am. Chem. Soc., 2012, 134, 4941 Search PubMed; (g) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed. Engl., 2012, 51, 767 Search PubMed.
- For selected examples, see: (a) R. Luciani, P. Saxena, S. Surade, M. Santucci, A. Venturelli, C. Borsari, G. Marverti, G. Ponterini, S. Ferrari, T. L. Blundell and M. P. Costi, J. Med. Chem., 2016, 59, 9269 CrossRef CAS PubMed; (b) M. Stefek, M. Soltesova Prnova, M. Majekova, C. Rechlin, A. Heine and G. Klebe, J. Med. Chem., 2015, 58, 2649 CrossRef CAS PubMed; (c) N. Oi, M. Tokunaga, M. Suzuki, Y. Nagai, Y. Nakatani, N. Yamamoto, J. Maeda, T. Minamimoto, M. R. Zhang, T. Suhara and M. Higuchi, J. Med. Chem., 2015, 58, 8444 CrossRef CAS PubMed; (d) F. Lopez-Tapia, K. A. Walker, C. Brotherton-Pleiss, J. Caroon, D. Nitzan, L. Lowrie, S. Gleason, S. H. Zhao, J. Berger, D. Cockayne, D. Phippard, R. Suttmann, W. L. Fitch, D. Bourdet, P. Rege, X. Huang, S. Broadbent, C. Dvorak, J. Zhu, P. Wagner, F. Padilla, B. Loe, A. Jahangir and A. Alker, J. Med. Chem., 2015, 58, 8413 CrossRef CAS PubMed; (e) N. Hin, B. Duvall, D. Ferraris, J. Alt, A. G. Thomas, R. Rais, C. Rojas, Y. Wu, K. M. Wozniak, B. S. Slusher and T. Tsukamoto, J. Med. Chem., 2015, 58, 7258 CrossRef CAS PubMed; (f) H. L. Maslen, D. Hughes, M. Hursthouse, E. De Clercq, J. Balzarini and C. Simons, J. Med. Chem., 2004, 47, 5482 CrossRef CAS PubMed.
- CCDC 1508810 contains the supplementary crystallographic data for compound 3aa.
- Z. Yang, N. Chen and J. Xu, J. Org. Chem., 2015, 80, 3611 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR spectra for all products related to this article; X-ray single crystal structure analysis data for 3aa. CCDC 1508810. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra27767e |
| This journal is © The Royal Society of Chemistry 2017 |