Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
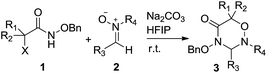
Facile access to novel 1,2,4-oxadiazinan-5-ones via [3 + 3] cycloaddition of in situ generated azaoxyallyl cations with nitrones†
Hong-Wu Zhao *,
Yu-Di Zhao
*,
Yu-Di Zhao ,
Yue-Yang Liu
,
Yue-Yang Liu ,
Li-Jiao Zhao*,
Ning-Ning Feng
,
Li-Jiao Zhao*,
Ning-Ning Feng ,
Hai-Liang Pang
,
Hai-Liang Pang ,
Xiao-Qin Chen
,
Xiao-Qin Chen ,
Xiu-Qing Song and
Juan Du
,
Xiu-Qing Song and
Juan Du
College of Life Science and Bio-engineering, Beijing University of Technology, No. 100 Pingleyuan, Chaoyang District, Beijing 100124, P. R. China. E-mail: hwzhao@bjut.edu.cn
First published on 24th February 2017
Abstract
In the presence of Na2CO3, azaoxyallyl cations in situ generated from α-halohydroxamates with nitrones readily underwent [3 + 3] cycloaddition, and gave rise to 1,2,4-oxadiazinan-5-one derivatives in 56–99% chemical yields. The chemical structure of the title compounds was unambiguously identified by X-ray single crystal structure analysis.
1. Introduction
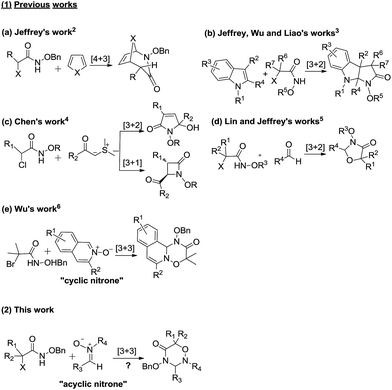
Azaoxyallyl cations represent a family of versatile and powerful synthetic synthons, which are generally in situ generated from α-halohydroxamates in the presence of organic or inorganic bases.1 Owing to the unique structural features and reactivities of azaoxyallyl cations, some various efforts have been made to enrich the synthetic methodology of azaoxyallyl cations (Scheme 1, 1). In 2011, Jeffrey and co-workers pioneeringly reported the [4 + 3] cycloaddition between azaoxyallyl cations and cyclic dienes (Scheme 1, 1a).2 Since then, the research groups of Jeffrey, Wu and Liao independently devised similar [3 + 2] cycloadditions of azaoxyallyl cations with differently substituted indoles for the preparation of pyrroloindolines (Scheme 1, 1b).3 Moreover, Chen's group discovered the [3 + 1] and [3 + 2] cycloadditions of azaoxyallyl cations with sulfur ylides delivering β- and γ-lactams (Scheme 1, 1c).4 In 2016, Lin and Jeffrey's groups individually successfully applied the [3 + 2] cycloaddition of azaoxyallyl cations with aldehydes in the synthesis of oxazolidin-4-ones (Scheme 1, 1d).5 Additionally, in the same year, Wu and co-workers established the [3 + 3] cycloaddition of isoquinoline N-oxides as cyclic nitrones with azaoxyallyl cations (Scheme 1, 1e).6 Even though the important and elegant advances in the synthetic methodology of azaoxyallyl cations, it remains highly demanded to develop novel and efficient synthetic methodologies of azaoxyallyl cations for the synthesis of structurally diverse heterocycles.Encouraged by the previous works on the synthetic methodology of azaoxyallyl cations, we designed the novel [3 + 3] cycloaddition of the azaoxyallyl cations in situ generated from α-halohydroxamates with acyclic nitrones as 1,3-dipoles with a purpose to prepare potentially bioactive 1,2,4-oxadiazine-5-ones (Scheme 1, 2).7 Pleasantly, the [3 + 3] cycloaddition between azaoxyallyl cations and acyclic nitrones proceeded readily under mild reaction conditions, and gave the title target molecules in the desirable chemical yields. To the best of our knowledge, no such a work has been reported in the literature to date.
2. Results and discussion
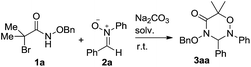
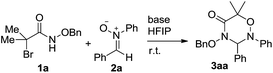
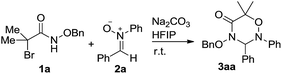
Initially, we screened the solvent effects on the [3 + 3] cycloaddition of α-halohydroxamate 1a with nitrone 2a as outlined in Table 1. Noticeably, the use of the different solvents significantly affected the chemical yield of the [3 + 3] cycloaddition. When EtOH was tested as polar protonic solvent, product 3aa was produced in a trace amount in 48 h (entry 6). Compared with the former case, the use of TFE, toluene and DCM as solvents differently increased the chemical yield of the [3 + 3] cycloaddition (entries 2 and 4–5 vs. 6). Moreover, the significant increase in the chemical yield of product 3aa was observed by using CH3CN as a polar aprotonic solvent (entry 3). Finally, the [3 + 3] cycloaddition underwent more efficiently in HFIP as a polar fluorinated solvent, and provided product 3aa in the highest chemical yield (entry 1).| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reactions were carried out with 0.2 mmol of 1a (54.2 mg) and 0.1 mmol of 2a (19.7 mg) in the presence of 0.2 mmol of Na2CO3 (21.2 mg) in 0.5 mL of the indicated solvents at room temperature.b Isolated yield. | |||
| 1 | HFIP | 1 | 98 |
| 2 | TFE | 1.5 | 40 |
| 3 | CH3CN | 4 | 90 |
| 4 | Toluene | 48 | 30 |
| 5 | DCM | 48 | 26 |
| 6 | EtOH | 48 | Trace |
Then, we examined a variety of bases bearing the various basic strength to clarify their effects on the [3 + 3] cycloaddition of α-halohydroxamate 1a with nitrone 2a using HFIP as solvent as summarized in Table 2. Remarkably, the chemical yield of the [3 + 3] cycloaddition highly depended on the used bases. As for NaHCO3 as base, it provided 3aa in 13% chemical yield (entry 6). By comparison, the use of K2CO3, Cs2CO3 and MeONa bases enhanced the chemical yield of the [3 + 3] cycloaddition differently (entries 2–3 & 7 vs. 6). As far as other examined bases such as Na2CO3, Et3N, KOH and DBU were concerned, they could promote the [3 + 3] cycloaddition efficiently, and delivered product 3aa in excellent chemical yields (entries 1, 4–5 & 8). Accordingly, in the presence of by Na2CO3 as an inorganic base, the [3 + 3] cycloaddition proceeded most efficiently, and produced product 3aa in the highest chemical yield (98%, entry 1).
| Entry | Base | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reactions were carried out with 0.2 mmol of 1a (54.2 mg) and 0.1 mmol of 2a (19.7 mg) in the presence of 0.2 mmol of the indicated bases in 0.5 mL of HFIP at room temperature.b Isolated yield. | |||
| 1 | Na2CO3 | 1 | 98 |
| 2 | K2CO3 | 1 | 78 |
| 3 | Cs2CO3 | 1 | 82 |
| 4 | Et3N | 1 | 98 |
| 5 | KOH | 1 | 92 |
| 6 | NaHCO3 | 1 | 13 |
| 7 | MeONa | 1 | 48 |
| 8 | DBU | 1 | 98 |
Meanwhile, we also investigated the effect of the equivalent ratio of 1a/2a/Na2CO3 on the [3 + 3] cycloaddition of α-halohydroxamate 1a with nitrone 2a as shown in Table 3. Apparently, the used equivalent ratio of 1a/2a/Na2CO3 dramatically influenced the chemical yield of the [3 + 3] cycloaddition. The application of the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the [3 + 3] cycloaddition formed product 3aa in 59% chemical yield (entry 1). In regard to the ratios such as 1.5
1 in the [3 + 3] cycloaddition formed product 3aa in 59% chemical yield (entry 1). In regard to the ratios such as 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, they provided product 3aa in the increased chemical yields (entries 1 vs. 2 & 4–5). Moreover, it was found that product 3aa was obtained in excellent chemical yields with the use of ratios of 2
1, they provided product 3aa in the increased chemical yields (entries 1 vs. 2 & 4–5). Moreover, it was found that product 3aa was obtained in excellent chemical yields with the use of ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the [3 + 3] cycloaddition (entries 3 & 6). Noticeably, among all the screened ratios of 1a/2a/Na2CO3, the ratio of 2
1 in the [3 + 3] cycloaddition (entries 3 & 6). Noticeably, among all the screened ratios of 1a/2a/Na2CO3, the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 should be the most optimal for the [3 + 3] cycloaddition, and furnished product 3aa in 98% chemical yield (entry 3).
2 should be the most optimal for the [3 + 3] cycloaddition, and furnished product 3aa in 98% chemical yield (entry 3).
| Entry | Equivalentratio (1a/2a/Na2CO3) | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reactions were carried out with 1a and 2a in the presence of Na2CO3 in 0.5 mL of HFIP at the indicated equivalent ratios of 1a/2a/Na2CO3 at room temperature.b Isolated yield. | |||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 59 |
| 2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1 | 85 |
| 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 | 98 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 68 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 78 |
| 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 92 |
Finally, we broadened the reaction scope of the [3 + 3] cycloaddition under the optimal reaction conditions by employing structurally different α-halohydroxamates 1 and nitrones 2 as summarized in Table 4. Obviously, the variations of R1–R4 groups of substrates 1 and 2 significantly affected the chemical yield of the [3 + 3] cycloaddition. Nitrones 2a–2l reacted easily with 1a bearing two methyl groups at the α-position, and gave products 3aa–3al in 76–99% chemical yields (entries 1–12). Basically, with respect to the [3 + 3] cycloaddition with 1a, the nitrones 2 could well tolerate the existence of electron-poor or electron-rich phenyl rings as R3 group, and furnished products 3 in excellent chemical yields (entries 2–4, 8 and 9, 11 and 12). Moreover, the nitrones 2b, 2e and 2g, involving a 4-, 3- or 2-MeO-substituted phenyl ring as R3 group respectively, afforded products 3ab, 3ae and 3ag in the quite different chemical yields in [3 + 3] the cycloaddition with 1a (entries 2, 5 & 7); in contrast, the nitrones 2d, 2f and 2h, including a 4-, 3- or 2-Cl-substituted phenyl ring as R3 group individually, provide products 3ad, 3af and 3ah in the same chemical yields (entries 4, 6 & 8).
| Entry | 1 (R1, R2, X) | 2 (R3, R4) | 3 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were carried out with 0.2 mmol of 1 and 0.1 mmol of 2 in the presence of 0.2 mmol of Na2CO3 (21.2 mg) in 0.5 mL of HFIP at room temperature.b Isolated yield.c No reaction. | |||||
| 1 | 1a (Me, Me, Br) | 2a (Ph, Ph) | 3aa | 1 | 98 |
| 2 | 1a (Me, Me, Br) | 2b (4-MeOC6H4, Ph) | 3ab | 1 | 96 |
| 3 | 1a (Me, Me, Br) | 2c (4-MeC6H4, Ph) | 3ac | 1 | 93 |
| 4 | 1a (Me, Me, Br) | 2d (4-ClC6H4, Ph) | 3ad | 1 | 99 |
| 5 | 1a (Me, Me, Br) | 2e (3-MeOC6H4, Ph) | 3ae | 1 | 89 |
| 6 | 1a (Me, Me, Br) | 2f (3-ClC6H4, Ph) | 3af | 1 | 99 |
| 7 | 1a (Me, Me, Br) | 2g (2-MeOC6H4, Ph) | 3ag | 1 | 76 |
| 8 | 1a (Me, Me, Br) | 2h (2-ClC6H4, Ph) | 3ah | 1 | 99 |
| 9 | 1a (Me, Me, Br) | 2i (4-BrC6H4, Ph) | 3ai | 1 | 97 |
| 10 | 1a (Me, Me, Br) | 2j (4-FC6H4, Ph) | 3aj | 1 | 87 |
| 11 | 1a (Me, Me, Br) | 2k (4-CNC6H4, Ph) | 3ak | 1 | 99 |
| 12 | 1a (Me, Me, Br) | 2l (4-NO2C6H4, Ph) | 3al | 1 | 97 |
| 13 | 1a (Me, Me, Br) | 2m (Ph, Bn) | 3am | 1 | 81 |
| 14 | 1a (Me, Me, Br) | 2n (Et, Bn) | 3an | 1 | 77 |
| 15 | 1a (Me, Me, Br) | 2o (Ph, Me) | 3ao | 1 | 93 |
| 16 | 1a (Me, Me, Br) | 2p (2-naphthyl, Ph) | 3ap | 1 | 92 |
| 17 | 1a (Me, Me, Br) | 2q (2-furyl, Ph) | 3aq | 1 | 99 |
| 18 | 1b (H, Et, Br) | 2a (Ph, Ph) | 3ba | 1 | nrc |
| 19 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2a (Ph, Ph) | 3ca | 1 | 92 |
| 20 | 1d (H, Cl, Cl) | 2a (Ph, Ph) | 3da | 12 | nrc |
| 21 | 1e (H, phenyl, Cl) | 2a (Ph, Ph) | 3ea | 1 | 56 |
| 22 | 1f  |
2a (Ph, Ph) | 3fa | 12 | nrc |
| 23 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2m (Ph, Bn) | 3cm | 1 | 78 |
| 24 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2g (2-MeOC6H4, Ph) | 3cg | 1 | 67 |
| 25 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2h (2-ClC6H4, Ph) | 3ch | 1 | 86 |
| 26 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2p (2-naphthyl, Ph) | 3cp | 1 | 90 |
| 27 | 1c (R1, R2 = –CH2(CH2)3CH2–, X = Br) | 2q (2-furyl, Ph) | 3cq | 1 | 61 |
Simultaneously, the nitrones 2 well endured the varying bulky size of R3 and R4 groups in the cycloaddition with 1a, and gave the corresponding products 3 in 77–99% chemical yields (entries 13–17). By comparison, in the cycloaddition with 2a, the α-halohydroxamates 1 could not widely tolerate the structural change of R1 and R2 groups. For example, the [3 + 3] cycloaddition of substrates 1b or 1d with 2a did not take place at all (entries 18 & 20); however, substrates 1c and 1e could well react with 2a, and furnished products 3ca and 3ea in 92% and 56% chemical yields, respectively (entries 19 & 21). In addition, we performed the [3 + 3] cycloaddition of α-halohydroxamate 1c containing a cyclohexyl subunit with the nitrones 2 with different R3 or R4 groups, and discovered that all these [3 + 3] cycloadditions proceeded ready to form products 3 in the reasonable chemical yields (entries 23–27). Finally, the chemical structure of 3ad was unambiguously determined by single crystal X-ray analysis as depicted in Fig. 1.8
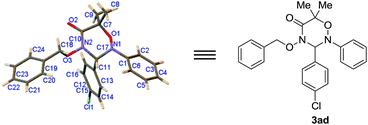 | ||
| Fig. 1 X-ray single crystal structure of 3ad (with thermal ellipsoils shown at the 50% probability level). | ||
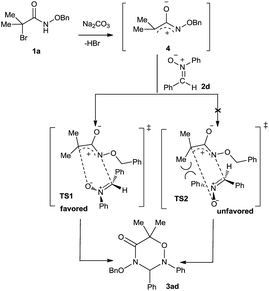
Finally, we proposed the reaction mechanism for the formation of 3ad (Scheme 2). In the presence of Na2CO3, the elimination reaction of 1a takes place to give azaoxyallyl cation 4. Then, two possible transition states TS1 and TS2 will be produced for the [3 + 3] cycloaddition between 4 and 2d. With the aid of the molecular model, it was found that in TS2 phenyl group at nitrogen atom of 2d sterically repulses α-methyl group of 4 severely; whereas, this strong destabilizing interaction does not exist in TS1 at all. Therefore, the transition state TS1 is more stable than the transition state TS2, and mainly accounts for the formation of the desired cycloadduct 3ad.
3. Conclusions
In conclusion, the [3 + 3] cycloadditions of in situ generated azaoxyallyl cations with nitrones underwent efficiently, and provided an easy access to the novel potentially bioactive 1,2,4-oxadiazinan-5-ones in reasonable chemical yields. Furthermore, the exploration of other novel cycloadditions of azaoxyallyl cations with various 1,3-, 1,4- and 1,5-dipoles is ongoing in our organic lab, and will be reported in due course.4. Experimental section
4.1 General information
Proton (1H) and carbon (13C) NMR spectra were recorded on 400 MHz instrument (400 MHz for 1H NMR, 100 MHz for 13C NMR) and calibrated using tetramethylsilane (TMS) as internal reference. High resolution mass spectra (HRMS) were recorded under electrospray ionization (ESI) conditions. The melting point of compounds was determined by a melting point instrument. Flash column chromatography was performed on silica gel (0.035–0.070 mm) using compressed air. Thin layer chromatography (TLC) was carried out on 0.25 mm SDS silica gel coated glass plates (60F254). Eluted plates were visualized using a 254 nm UV lamp. Unless otherwise indicated, all reagents were commercially available and used without further purification. All solvents were distilled from the appropriate drying agents immediately before using. α-halohydroxamates 1a–1e and α-haloamide 1f and nitrone 2a–2q were prepared according to literature procedures.2,3c,94.2 Procedure for the synthesis of products 3
A mixture of α-halohydroxamate 1 (2.0 equiv., 0.2 mmol), nitrone 2 (1.0 equiv., 0.1 mmol) and Na2CO3 (2.0 equiv., 0.2 mmol) in 0.5 mL of HFIP was stirred at room temperature for 1 h. After the reaction was completed as indicated by TLC plate, the solvent was removed by evaporation and the crude product was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products 3 as white solid (56–99% yields).
1) to afford the pure products 3 as white solid (56–99% yields).
Acknowledgements
We thank Beijing Municipal Commission of Education (No. JC015001200902), Beijing Municipal Natural Science Foundation (No. 7102010, No. 2122008), Basic Research Foundation of Beijing University of Technology (X4015001201101), Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (No. PHR201008025), Doctoral Scientific Research Start-up Foundation of Beijing University of Technology (No. 52015001200701) for financial supports.References
- For a review, see: K. L. Barnes, A. K. Koster and C. S. Jeffrey, Tetrahedron Lett., 2014, 55, 4690–4696 CrossRef CAS.
- C. S. Jeffrey, K. L. Barnes, J. A. Eickhoff and C. R. Carson, J. Am. Chem. Soc., 2011, 133, 7688–7691 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Acharya, D. Anumandla and C. S. Jeffrey, J. Am. Chem. Soc., 2015, 137, 14858–14860 CrossRef CAS PubMed; (b) M. C. DiPoto, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2015, 137, 14861–14864 CrossRef CAS PubMed; (c) W. Ji, L. Yao and X. Liao, Org. Lett., 2016, 18, 628–630 CrossRef CAS PubMed.
- C. Li, K. Jiang, Q. Ouyang, T. Y. Liu and Y. C. Chen, Org. Lett., 2016, 18, 2738–2741 CrossRef CAS PubMed.
- (a) K. Zhang, C. Yang, H. Yao and A. Lin, Org. Lett., 2016, 18, 4618–4621 CrossRef CAS PubMed; (b) A. Acharya, K. Montes and C. S. Jeffrey, Org. Lett., 2016, 18, 6082–6085 CrossRef CAS PubMed.
- Y. An, H. Xia and J. Wu, Chem. Commun., 2016, 52, 10415–10418 RSC.
- For selected examples, see: (a) H. M. Elokdah and T. S. Sulkowski, PCT Int. Appl., WO2002028845, 2002; (b) H. Kouji and T. Odagami, PCT Int. Appl., WO2015056104, 2015; (c) L. Urogdi, A. Patthy, C. Vezer and L. Kisfaludy, Org. Chem., 1984, 18, 323–327 CAS; (d) H. N. Weller, A. V. Miller, K. E. J. Dickinson and S. A. Hedberg, Heterocycles, 1993, 36, 1027–1038 CrossRef CAS; (e) M. Palfi-Ledniczky, I. Szinai, K. Ujszaszy and S. Holly, Org. Chem., 1984, 18, 329–333 CAS; (f) N. Arikan, D. Sumengen and B. Dulger, Turk. J. Chem., 2008, 32, 147–155 CAS; (g) L. Ueroegdi, A. Patthy, L. Kisfaludy and E. Moravcsik, Ger. Pat. DD207202, A119840222, 1984; (h) L. Urogdi, A. Patthy, L. Kisfaludy and E. Moravcsik, Eur. Pat., EP55484, A119820707, 1982.
- CCDC 1504786 contains the supplementary crystallographic data for compound 3ad.
- D. A. Evans, H. J. Song and K. R. Fandrick, Org. Lett., 2006, 8, 3351–3354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR spectra for all products related to this article; X-ray single crystal structure analysis data for 3ad. CCDC 1504786. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra27440d |
| This journal is © The Royal Society of Chemistry 2017 |