Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of the anti-cancer activity of the triterpenoidal saponin fraction isolated from the traditional Chinese medicine Conyza blinii H. Lév.†
Long Ma*ab,
Haiyan Liua,
Lingpei Menga,
Ping Qina,
Botao Zhanga,
Yuyin Lia,
Shuli Mana,
Zhen Liua,
Zhenxing Liua and
Aipo Diaoab
aKey Laboratory of Industrial Fermentation Microbiology, Ministry of Education, School of Biotechnology, Tianjin University of Science & Technology, Tianjin 300457, China. E-mail: malong@tust.edu.cn; woshimalong1983@163.com; Fax: +86 22 60602298; Tel: +86 22 60602948
bTianjin Key Laboratory of Industry Microbiology, School of Biotechnology, Tianjin University of Science & Technology, Tianjin 300457, China. Tel: +86 01 334467254
First published on 13th January 2017
Abstract
Conyza blinii H. Lév. is traditionally used in China as a medicinal plant to treat inflammation. We show here that the active fraction of this plant, Conyza blinii saponin (CBS), also has potent anti-cancer activity both in vitro and in vivo. Cell-based experiments showed that CBS suppresses the growth of cancer cells in a number of ways. CBS induces apoptosis in a dose-dependent manner. The mitochondrial membrane potential (ΔΨm) can be disrupted by CBS, which indicates activation of the mitochondrial apoptosis pathway. This was confirmed by an increase in the number of apoptotic bodies seen with scanning electron microscopy. CBS is capable of inhibiting cancer cell migration and invasion. Flow cytometry experiments demonstrated that CBS causes S phase arrest in cancer cells. These effects collectively led to an inhibitory activity against HeLa cells. In vivo animal experiments were carried and showed that 15 mg kg−1 CBS is a tolerable dose and reduced the tumour weight by 70% in a 10 day administration regimen.
Conyza blinii H. Lév. is a herbaceous plant of the Compositae family. It is native to SW China (mainly Sichuan and Yunnan provinces) and is commonly called Jin Long Dan Cao. The dried above-ground parts of the plant are used in traditional Chinese medicine (TCM) to treat chronic bronchitis, gastroenteritis and other inflammatory diseases.1 Ethnopharmacological and clinical studies have indicated that Conyza blini H. Lév. has antibacterial, anti-inflammatory and expectorant properties, which are well recorded in official guides to TCM, including the Pharmacopoeia of the People's Republic of China (2010 edition).2,3
Chemical profiles of this medical plant have revealed a variety of natural products, including diterpenoids, flavonoids, triterpenoids and saponins, which may be responsible for its activity.4 A total of 15 triterpene saponins have been isolated and identified from this plant.1,5,6 Conyza blinii saponin (CBS), composed of triterpenoidal saponins, represents the total saponin content of Conyza blinii H. Lév. Thirty-five saponins have been characterized based on their retention times and HPLC-high-resolution tandem MS (MSn, n = 2–4), analyses.7 We have previously reported that CBS has a protective activity against acute gastric ulcers induced by ethanol as a result of anti-lipid peroxidation and free radical clearance.8 A series of saponins often exists in one plant with identical skeletons, but slightly altered sugar components. It is believed that triterpenoidal saponins have a wide range of bioactivity, e.g. anti-cancer, anti-virus and anti-anaphylaxis.9 Invasive cancer is the leading cause of death in developed countries and the second leading cause of death in developing countries.10 This creates an urgent need to discover anti-cancer drugs with a better therapeutic index. Natural products have an unparalleled diversity with regard to their structures and bio-function.11,12 This makes them a rich reservoir for lead drug discoveries.
Saponins from C. blinii are biosynthesized through a specific pathway and their structures have remarkable characteristics. Fig. 1 shows the skeleton for all saponins characterized in CBS. The aglycones of all the identified saponins from this species are either 2β,23-dihydroxyoleanolic acid or 2β,16α,23-trihydroxyoleanolic acid. The aglycones are substituted with oligosaccharides at C-3 and C-28 to form bisdesmosidic triterpene saponins. The saccharide sequences are highly modular. All the α-chains (sugar chain at C-28) start with -Ara(2,1)-Rha(4,1)-Xyl, which, in some cases, are further substituted with Api, Rha, Xyl or Gal. The β-chain (sugar chain at C-3) is much simpler and has only three combinations: -Glc, -Glc(3,1)-Glc, or -Glc(3,1)-Xyl. C-16 is either an –OH or –H substitution. Fig. SI-1† shows the preparation of CBS.
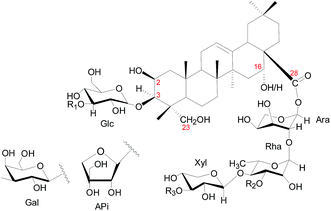 | ||
| Fig. 1 Structural skeleton of components in CBS. Numbers in red indicate the position of carbon. Glc = glucose; Ara = arabinose; Rha = rhamnose; Xyl = xylose; Api = apiose. | ||
We explored the in vitro anti-cancer activities of CBS using cell-based models with HeLa (human cervical cancer) and A549 (human non-small cell lung cancer) cells. Fig. 2 clearly shows that CBS had an inhibitory effect against both cell lines in a time- and dose-dependent manner. A concentration of CBS as low as 3.75 μg mL−1 started to show anti-cancer effects. A concentration of 15 μg mL−1 of CBS was able to inhibit cancer cell growth for both cell lines, suppressing the cell survival rate by 50%. Based on our calculation, CBS had IC50 values of 19.4 ± 1.94 μg mL−1 (24 h) and 8.56 ± 1.21 μg mL−1 (48 h), respectively, against HeLa cells and IC50 values of 14.6 ± 1.67 μg mL−1 (24 h) and 12.9 ± 1.45 μg mL−1 (48 h) against A549 cells.
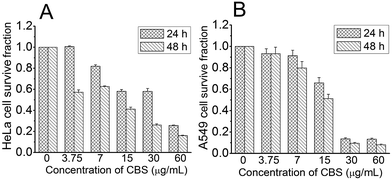 | ||
| Fig. 2 Effect of CBS on the viability of HeLa (A) and A549 (B) cells at different concentrations and time points (n = 3). | ||
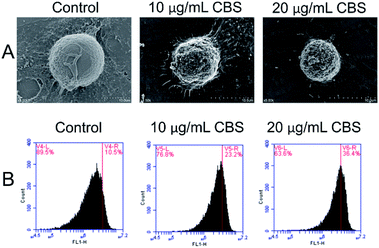
The morphology of HeLa cells in the absence or presence CBS was studied using SEM (Fig. 3A). For the HeLa cells in the control group, dense microvilli with minor protrusions were seen. The cell shrinkage was more severe for HeLa cells treated with 10 μg mL−1 CBS and the microvilli became thinner. The outer cell membranes showed remarkably increased protrusions and blebbing. All these features are characteristic of apoptotic cells. In the presence of 20 μg mL−1 CBS, deeper wrinkles were formed on the surface of the cells and apoptotic bodies were formed on and around the spherical cells. All these features suggest that CBS is an effective inducer of apoptosis in cancer cells. To examine whether CBS induced programmed cell death through a mitochondria-dependent pathway, we used rhodamine-123 to measure the mitochondrial membrane potential (ΔΨm). Fig. 3 shows the enhanced fluorescence intensity, which indicates distinct changes in the mitochondrial membrane potential (ΔΨm) caused by damage to the integrity of the mitochondrial membrane, which is a marker of apoptosis.13
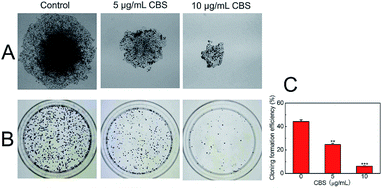
To determine the proliferation of HeLa cells with and without CBS, a colony formation assay was carried out (Fig. 4). The ability of cancer cells to produce colonies when plated at low densities is associated with their proliferation potential. This cell counting technique allowed us to estimate the ability of CBS to inhibit the proliferation of cancer cells.14 We used HeLa cells without the addition of CBS as a control and found that the number of colonies formed was much greater than in HeLa cells treated with 5 (P < 0.01) and 10 μg mL−1 CBS (P < 0.001). The size of the colonies of CBS-treated cells was much smaller than that of the control colonies. These observations suggest that CBS is an effective suppressor of the proliferation of HeLa cells.
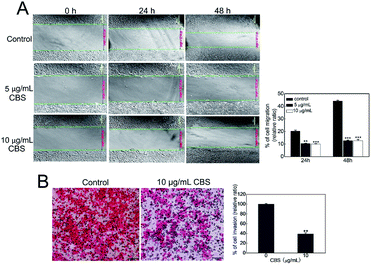
We used an in vitro wound-healing assay to evaluate how CBS affects the migration of HeLa cells.15 Fig. 5A shows that the control HeLa cells continued to grow and that the scratch was narrower at 48 h than at 24 h. This shows the capability of the cancer cells to migrate. The cells in the control group had migrated much further than the two CBS-treated groups at both 24 (P < 0.01) and 48 h (P < 0.001). The cells treated with 5 or 10 μg mL−1 CBS migrated more slowly and in a dose-dependent manner. HeLa cell migration was been largely restricted when CBS was added, which illustrated the inhibitory effect of CBS on cell migration. We also carried out a transwell migration assay as described previously16 to investigate the invasion of HeLa cells in response to CBS. Fig. 5B shows that the cells in the control group was able to pass through the Matrigel transwell coated membranes, suggesting that they were highly invasive. The HeLa cells in the group treated with 10 μg mL−1 CBS displayed reduced graded invasion (P < 0.001), which suggested that CBS was capable of significantly weakening invasion by HeLa cells.
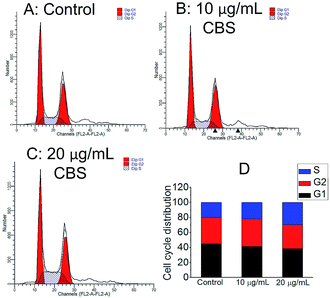
The cell cycle distribution of HeLa cells was measured by flow cytometry. Fig. 6 shows the effect of 24 h of CBS treatment on the phase distribution in the cell cycle. Compared with the control samples, a low dose of 10 μg mL−1 CBS elicited only marginal accumulation in the S phase, whereas a higher dose of CBS (20 μg mL−1) resulted in an marked increase in the S phase by 45.6%. These results indicate that the reduced growth of HeLa cells by CBS was related to cell cycle arrest in S phase.
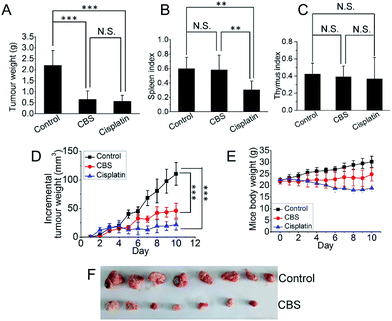
To investigate the anti-tumour effect of CBS in vivo, mouse hepatoma H22 xenografts were established in the flanks of a mouse models. Two days after subcutaneous implantation, the mice were assigned to three groups: (1) physiological saline; (2) CBS (10 mg kg−1); and (3) cisplatin (5 mg kg−1). Each group was composed of eight mice and all were dosed using an intraperitoneal injection for 10 consecutive days, two days each injection. On the 11th day of the experiment, all the mice were killed and the xenografts were collected. The tumour weights were measured. Fig. 7A shows that tumour growth was significantly suppressed in both the CBS- and cisplatin-treated groups compared with the controls (for each CBS treatment, P < 0.001). The CBS and cisplatin treatments led to a 70.5 and 74.4% reduction in tumour weight, respectively, compared with the controls. This was also reflected by the daily increase in tumour size (Fig. 7D). However, for the cisplatin group, the spleen index for the mice was lower than for the control, indicating some adverse effects. By contrast, CBS did not show any appreciable toxicity during the experiment, as supported by the spleen and thymus indexes (Fig. 7B and C). A thorough necropsy was carried out and no obvious abnormality was found in the main organs (heart, liver, stomach, kidney, lung and spleen) for the CBS-treated mice. Fig. 7E shows that the body weight of the control mice increased normally. For the CBS-treated group, the body weight was almost unchanged, whereas the body weight of the mice in the cisplatin-treated group decreased considerably. This indicated that CBS was better tolerated than cisplatin in this animal model.
We tested the efficacy of CBS as an anti-cancer treatment. MTT assays showed that CBS inhibited the growth of both HeLa and A549 cells in a time- and dose-dependent manner. Based on our calculations, 15 μg mL−1 CBS suppresses cell growth by at least 50% at 48 h for both HeLa and A549 cells. A decrease in the mitochondrial membrane potential (ΔΨm) is a diagnostic marker of cell apoptosis and we found that ΔΨm decreased after the administration of CBS. This implies an apoptosis induction effect, which was confirmed by an increase in the number of apoptotic bodies after the addition of CBS as shown by SEM. The anti-proliferation effect of CBS was evidenced by a colony formation assay; 5 μg mL−1 CBS significantly decreased the proliferation of HeLa cells. Cell cycle arrest of HeLa cells in the S phase by CBS was demonstrated by flow cytometry. Cancer cells vigorously replicate their own DNA in the S phase in order to propagate. Blockage in the S phase is thought to greatly inhibit cancer cells.17 CBS also suppressed the migration and invasion of HeLa cells. Resistance to cell death, limitless replicative potential, migration and tissue invasion are striking features of cancer cells.18 Malignant cancers are the consequence of these features and therefore targeting these crucial processes provides a realistic strategy to control cancers. Tumour xenograft mouse models confirmed the anti-neoplastic effect of CBS in vivo and no obvious toxicity was observed. A mixture of compounds usually shows stronger and more effective anti-tumour effects than a single compound.19 Increasing evidence suggests that the mixtures of interacting ingredients produced by plants may provide collective therapies that simultaneously act on multiple targets or gene networks. This leads to a synergistic efficacy that cannot be obtained by any one ingredient alone.20 This is a potential advantage of botanical/herbal drugs over single-agent treatments.
We have shown that CBS is able to suppress cancers both in vitro and in vivo and shows promise as a novel therapeutic modality. Our novel findings expand the utility of saponins and verify that exploring bioactive compounds from natural products is a rational approach for anti-cancer drug research.21–26 In addition to the anti-inflammation properties of the herbal medicine Conyza blinii H. Lév., we have also shown its potential as a botanical drug in cancer treatment.
Live subject statement
Animal welfare and the experimental procedures were carried out in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals, approved by the State Council of People's Republic of China, and the Ethical Regulations on the Care and Use of Laboratory Animals of Tianjin University of Science and Technology, approved by the university committee for animal experiments.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81503086 and 21672161 to LM; 81673647 to SLM), a starting funding (No. 20140520) from Tianjin University of Science & Technology (TUST), research funding of “1000 Talents Plan” of Tianjin (to LM) and funding from the Foundation of Key Laboratory of Industrial Fermentation Microbiology of Ministry of Education and Tianjin Key Lab of Industrial Microbiology (No. 2015IM106). We also thank Professor Peng Yu (TUST) for giving us access to facilities for the animal experiments. We would like to thank Prof. Yanfang Su (Tianjin University) for providing CBS sample.Notes and references
- Y. Su, D. Guo, H. Guo, J. Liu, J. Zheng, K. Koike and T. Nikaido, J. Nat. Prod., 2001, 64, 32–36 CrossRef CAS.
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, People's Medical Publishing House, Beijing, China, 2010, vol. I, p. 201 Search PubMed.
- The Research Institute for Medicinal Plants, Chinese Academy of Medical Sciences, Materia Medica, Public Health Press, Beijing, China, 1988, vol. 4, pp. 488–491 Search PubMed.
- L. Xu, D. Guo, J. Liu, J. Zheng, K. Koike, Z. Jia and T. Nikaido, Heterocycles, 1999, 51, 605–609 CrossRef CAS.
- Y. Su, K. Koike, D. Guo, T. Satou, J. Liu, J. Zheng and T. Nikaido, Tetrahedron, 2001, 57, 6721–6726 CrossRef CAS.
- Y. Su, K. Koike, T. Nikaido, J. Liu, J. Zheng and D. Guo, J. Nat. Prod., 2003, 66, 1593–1599 CrossRef CAS PubMed.
- X. Qiao, X. Zhang, M. Ye, Y. F. Su, J. Dong, J. Han, J. Yin and D. A. Guo, Rapid Commun. Mass Spectrom., 2010, 24, 3340–3350 CrossRef CAS PubMed.
- L. Ma and J. Liu, J. Ethnopharmacol., 2014, 158, 358–363 CrossRef CAS PubMed.
- S. Sparg, M. Light and J. Van Staden, J. Ethnopharmacol., 2004, 94, 219–243 CrossRef CAS PubMed.
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, Ca-Cancer J. Clin., 2011, 61, 69–90 CrossRef PubMed.
- H. Deng, L. Ma, N. Bandaranayaka, Z. Qin, G. Mann, K. Kyeremeh, Y. Yu, T. Shepherd, J. H. Naismith and D. O'Hagan, ChemBioChem, 2014, 15, 364–368 CrossRef CAS PubMed.
- L. Ma, Y. Li, L. Meng, H. Deng, Y. Li, Q. Zhang and A. Diao, RSC Adv., 2016, 6, 27047–27051 RSC.
- R. C. Scaduto and L. W. Grotyohann, Biophys. J., 1999, 76, 469–477 CrossRef CAS PubMed.
- S. Horibata, T. V. Vo, V. Subramanian, P. R. Thompson and S. A. Coonrod, J. Visualized Exp., 2015, e52727 Search PubMed.
- R. Riahi, Y. Yang, D. D. Zhang and P. K. Wong, J. Lab. Autom., 2012, 17, 59–65 CrossRef CAS PubMed.
- N. Kramer, A. Walzl, C. Unger, M. Rosner, G. Krupitza, M. Hengstschläger and H. Dolznig, Mutat. Res., Rev. Mutat. Res., 2013, 752, 10–24 CrossRef CAS PubMed.
- A. K. Joe, H. Liu, M. Suzui, M. E. Vural, D. Xiao and I. B. Weinstein, Clin. Cancer Res., 2002, 8, 893–903 CAS.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed.
- F. Herrmann and M. Wink, Phytomedicine, 2011, 18, 1191–1196 CrossRef CAS PubMed.
- B. M. Schmidt, D. M. Ribnicky, P. E. Lipsky and I. Raskin, Nat. Chem. Biol., 2007, 3, 360–366 CrossRef CAS PubMed.
- L. Ma, A. Bartholome, M. H. Tong, Z. Qin, Y. Yu, T. Shepherd, K. Kyeremeh, H. Deng and D. O'Hagan, Chem. Sci., 2015, 6, 1414–1419 RSC.
- L. Ma and A. Diao, Anti-Cancer Agents Med. Chem., 2015, 15, 298–306 CrossRef CAS PubMed.
- S. Huang, L. Ma, M. H. Tong, Y. Yu, D. O'Hagan and H. Deng, Org. Biomol. Chem., 2014, 12, 4828–4831 CAS.
- L. Ma, H. Liu, P. Qin, C. Hu, S. Man, Y. Li, Z. Liu, Z. Liu and A. Diao, Biochem. Biophys. Res. Commun., 2016 Search PubMed , in press.
- J. Liu, Z. Liu, S. Man, H. Chai, L. Ma and W. Gao, RSC Adv., 2016, 6, 92330–92334 RSC.
- P. Qiu, S. Man, H. Yang, Y. Liu, Z. Liu, L. Ma, P. Yu and W. Gao, RSC Adv., 2016, 6, 115029–115038 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26361e |
| This journal is © The Royal Society of Chemistry 2017 |