Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
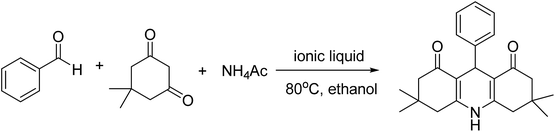
Betainium-based ionic liquids catalyzed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones†
Anlian Zhu *a,
Ruixia Liub,
Chunyan Dua and
Lingjun Lia
*a,
Ruixia Liub,
Chunyan Dua and
Lingjun Lia
aSchool of Chemistry and Chemical Engineering, Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: alzhuchem@126.com; Fax: +86-0373-3329030; Tel: +86-0373-3325805
bLuohe Vocational College of Food, Henan 462300, P. R. China
First published on 20th January 2017
Abstract
In this study, a series of betainium-based ionic liquids with various anions have been synthesized and their catalytic performances for the Hantzsch reactions have been investigated. It is shown that these ionic liquids have high selectivity for the one-pot production of acridinediones through Hantzsch reactions under mild conditions, and betainium lactate has the highest catalytic activity. This ionic liquid catalyst is very cheap and easy to prepare; the catalytic procedure is very simple and the catalyst can be recycled and reutilized. In addition, the biocompatibility of the raw material of these ionic liquids suggests that this catalytic system may have great industrial potential applications.
Introduction
Multicomponent reactions (MCRs) have received a great deal of attention in organic synthesis and combinatorial chemistry due to their wide supplement of molecular diversity and high atom economic efficiency.1,2 Although MCRs can provide high throughput generation of organic compounds, often in a one-pot strategy, they still suffer from the utilization of excessive reagents and toxic solvents, harsh reaction conditions and long reaction time. Therefore, appropriately selected catalyst or reaction medium for special MCRs plays a critical role towards the development of green processes. Ionic liquids which are composed of organic cations and organic or inorganic anions have been widely used in organic synthesis and catalysis in the past two decades.3–6 The ability to remain a liquid under a wide range of temperatures, negligible vapor pressure and facile functionalization of ionic liquids make them attractive alternatives as the reaction medium or catalyst for MCRs, and numerous MCRs have been performed efficiently with the help of acidic or basic ionic liquids, acting as both reaction media and catalysts.7,8Acridinediones are an important class of heterocyclic compounds found in much complex compounds that exhibit antimalarial, antibacterial, anticancer and antimicrobial activities.9,10 Moreover, some of their derivatives have been used as laser dyes and photoinitiators.11 The multicomponent Hantzsch reaction is an efficient method for the synthesis of acridinediones using aldehydes, dimedones and different nitrogen sources like urea, methyl amine, aniline or ammonium acetate as starting materials. The reported catalysts include amberlyst-15,12 silica-supported polyphosphoric acid,13 CdO nanoparticles,14 Brønsted acidic imidazolium ionic liquids,15 triethylbenzylammonium chloride,16 sulfonic acid functionalized silica,17 Zn(OAc)2·2H2O or proline,18 cetyl trimethyl ammonium bromide (CTAB),19 heteropolyacid functionalized ionic liquid,20 p-sulfonic acid calix[4]arene,21 acetate acid,22 cellulose sulfuric acid,23 ceric ammonium nitrate (CAN) in PEG,24 CeCl3·7H2O in ionic liquid25 or ferric hydrogen sulfate supported on silica-coated nickel ferrite nanoparticles.26 However, almost all of these catalyst systems have limitations, such as the use of hazardous solvents, prolonged reaction time, expensive reagents, a tedious or high cost catalyst preparation procedure, and the impossibility to recycle the catalyst. Thus, it is still necessary to develop efficient methods for the synthesis of acridinediones using low cost and reusable catalysts.
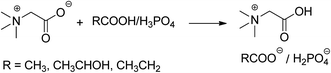
In our previous study, we have developed a series of hydroxyl functionalized task-specific ionic liquids used to catalyze organic reactions with aldehydes, including Knoevenagel reactions,27 Biginelli reactions,28 Pechmann reactions29 and Domino Knoevenagel–Michael reactions.30 In those studies, we found that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the aldehyde could be activated by the hydrogen-bond donor groups on the cations of ionic liquids, and increasing the hydrogen-bond donor ability benefits their catalytic activities. In this study, a series of ionic liquids based on a betainium cation was synthesized. It is known that the carboxyl acid group on the betainium cation has stronger hydrogen-bond donor ability than that of the hydroxyl group, and therefore the ionic liquids based on betainium are expected to show stronger activation ability towards C
O group of the aldehyde could be activated by the hydrogen-bond donor groups on the cations of ionic liquids, and increasing the hydrogen-bond donor ability benefits their catalytic activities. In this study, a series of ionic liquids based on a betainium cation was synthesized. It is known that the carboxyl acid group on the betainium cation has stronger hydrogen-bond donor ability than that of the hydroxyl group, and therefore the ionic liquids based on betainium are expected to show stronger activation ability towards C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Then, we investigated the catalytic activities of these synthesized betainium-based ionic liquids on the multicomponent Hantzsch reactions. The results showed that various aryl aldehydes with electron withdrawing or electron donating groups, heterocyclic aromatic aldehydes and allyl aldehyde could react smoothly with dimedone and ammonium acetate under mild conditions to give the target acridinediones in good to excellent isolated yields. Moreover, the betainium-based ionic liquids are easy to prepare and the raw materials like anhydrous betain and acetate acids are both biocompatible and very cheap. In addition, their ability to be reused in this catalytic procedure, further supports their use as a greener catalyst for the synthesis of acridinediones through one-pot Hantzsch reactions.
O. Then, we investigated the catalytic activities of these synthesized betainium-based ionic liquids on the multicomponent Hantzsch reactions. The results showed that various aryl aldehydes with electron withdrawing or electron donating groups, heterocyclic aromatic aldehydes and allyl aldehyde could react smoothly with dimedone and ammonium acetate under mild conditions to give the target acridinediones in good to excellent isolated yields. Moreover, the betainium-based ionic liquids are easy to prepare and the raw materials like anhydrous betain and acetate acids are both biocompatible and very cheap. In addition, their ability to be reused in this catalytic procedure, further supports their use as a greener catalyst for the synthesis of acridinediones through one-pot Hantzsch reactions.
Results and discussion
Different catalytic performances of the ionic liquids
A series of ionic liquids based on betainium cation (Hbet) with different anions, such as lactic (Lac), acetate (Ac), propionate (Pr) and dihydrogen phosphate (H2PO4), were synthesized and their catalytic performances were investigated using the reaction among benzaldehyde, 5,5-dimethyl-1,3-cyclohexanedione and NH4Ac as a model. For the sake of comparison, anhydrous betain, lactic acid and the ionic liquids based on cholinium cation with anions such as Lac and H2PO4 were also used in the model reactions. The results were collected in Table 1. It can be seen that although anhydrous betain and lactic acid can promote this reaction, the ionic liquids have high catalytic activity and ionic liquids with betanium cations have higher chemo-selectivity for the one-pot Hantzsch reactions, whereas the ionic liquids with cholinium cations prefer to produce the two component reaction product between benzaldehyde and 5,5-dimethyl-1,3-cyclohexanedione. The ionic liquid with betanium cation and lactate anion, [Hbet][Lac], has the highest catalytic activity and selectivity.| Entrya | Catalyst | Reaction time (h) | Yieldb (%) |
|---|---|---|---|
| a 0.5 mmol benzaldehyde, 1 mmol 5,5-dimethyl-1,3-cyclohexanedione, 0.75 mmol NH4Ac and 0.15 mmol ionic liquid were mixed with 1 ml ethanol, and heated at 80 °C for the desired reaction time.b Isolated yields.c The yield for the product of benzaldehyde and 5,5-dimethyl-1,3-cyclohexanedione. | |||
| 1 | — | 3 | Trace |
| 2 | Betain | 3 | 50 |
| 3 | HLac | 3 | 35 |
| 4 | [Hbet][Lac] | 3 | 90 |
| 5 | [Hbet][Ac] | 3 | 75 |
| 6 | [Hbet][Pr] | 3 | 65 |
| 7 | [Hbet][H2PO4] | 3 | 70 |
| 8 | [Choline][Lac] | 4 | 25 (50c) |
| 9 | [Choline][H2PO4] | 4 | 25 (45c) |
The influences of solvent, catalyst amount and reaction temperature
The influences of solvent, catalyst amount and reaction temperature were investigated using the model reaction. It can be seen from Table 2 that solvents have a significant effect on the catalytic performances of ionic liquids and ethanol is the proper solvent. The results in Table 3 showed that the increase of the ionic liquid amount from 0 to 30 mol% was beneficial to the reaction, but further increasing the amount to 100 mol% had a negligible effect. Therefore, 30 mol% [Hbet][Lac] based on aldehyde was selected as the optimal catalyst amount. Then, the influence of the reaction temperature on the model reaction was investigated under the catalysis of the optimized amount of ionic liquid, and the results are given in Table 4. It is clear that 80 °C is the most favorable reaction temperature. Table 5 has also collected the reaction conditions of the other reported catalytic systems.| Entrya | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a 0.5 mmol benzaldehyde, 1 mmol 5,5-dimethyl-1,3-cyclohexanedione, 0.75 mmol NH4Ac and 0.15 mmol ionic liquid were mixed with 1 ml solvent, and then heated at 80 °C for the desired reaction time.b Isolated yields. | |||
| 1 | MeOH | 3 | 55 |
| 2 | EtOH | 3 | 90 |
| 3 | CH3CN | 3 | 70 |
| 4 | H2O | 3 | Trace |
| 5 | Solventless | 3 | Trace |
| 6 | [Hbet][Lac] | 3 | 55 |
| Entrya | Catalyst amount (mol%) | Time | Yieldb (%) |
|---|---|---|---|
| a 0.5 mmol benzaldehyde, 1 mmol 5,5-dimethyl-1,3-cyclohexanedione, 0.75 mmol NH4Ac and corresponding amount of ionic liquid were mixed with 1 ml ethanol, and then heated at 80 °C for the desired reaction time.b Isolated yields. | |||
| 1 | 0 | 6 h | Trace |
| 2 | 5 | 3 h | 55 |
| 3 | 10 | 3 h | 75 |
| 4 | 30 | 3 h | 90 |
| 5 | 50 | 3 h | 90 |
| 6 | 100 | 3 h | 90 |
| Entry | T (°C) | Time | Yielda,b (%) |
|---|---|---|---|
| a 0.5 mmol benzaldehyde, 1 mmol 5,5-dimethyl-1,3-cyclohexanedione, 0.75 mmol NH4Ac and 30 mol% of ionic liquid were mixed with 1 ml ethanol, and then heated at corresponding reaction temperature for the desired reaction time.b Isolated yields. | |||
| 1 | r.t. | 3 h | — |
| 2 | 40 | 3 h | Trace |
| 3 | 50 | 3 h | 30 |
| 4 | 80 | 3 h | 90 |
| 5 | 100 | 3 h | 90 |
| Entry | Catalyst | Solvent/condition | Time | Yields | Ref. |
|---|---|---|---|---|---|
| 1 | L-Proline | H2O, reflux | 3 h | 84 | 18 |
| 2 | MSI3PW | BMINTf2, 90 °C | 4 h | 98 | 20 |
| 3 | CdO | Solvent free, 120 °C | 8 min | 92 | 14 |
| 4 | Glycol, NaOAC | H2O, microwave | 10 min | 78–94 | 11 |
| 5 | Cellulose sulfuric | Solvent free, 100 °C | 5 h | 80 | 23 |
| 6 | CAN | PEG-400, 50 °C | 4–8 h | 91 | 24 |
| 7 | Triethylbenzylammonium chloride | Solvent free, 85 °C | 20 min | 85 | 16 |
| 8 | [CMIM][CF3COO] | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
1.5 h | 85 | 17 |
| 9 | CeCl3·7H2O | [Bmim][BF4], 100 °C | 3 h | 87 | 25 |
| 10 | NiFe2O4@SiO2–FHS | Solvent free, 80 °C | 15–30 min | 90 | 26 |
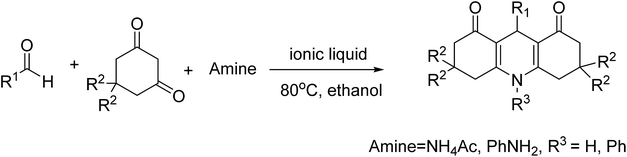
Next, the substrate tolerance of this catalyst for the synthesis of acridinediones through Hantzsch reactions was investigated using different aldehydes under optimized conditions, and the results are displayed in Table 6. It is evident that various aromatic aldehydes with electron donating or electron withdrawing substituent groups could react with 5,5-dimethyl-1,3-cyclohexanedione and NH4Ac smoothly to give the target compounds with good to excellent isolated yields. An allyl aldehyde like cinnamaldehyde, heteroaromatic aldehydes like pyridylaldehyde and furfuraldehyde could also be converted to the corresponding acridinediones with excellent isolated yields within 3 hours.
| Entry | R1 | R2 | Amine | Time (h) | Yielda (%) | Mp (°C) (obs) | Mp (°C) (lit) |
|---|---|---|---|---|---|---|---|
| a Isolated yield. | |||||||
| 1 | C6H5 | CH3 | NH4Ac | 3 | 90 | 272 | 277–278 (ref. 31) |
| 2 | 4-NO2C6H4 | CH3 | NH4Ac | 8 | 85 | 268–270 | 261–262 (ref. 32) |
| 3 | 3-NO2C6H4 | CH3 | NH4Ac | 8 | 80 | 298–300 | 287–289 (ref. 31) |
| 4 | 4-BrC6H4 | CH3 | NH4Ac | 5 | 85 | >300 | 330–332 (ref. 33) |
| 5 | 4-CH3OC6H4 | CH3 | NH4Ac | 3 | 96 | >300 | 275–277 (ref. 8) |
| 6 | 4-HOC6H4 | CH3 | NH4Ac | 3 | 95 | >300 | >300 (ref. 31) |
| 7 | 4-CH3C6H4 | CH3 | NH4Ac | 3 | 90 | >300 | 270–275 (ref. 8) |
| 8 | 4-CF3C6H4 | CH3 | NH4Ac | 3 | 95 | 258–260 | >300 (ref. 8) |
| 9 | 4-ClC6H4 | CH3 | NH4Ac | 3.5 | 85 | 302–303 | 300–301 (ref. 34) |
| 10 | 4-FC6H4 | CH3 | NH4Ac | 3.5 | 85 | 246–248 | 275–276 (ref. 16) |
| 11 | 3-ClC6H4 | CH3 | NH4Ac | 3.5 | 85 | 290 | 283–285 (ref. 8) |
| 12 | 4-OH-3-CH3OC6H3 | CH3 | NH4Ac | 8 | 90 | 300 | 295–298 (ref. 8) |
| 13 | 2-ClC6H4 | CH3 | NH4Ac | 6 | 87 | 290–292 | 263–264 (ref. 8) |
| 14 | C6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
CH3 | NH4Ac | 1 | 88 | 217 | — |
| 15 | 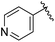 |
CH3 | NH4Ac | 1 | 95 | 248–250 | — |
| 16 | 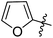 |
CH3 | NH4Ac | 3 | 95 | 292 | — |
| 17 | C6H5 | H | NH4Ac | 3 | 95 | 310 | 279–281 (ref. 17) |
| 18 | 4-CH3OC6H4 | H | NH4Ac | 3 | 92 | >300 | 306–308 (ref. 17) |
| 19 | 4-HOC6H4 | H | NH4Ac | 3 | 85 | >300 | 305–307 (ref. 17) |
| 20 | 4-CH3C6H4 | H | NH4Ac | 2 | 95 | 300 | — |
| 21 | 4-ClC6H4 | H | NH4Ac | 3 | 95 | 300 | 298–299 (ref. 17) |
| 22 | 4-CH3OC6H4 | CH3 | PhNH2 | 3 | 80 | 290–292 | 291–293 (ref. 19) |
| 23 | 3-NO2C6H4 | CH3 | PhNH2 | 3 | 75 | 281–283 | 281–283 (ref. 19) |
| 24 | 4-ClC6H4 | CH3 | PhNH2 | 3 | 75 | 237–239 | 243–245 (ref. 19) |
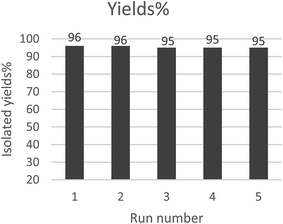
The reusability of the ionic liquids was also investigated using the reaction between 4-methoxy-benzaldehyde, 5,5-dimethyl-1,3-cyclohexanedione and NH4Ac as a model system, and the results are illustrated in Fig. 1. It was shown that after five cycles the catalytic activity had a negligible decrease, suggesting the excellent recyclability of this ionic liquid in Hantzsch reactions.
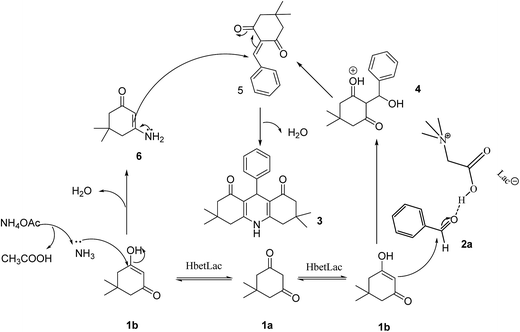
The plausible reaction mechanism was proposed and is illustrated in Fig. 2. The hydrogen bond donor ability of the ionic liquid may increase the enol (1b) content in the keto–enol equilibrium of dimitone. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the aldehyde was polarized by the formation of hydrogen bonds with the carboxyl group on the cation of the ionic liquid, which was then attacked by 1b to form intermediate 4 and then dehydrated to 5. NH4OAc was decomposed to NH3 and CH3COOH, and NH3 attacked enol 1b to form intermediate 6. The intermediate 6 reacted with 5 to form the product 3.
O of the aldehyde was polarized by the formation of hydrogen bonds with the carboxyl group on the cation of the ionic liquid, which was then attacked by 1b to form intermediate 4 and then dehydrated to 5. NH4OAc was decomposed to NH3 and CH3COOH, and NH3 attacked enol 1b to form intermediate 6. The intermediate 6 reacted with 5 to form the product 3.
Conclusion
The biocompatible ionic liquid [Hbet][Lac] was found to be an efficient and reusable catalyst for the Hantzsch reactions under mild conditions. Various aromatic aldehydes, allyl aldehyde and heteroaromatic aldehydes could be converted to the corresponding acridinediones with good to excellent isolated yields. The operation and work-up procedures were very simple and no column chromatography purification was needed. In addition, this ionic liquid catalyst is very cheap, easy to prepare, and its raw materials such as anhydrous betain and lactic acid are highly biocompatible and biodegradable, which make the preparation and utilization procedures more safe and green.Experimental
Chemical and instrument
All of the reagents purchased were of AR grade and used without further purification. Melting points were detected with a XTC-1 Microscopic Melting Point Measurer (Sichuan University Instrument Factory) without correction; 1H-NMR and 13C-NMR spectra were recorded on a Bruker AV-400 instrument with CDCl3 as solvent.Synthesis of betainium ionic liquids
0.1 mol carboxyl acid dissolved in 10 ml methanol was drop-wise added to the 100 ml flask containing 0.1 mol anhydrous betain in 10 ml methanol under stirring. The solution was stirred at room temperature for 24 h to complete the reaction, and then the methanol was removed at 50 °C to get the target ionic liquids with white solid or pale yellow liquid status (Scheme 1).General procedures for the synthesis of 1,8-dioxodecahydroacridines
In a 10 ml round bottom flask, the mixture of aromatic aldehyde (0.5 mmol), 5,5-dimethyl-1,3-cyclohexanedione (1 mmol), ammonium acetate (0.75 mmol) and [Hbet][Lac] (30 mol%) in ethanol (1 ml) was stirred at 80 °C for the desired time. The process of reaction was monitored using TLC (the solvent for the TLC is the mixture of ethyl acetate and petroleum ether (b.p. 60–90 °C)). After completion of the reaction, the mixture was gradually cooled to room temperature and ice water was added under stirring, and then the product could be isolated as solid precipitate. The solid product was filtered and washed using ice aqueous ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) or recrystallized in ethanol to get the pure products.
1 in volume) or recrystallized in ethanol to get the pure products.
Reutilization of the ionic liquid
The reusability of [Hbet][Lac] was investigated from the reaction between p-methoxybenzaldehyde, 5,5-dimethyl-1,3-cyclohexanedione and ammonium acetate as a model. After the completion of the reaction, ice water was added to the reaction mixtures. The solid product was filtered and the catalyst [Hbet][Lac] was recovered from the filtrate. The containing water was evaporated, and then the ionic liquid was dried in vacuum at 50 °C for 12 h. The recovered ionic liquid can then be reused for additional runs without activation.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21373079) and Program for Science & Technology Innovation Talents in Universities of Henan Province (14HASTIT015).References
- B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef CAS PubMed.
- A. Shaabani, A. Sarvary, S. Ghasemi, A. H. Rezayan, R. Ghadari and S. W. Ng, Green Chem., 2011, 13, 582 RSC.
- M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16 RSC.
- Y. L. Gu, Green Chem., 2012, 14, 2091 RSC.
- J. Dupont, R. F. D. Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed.
- J. W. Lee, J. Y. Shin, Y. S. Chun, J. H. Bin, C. E. Song and S. G. Lee, Acc. Chem. Res., 2010, 43, 985 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed.
- H. P. Steinrueck and P. Wasserscheid, Catal. Lett., 2015, 145, 380 CrossRef CAS.
- H. W. Lee, S. J. Shin, H. Yu, S. K. Kang and C. L. Yoo, Org. Process Res. Dev., 2009, 13, 1382 CrossRef CAS.
- S. A. Gamage, J. A. Spicer, G. J. Atwell, G. J. Finlay, B. C. Baguley and W. A. Denny, J. Med. Chem., 1999, 42, 2383 CrossRef CAS PubMed.
- S. J. Tu, X. J. Zhang, F. Shi, T. J. Li, Q. Wang, X. T. Zhu, J. P. Zhang and J. N. Xu, J. Heterocycl. Chem., 2005, 42, 1155 CrossRef CAS.
- M. Kaya, Y. Yıldırır and G. Y. Çelik, Med. Chem. Res., 2011, 20, 293 CrossRef CAS.
- Z. Nasresfahani and M. Z. Kassaee, Catal. Commun., 2015, 60, 100 CrossRef CAS.
- A. V. Borhade, B. K. Uphade and A. G. Gadhave, Res. Chem. Intermed., 2015, 41, 1447 CrossRef CAS.
- D. Patil, D. Chandam, A. Mulik, P. Patil, S. Jagadale, R. Kant, V. Gupta and M. Deshmukh, Catal. Lett., 2014, 144, 949 CrossRef CAS.
- X. S. Wang, D. Q. Shi, Y. F. Zhang, S. H. Wang and S. J. Tu, Chin. J. Org. Chem., 2004, 24, 430 CAS.
- M. Seyyedhamzeh, P. Mirzaei and A. Bazgir, Dyes Pigm., 2008, 76, 836 CrossRef CAS.
- K. Venkatesan, S. S. Pujari and K. V. Srinivasan, Synth. Commun., 2009, 39, 228 CrossRef CAS.
- J. J. Xia and K. H. Zhang, Molecules, 2012, 17, 5339 CrossRef CAS PubMed.
- H. G. O. Alvima, G. A. Bataglion, L. M. Ramos, A. L. de Oliveira, H. C. B. de Oliveira, M. N. Eberlin, J. L. de Macedo, W. A. da Silva and B. A. D. Neto, Tetrahedron, 2014, 70, 3306 CrossRef.
- N. Hazeri, A. Masoumania, M. T. Mghsoodlou, S. Salahi, M. Kangani, S. Kianpour, S. kiaee and J. Abonajmi, Res. Chem. Intermed., 2015, 41, 4123 CrossRef CAS.
- S. M. Baghbanian, G. Khanzad, S. M. Vahdat and H. Tashakkorian, Res. Chem. Intermed., 2015, 41, 9951 CrossRef CAS.
- Y. L. N. Murthy, A. Rajack, M. T. Ramji, J. J. babu, C. Praveen and K. A. Lakshmi, Bioorg. Med. Chem. Lett., 2012, 22, 6016 CrossRef CAS PubMed.
- M. Kidwai and D. Bhatnagar, Chem. Pap., 2010, 64, 825 CAS.
- X. Fan, Y. Li, X. Zhang, G. Qu and J. Wang, Heteroat. Chem., 2007, 18, 786 CrossRef CAS.
- A. Khojastehnezhad, M. Rahimizadeh, H. Eshghi, F. Moeinpour and M. Bakavoli, Chin. J. Catal., 2014, 35, 376 CrossRef CAS.
- A. L. Zhu, R. X. Liu, L. J. Li, L. Y. Li, L. Wang and J. J. Wang, Catal. Today, 2013, 200, 17 CrossRef CAS.
- A. L. Zhu, Q. Q. Li, L. J. Li and J. J. Wang, Catal. Lett., 2013, 143, 463 CrossRef CAS.
- Y. H. Zhang, A. L. Zhu, L. J. Li, Y. Zhao and J. J. Wang, RSC Adv., 2014, 4, 22946 RSC.
- A. L. Zhu, S. K. Bai, W. Jin, R. X. Liu, L. J. Li, Y. Zhao and J. J. Wang, RSC Adv., 2014, 4, 36031 RSC.
- Y. B. Shen and G. W. Wang, ARKIVOC, 2008, 16, 1 Search PubMed.
- X. S. Wang, M. M. Zhang, Z. S. Zeng, D. Q. Shi, S. J. Tu, X. Y. Wei and Z. M. Zong, ARKIVOC, 2009, 117 Search PubMed.
- X. S. Fan, Z. Li, Y. X. Y. Zhang, G. R. Qu and J. J. Wang, Heteroat. Chem., 2007, 18, 786 CrossRef CAS.
- A. Khojastehnezhad, M. Rahimizadeh, H. Eshghi and F. Moeinpour, Chin. J. Catal., 2014, 35, 376–382 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25709g |
| This journal is © The Royal Society of Chemistry 2017 |