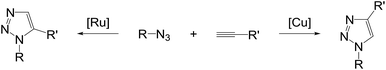Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of steric demand on ruthenium-catalyzed cycloaddition of sterically hindered azides†
Venkata S. Sadu a,
Sirisha Sadu
a,
Sirisha Sadu a,
Seji Kim
a,
Seji Kim b,
In-Taek Hwang
b,
In-Taek Hwang b,
Ki-Jeong Kong
b,
Ki-Jeong Kong b and
Kee-In Lee
b and
Kee-In Lee *ab
*ab
aMajor of Green Chemistry and Environmental Biotechnology, University of Science & Technology, Taejon 305-350, Korea. E-mail: kilee@krict.re.kr
bGreen Chemistry Division, Korea Research Institute of Chemical Technology, Taejon 305-600, Korea
First published on 13th January 2017
Abstract
The RuAAC of sterically hindered 2,2-diaryl-2-azidoamines and terminal alkynes resulted in the unprecedented formation of 1,4-disubstituted-1,2,3-triazoles. A control experiment with 2-(azidomethyl)pyrrolidine revealed the usual selectivity with RuAAC and the reactions of azides with intermediate bulkiness gave mixtures of 1,4- and 1,5-regioisomers. The results suggest that the steric demands could annul the preference and influence on the regioselectivity of RuAAC substantially.
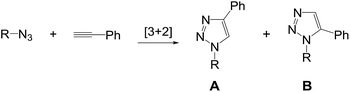
The Huisgen 1,3-dipolar cycloaddition between azides and alkynes is the most convenient way to access 1,2,3-triazoles, but it produces a mixture of 1,4- and 1,5-disubstituted triazoles and may require elevated temperatures.1 After the discovery of copper-catalyzed azide-alkyne cycloaddition (CuAAC) leading to the regioselective formation of 1,4-disubstituted 1,2,3-triazoles,2 the reaction enabled the rapid spread of triazole chemistry across multilateral disciplines including medicinal chemistry, chemical biology and material sciences due to its high degree of reliability, specificity and biocompatibility.3 In addition, recent advances in ruthenium-catalyzed azide-alkyne cycloaddition (RuAAC) allow entry to 1,5-regioisomers as the complementary version to the copper catalysis. Among them, ruthenium complexes containing the pentamethylcyclopentadienyl ligand (Cp*) are the most prominent class of catalysts showing high levels of regioselectivity for a broad substrate scope.4 Particularly interesting are approaches featuring the complementary pairings between CuAAC and RuAAC to the same substrates as illustrated in Scheme 1, so that the pairs could be used for comparison and validation studies in many different ways.5
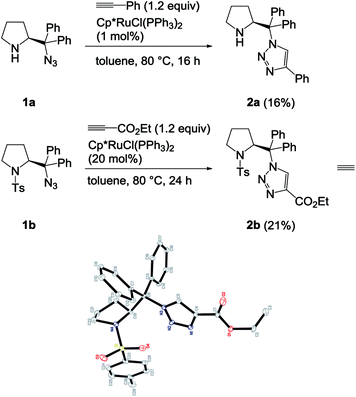
We envisioned that incorporation of triazole functionality into the backbone of diarylprolinol may serve as a proline surrogate potentially useful for organocatalysis.6 Previously, the sequential synthesis of highly hindered β-amino triazoles bearing a gem-diaryl group has been accomplished from 1,1-diaryl-2-aminoethanols in part employing the copper catalysis.7 Attention next turned to the construction of pairwise surrogates. During the investigation, we found an exclusive formation of 1,4-disubstituted triazoles from ruthenium-catalyzed cycloaddition particularly between sterically hindered azides and terminal alkynes even under the Cp*RuCl(PPh3)2 catalyst. Herein we wish to discuss the effect of steric factors on the regioselectivity of the RuAAC using azide partners embedded in the bulky group.
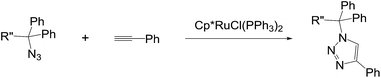
The first choice was Cp*RuCl(PPh3)2 as it has been widely used for the construction of 1,5-disubstituted triazoles. However, surprisingly the product 2a from the reaction of 1a with phenylacetylene was completely matched with the triazole from CuAAC. The result is in contrast with the reported regioselectivity of the RuAAC of terminal alkynes with Cp*RuCl(PPh3)2 that show the preference for forming the 1,5-regioisomers. It seems we needed to clarify an ambiguity of a possible coordination of the secondary amine to a metal centre that may cause a change in the regioselectivity. Hence, the reaction between N-tosyl-protected 1b and ethyl propiolate was subjected and X-ray crystal structure of 2b clearly revealed the 1,4-rigioisomer as shown in Scheme 2.† Even both reactions were not completed in toluene at given times, the reverse regioselectivity is quite unusual behaviour, as seen previously in the ruthenium complexes containing the Cp* ligand.
We next questioned that the observed selectivity is general when employing different ruthenium complexes, which have been addressed on the regioselective formation of the triazoles in the literature. The Cp*-containing ruthenium catalysts such as Cp*RuCl(PPh3)2,4a Cp*RuCl(COD),4a,b and [Cp*RuCl]4 (ref. 4c and d) give 1,5-disubstituted triazoles with high regioselectivity, whereas CpRuCl(PPh3)2 (ref. 4b) containing a cyclopentadienyl ligand (Cp) shows marginal regioselectivity and catalytic activity towards 1,5-regioisomers. On the other hand, Cp*/Cp-lacking RuH2(CO)(PPh3)3 (ref. 8a) and RuH(η2-BH4)(CO)(PCy)2 (ref. 8b) afford 1,4-regioisomers selectively. It seems clear that the Cp* ligand serves as the key element responsible for the high 1,5-selectivity in the ruthenium catalysis.
Examinations were thus undertaken with different Cp*-containing catalysts, where DMF was the best choice and the reactions were completed within 20 h. The tertiary azide proved to be a relatively poor substrate quite possibly due to the steric hindrance imposed by the two phenyl groups, however, the RuAAC reactions were equally effective for the generation of 1,4-regioisomer as the sole product (Table 1, entries 1–3). The catalysts RuH2(CO)(PPh3)3 and Tp(PPh3)(EtNH2)RuN3 (Tp = HB(pz)3, pz = pyrazolyl)9 also produced the 1,4-disubstituted triazole but with slightly lower yield than the Cp*-containing catalysts (entries 4 and 5). Regardless of the ruthenium catalysts tested, the reactions exhibited 1,4-selectivity exclusively. Due to the unique outcomes, we then needed to measure the thermal contribution to the reaction and the result suggests that there is no contribution from the thermal to the observed regioselectivity (entry 6). By the way, supplementary attempts to obtain 1,5-isomers were not fruitful, as applying alternative protocols based on the generation of magnesium acetylide10a or the presence of catalytic tetramethylammonium hydroxide,10b respectively.
| Entry | Ru catalyst | Equiv. of alkyne | Reaction timeb (h) | Yield of 2ac (%) |
|---|---|---|---|---|
| a Unless otherwise indicated, reaction conditions are as follows: 1a (1 mmol), Ru catalyst (1 mol%), phenylacetylene (2–3 equiv.), DMF, 150 °C.b At a given time, 1a was completely consumed or decomposed.c No 1,5-regioisomer observed.d Thermal Huisgen reaction performed without the catalyst.e No reaction observed. | ||||
| 1 | Cp*RuCl(PPh3)2 | 2 | 16 | 46 |
| 2 | Cp*RuCl(COD) | 2 | 20 | 41 |
| 3 | [Cp*RuCl]4 | 3 | 16 | 47 |
| 4 | RuH2(CO)(PPh3)3 | 3 | 20 | 37 |
| 5 | Tp(PPh3)(EtNH2)RuN3 | 2 | 24 | 23 |
| 6d | — | 3 | 72 | —e |
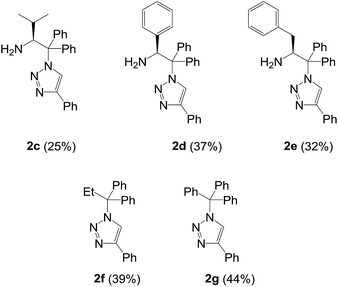
We next turned our attention to other substrates as shown in Table 2, which used previously in the CuAAC affording the exclusive formation of 1,4-triazoles. The RuAAC reactions of 2,2-diaryl-2-azidoamines derived from valine, phenylglycine, and phenylalanine reconfirmed the exclusive formation of 1,4-regioisomers as revealed by 1H and 13C NMR analysis (2c–2e). On the other hand, interestingly, internal alkynes such as 1-phenyl-1-propyne11 and 3-phenyl-2-propyn-1-ol4b with 1a were totally inactive in the RuAAC. Over again, the azides lacking the amino group also produced the single regioisomer (2f and 2g). Accordingly, we could rule out the possible coordination of the amine moiety with metal centre which may alter the catalytic activity.
| a The reaction conditions are as follows: azide (1 mmol), phenylacetylene (2.5 equiv.), Cp*RuCl(PPh3)2 (1 mol%), DMF, 150 °C, 20 h. |
|---|
 |
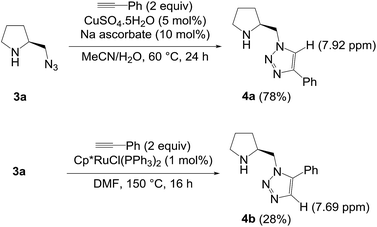
To understand influence of the steric demand in the regioselectivity, 2-(azidomethyl)pyrrolidine 3a was chosen and subjected to the cycloadditions under the copper and ruthenium catalysts, respectively, as shown in Scheme 3. Regioselective formation of isomeric triazoles could be viable, as the CuAAC of 3a produced 1,4-regioisomer 4a6d and the corresponding 1,5-isomer 4b was obtained by the RuAAC under the catalyst Cp*RuCl(PPh3)2. Two regioisomers are distinguished by the comparison to NMR spectroscopy. The contrasting results from 1a and 3a reveal the substantial influence on the regioselectivity of the RuAAC, and thus suggest that the preference could be overridden by steric demands imposed by bulky groups on the azide partners.
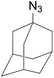
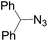
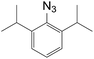
Subsequently, we selected the substrates representing a structural intermediacy in bulkiness with the intention of deciphering the relationship between steric factors and selectivity by the determination of product population. Thus, three different types of 1,3-dipolar cycloaddition were undertaken separately (Table 3). All CuAAC reactions of sterically hindered azides solely afforded 1,4-triazoles in good yields. In contrary, when benzhydryl azide and 2,6-diisopropyl-1-azidobenzene were treated with the ruthenium catalyst, arguably, 1,4-regioisomers appeared in both cases. For the same substrates, the thermal reactions gave the regioisomeric mixtures at the ratio of 1/1, but only in very low yield. In particular to adamantyl azide, the RuAAC solely gave 1,5-regioisomers as the azide group can be freely exposed to the metal centre presumably due to the configurationally constrained tricyclic backbone. It is now obvious that steric effect plays an important role in the regioselectivity in the RuAAC.12
| Azide | Triazole (yield, ratio) | ||
|---|---|---|---|
| By CuAACa | By RuAACb | By heatingc,d | |
| a Reaction conditions: azide (1 mmol), phenylacetylene (1.2 equiv.), CuSO4·5H2O (10 mol%), Na ascorbate (20 mol%), MeCN/H2O = 1/1 (5 mL), rt, 16 h.b Azide (1 mmol), phenylacetylene (2.5 equiv.), Cp*RuCl(PPh3)2 (1 mol%), DMF (5 mL), 150 °C, 16 h.c Azide (1 mmol), phenylacetylene (2.5 equiv.), DMF (5 mL), 150 °C, 72 h.d Reaction not completed after 72 h.e Reaction not performed.f Isolated as mixtures and ratio based on NMR. | |||
 |
5A (82%) | 5B (52%) | —e |
 |
6A (77%) | 6A/6B (47%, 1/3)f | 6A/6B (10%, 1/1)f |
 |
7A (62%) | 7A/7B (54%, 1/3)f | 7A/7B (10%, 1/1)f |
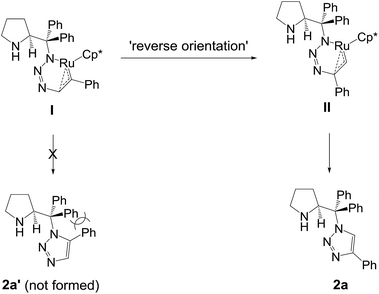
The RuAAC appears to proceed via oxidative coupling of the azide and the alkyne to give a ruthenacycle which exerts a complete control over regioselectivity. However, the formation of the ruthenacycle I might be hampered considerably by the stereo-demanding nature of steric bulkiness within such a highly strained intermediacy and this may explain the observed 1,5-regioisomer. Furthermore, an attempted product 2a′ would be extremely destabilised by the allylic strain exerting between two substituents, as illustrated in Scheme 4. The mechanistic considerations for this unprecedented observation need much more studies, but we propose that spatial crowding can alter the metal binding mode and eventually induce a reverse orientation to the incoming alkyne, a CuAAC-like ruthenacycle such as II. Likewise, inertness to internal alkyne also supports this proposal.‡
In summary, we observed the unprecedented formation of 1,4-disubstituted-1,2,3-triazoles from the reaction of 2,2-diaryl-2-azidoamines and terminal alkynes in the presence of ruthenium catalysts bearing Cp* ligand. The reversal in regioselectivity is presumably due to the steric congestion arose from the geminal diaryl groups of the azide, as evidenced from the control experiment with 2-(azidomethyl)pyrrolidine revealing the usual selectivity with RuAAC. The contrasting results suggest that the steric demands imposed by the bulky groups of the azides could annul the preference and influence the regioselectivity of RuAAC substantially. We thus surmise that the formation of the ruthenacycle allowing the 1,5-regioselectivity might be hampered considerably by the stereo-demanding nature within such a highly strained intermediacy causing the reverse orientation of the alkyne leading to the 1,4-regioisomer. Furthermore, RuAAc of the bulky azides was inactive to internal alkynes and this suggests that the mechanism is more likely to resemble CuAAC pathway. In addition, the RuAAC reactions of azides with intermediate bulkiness gave the mixtures of 1,4- and 1,5-regioisomers providing the additional support to this hypothesis.
Acknowledgements
This work was supported by the Creative Challenge Project (KK1607-C03) of the Korea Research Institute of Chemical Technology, Taejon, Korea.Notes and references
- R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565 CrossRef.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef.
- (a) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS PubMed; (b) K. Majumdar and K. Ray, Synthesis, 2011, 3767 CrossRef CAS; (c) P. Wu and V. V. Fokin, Aldrichimica Acta, 2007, 40, 7 CAS; (d) J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC; (e) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- (a) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed; (b) B. C. Boren, S. Narayan, L. K. Rasmussen, L. Zhang, H. Zhao, Z. Lin, G. Jia and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 8923 CrossRef CAS PubMed; (c) L. K. Rasmussen, B. C. Boren and V. V. Fokin, Org. Lett., 2007, 9, 5337 CrossRef CAS PubMed; (d) J. Zhang, J. Kemmink, D. T. S. Rijkersa and R. M. J. Liskamp, Chem. Commun., 2013, 49, 4498 RSC; (e) M. Lamberti, G. C. Fortman, A. Poater, J. Broggi, A. M. Z. Slawin, L. Cavallo and S. P. Nolan, Organometallics, 2012, 31, 756 CrossRef CAS.
- (a) U. Pradere, V. Roy, T. R. McBrayer, R. F. Schinazi and L. A. Agrofoglio, Tetrahedron, 2008, 64, 9044 CrossRef CAS; (b) J. M. Kwak, J. S. Moon, S. Seo, J. I. Choi, V. Sampath, H.-Y. Lee and H. Y. Koh, Bull. Korean Chem. Soc., 2014, 35, 3675 CrossRef CAS; (c) H. Nulwala, K. Takizawa, A. Odukale, A. Khan, R. J. Thibault, B. R. Taft, B. H. Lipshutz and C. J. Hawker, Macromolecules, 2009, 42, 6068 CrossRef CAS.
- (a) K. R. Knudsen, C. E. T. Mitchell and S. V. Ley, Chem. Commun., 2006, 1, 66 RSC; (b) T. Ishii, S. Fujioka, Y. Sekiguchi and H. Kotsuki, J. Am. Chem. Soc., 2004, 126, 9558 CrossRef CAS PubMed; (c) S. Luo, X. Mi, L. Zhang, S. Liu, H. Xu and J.-P. Cheng, Angew. Chem., Int. Ed., 2006, 45, 3093 CrossRef CAS PubMed; (d) S. Luo, H. Xu, X. Mi, J. Li, X. Zheng and J.-P. Cheng, J. Org. Chem., 2006, 71, 9244 CrossRef CAS PubMed.
- (a) H. Roy, A. Pitchaiah, M. Kim, I. T. Hwang and K. I. Lee, RSC Adv., 2013, 3, 3526 RSC; (b) V. S. Sadu, H. Roy, A. Pitchaiah, I. T. Hwang and K. I. Lee, Bull. Korean Chem. Soc., 2014, 35, 1605 CrossRef CAS.
- (a) P. N. Liu, L. Zhang, C. Shi and G. Jia, J. Org. Chem., 2012, 77, 5844 CrossRef CAS PubMed; (b) P. N. Liu, J. Li, H. S. Su, K. D. Ju, L. Zhang, C. Shi, H. H. Y. Sung, I. D. Williams, V. V. Fokin, Z. Lin and G. Jia, Organometallics, 2012, 31, 4904 CrossRef CAS.
- Tp-containing Ru catalyst is also known to give 1,5-regioselectivity: see, T.-H. Wang, F.-L. Wu, G.-R. Chiang, S.-T. He and Y.-H. Lo, J. Organomet. Chem., 2014, 774, 57 CrossRef CAS.
- (a) A. Krasiński, V. V. Fokin and K. B. Sharpless, Org. Lett., 2004, 6, 1237 CrossRef PubMed; (b) S. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov and V. V. Fokin, Org. Lett., 2010, 12, 4217 CrossRef CAS PubMed.
- M. M. Majireck and S. M. Weinreb, J. Org. Chem., 2006, 71, 8680 CrossRef CAS PubMed.
- Selected papers for steric effects on reactivity and selectivity: see, (a) V. P. Ananikov, R. Szilagyi, K. Morokuma and D. G. Musaev, Organometallics, 2005, 24, 1938 CrossRef CAS; (b) S. Yoshida, A. Shiraiah, K. Kanno, T. Matsushita, K. Johmoto, H. Uekusa and T. Hosoya, Sci. Rep., 2011, 1, 82 Search PubMed; (c) L. Wang and J. Xiao, Org. Chem. Front., 2016, 3, 635 RSC; (d) D. W. Crandell, S. Mazumdar, P. A. Evans and M.-H. Baik, Chem. Sci., 2015, 6, 6896 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1503841 X-ray crystallographic data of 2b have been deposited in the Cambridge Crystallographic Data Centre database with accession number. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra25403a |
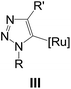
| ‡ Many attempts at intercepting a possible Ru-triazole intermediate such as III were unsuccessful; for the trapping of Cu-triazole intermediates: see, (a) W. Wang, F. Wei, Y. Ma, C.-H. Tung and Z. Xu, Org. Lett., 2016, 18, 4158; (b) Y.-M. Wu, J. Deng, Y. Li and Q.-Y. Chen, Synthesis, 2005, 1314; (c) X. Zhang, R. P. Hsung and H. Li, Chem. Commun., 2007, 2420. |
| This journal is © The Royal Society of Chemistry 2017 |