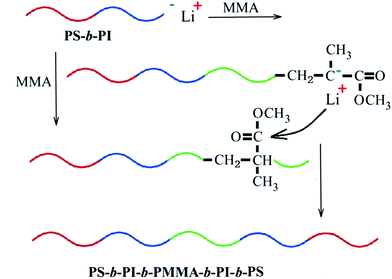
Open Access Article
Open Access ArticleA controlled synthesis method of polystyrene-b-polyisoprene-b-poly(methyl methacrylate) copolymer via anionic polymerization with trace amounts of THF having potential of a commercial scale
Zheng Li,
Jianding Chen,
Ling Su,
Bin Zou ,
Pengfei Zhan,
Yong Guan* and
Anna Zheng*
,
Pengfei Zhan,
Yong Guan* and
Anna Zheng*
Key Laboratory of Special Functional Polymeric Materials and Related Technology of the Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: yguan@ecust.edu.cn; zan@ecust.edu.cn
First published on 6th February 2017
Abstract
A controlled synthesis method for the polystyrene-b-polyisoprene-b-poly(methyl methacrylate) (PS-b-PI-b-PMMA) tri-block copolymer using the anionic copolymerization was innovated, in which being differ from previous researches for the anionic polymerization of polar monomers, nonpolar cyclohexane was used as solvent and only trace amounts tetrahydrofuran (THF) was adopted as a polar regulator. The sequential anionic copolymerization could be smoothly conducted at 0 °C and the conversion of all monomers reached 100%. The copolymer was characterized by gel permeation chromatography (GPC), proton nuclear magnetic resonance (1H-MNR), dynamic mechanical analysis (DMA) and differential scanning calorimetry (DSC). It was found that the number average molecular weights (Mn) of PS, PI and PMMA were 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760, 26
760, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 and 13
340 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 respectively. The 1,4-addition structure in PI block occupied 93% and its forming mechanism was explained. Furthermore, the produce of tri-block copolymers with PMMA block of different lengths confirmed this synthesis method controlled for definite structures, fewer side reactions and a appropriate temperature. Based on these results, the anionic block copolymerization containing polar monomer MMA at a commercial scale starts to become possible.
600 respectively. The 1,4-addition structure in PI block occupied 93% and its forming mechanism was explained. Furthermore, the produce of tri-block copolymers with PMMA block of different lengths confirmed this synthesis method controlled for definite structures, fewer side reactions and a appropriate temperature. Based on these results, the anionic block copolymerization containing polar monomer MMA at a commercial scale starts to become possible.
Introduction
Research on living anionic polymerization has never been interrupted since the polymerization mechanism was demonstrated by Scwarc in 1956,1–6 due to its remarkable advantages such as fast initiation and propagation, almost no termination, controllable molecular weight, narrow molecular weight distribution (MWD), and definite molecular structure.Decades of researches have realized the application of anionic polymerization in the industry, and products such as polystyrene-b-polyisoprene/polybutadiene-b-polystyrene (SIS/SBS) and the hydrogenation products of SBS and SIS (SEBS and SEPS) have been proposed subsequently.7–12 Y. Duan et al. synthesised a kind of tetrafunctional multigraft polystrene–polyisoprine copolymers by means of anionic polymerization and researched the morphology and deformation mechanism and tensile properties of the copolymers.13
However, to date, there is almost no one polar monomer, which could be used as the monomer in the anionic polymerization in industrial scale, also including the commonly used methyl methacrylate (MMA). Due to too more side reactions are likely to occur during the anionic polymerization of MMA.14
In order to overcome these side reactions, extremely low reaction temperature and addition of some inhibiting ligand have been adopted in recent years. Kim et al.15 used a plug flow reactor to synthesize the polystyrene and poly(methyl methacrylate) di-block copolymer (PS-b-PMMA) with high molecular weight in tetrahydrofuran (THF) at −78 °C. Lee et al.16 synthesized the polystyrene, polyisoprene and poly(methyl methacrylate) tri-block copolymer (PS-b-PI-b-PMMA) by anionic polymerization, in which the PS-b-PI di-block copolymer polymerized in toluene at room temperature at first. Then the PS-b-PI chains end-capped with DPE and the reactor was cooled down to −78 °C. After that, a certain amount of the solution of LiCl in THF was added in, for a while, the anionic polymerization of MMA was carried out. In this study, multimodal size exclusion chromatography (SEC) peak was observed, which might be caused by the inadvertent termination reaction of PS-b-PI living anions for the impurities secondly added in with THF solvent and MMA monomer. Unfortunately, in the synthesis method the extra-low temperature (−78 °C) was infaust to the industry realization. Similarly to the polymerization above, a well-defined novel block polymerization containing PMMA block was synthesized under the existence of potassium tert-butoxide (t-BuOK) by Shunsuke Tanaka et al.17 D. Baskaran researched the living anionic polymerization of MMA at −40 °C in THF and −78 °C in toluene/THF (9/1 v/v) using (1,1-diphenylhexyl) lithium as the initiator in the presence of lithium perchlorate (LiClO4) as the additive.18 However, the polymerization was conducted at −78 °C which could not be realized in industrialized conditions either.
Zheng et al.19,20 synthesized a compound named “P-Complex” which was used as an effective inhibitor to control the side reactions in the anionic polymerization of MMA and its copolymers at 0 °C. However, “P-Complex” cannot be dissolved in nonpolar solvent.
Group transfer polymerization (GTP)21,22 and metal-free anionic polymerization23,24 could synthesize PMMA under mild conditions, however, these methods cannot be applied in the industry because there exist some issues such as high cost, inherent termination reaction, incomplete initiation efficiency, etc.
As a fact that copolymers containing PMMA block have extensive potential applications.25–27 PS-b-PB-b-PMMA, for example, can be used as an excellent compatibilizer of PVC and SBS.28 Furthermore, a thermoplastic elastomer PS-b-PI-b-PMMA may be a new and excellent transparent toughening agent for PMMA, a widely used material only with brittleness weakness.
A series of thermoplastic elastomers consisted of unpolar monomers has been synthesized by living anionic polymerization in hydrocarbon solvent at room temperature by Wang et al.29 As mentioned above, the impurities in the polar solvents or monomers may a main factor to affect the side reactions during the anionic polymerization of polar monomers.
For example, there existed some effects of THF on the anionic polymerization initiated by organic lithium initiator, in which the reaction of THF with n-BuLi could generate and form the lithium enolate of acetaldehyde (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOLi).30,31 Lee et al. supported this idea, however, they adopted the s-BuLi as the initiator.32 It is regrettable in these researches that the reaction of THF with organic lithium is related to some impurities or not, such as water and alcohols. On the other hand, Chen20 and Su33 synthesized PMMA through the anionic polymerization with n-BuLi as the initiator at about room temperature with 100% conversion of MMA.
CHOLi).30,31 Lee et al. supported this idea, however, they adopted the s-BuLi as the initiator.32 It is regrettable in these researches that the reaction of THF with organic lithium is related to some impurities or not, such as water and alcohols. On the other hand, Chen20 and Su33 synthesized PMMA through the anionic polymerization with n-BuLi as the initiator at about room temperature with 100% conversion of MMA.
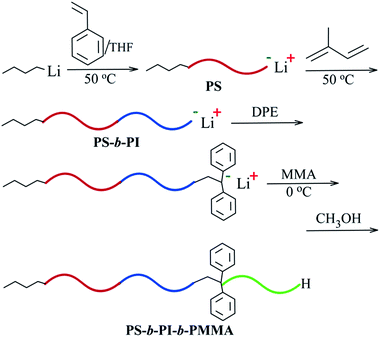
Therefore, in this work, a new method to synthesize a tri-block copolymer of PS-b-PI-b-PMMA was initiated, in which three monomers (St, Ip, MMA) were sequentially added in the polymerization system with n-BuLi in nonpolar cyclohexane (CH) solvent as the initiator. This is for decreasing the impurities accompanied by the polar solvents in the polymerization system by means of nonpolar CH. Furthermore, only trace amounts of THF was adopted as the polar regulator. By doing this, not only the polymerization rate could be greatly increased, but also the conformation of polyisoprene (PI) was unlikely changed.
Experimental
Materials
Styrene (St, Sinopharm Chemical Reagent Co., Ltd., China) and methyl methacrylate (MMA, Lingfeng Chemical Reagent Co., LTD., China) were purified by distillation in a vacuum system at 50 °C and 20 °C after stirring with calcium hydride for 48 h, and soaked with 4 Å molecular sieves for more than 24 h before use. Isoprene (Ip) was dried with calcium hydride for 48 h and distilled under normal atmosphere at 40 °C, and soaked with 4 Å molecular sieves as well before use. Cyclohexane (CH, Sinopharm Chemical Reagent Co., Ltd., China) used as solvent was refluxed with sodium at 81 °C for 24 h, distilled and soaked with 4 Å molecular sieves for more than 24 h. Tetrahydrofuran (THF, Sinopharm Chemical Reagent Co., Ltd., China) used as solvent was refluxed with sodium at 66 °C for 24 h, distilled and soaked with sodium. n-Butyl-lithium (n-BuLi, 2.4 mol L−1, in n-hexane, J&K Chemical Co., Ltd, Shanghai, China) was used as received. Argon (Ar, 99.99999%) was purified by flowing through two connected towers filled with 4 Å molecular sieves. 1,1-Diphenylethylene (DPE) (98%, TCI, Japan) was used as received.Characterization methods
The molecular weight and its distribution (Mw/Mn) was determined by multi-detector gel permeation chromatography (Water 1515 system; Waters corporation, America), equipped with an 18 angles laser scattering detector (LS signal) and a refractive detector (RI signal), using THF as the eluent at a flow rate of 1.0 mL min−1 at 25 °C.1H-NMR spectra were measured by a BRUKER AV400 spectrometer with CDCl3 as solvent and tetramethylsilane (TMS) as the internal reference.
Dynamic mechanical spectrometer (Q800, TA Co., USA) was used to study the dynamic mechanical properties of the copolymer. The samples were made into rectangular strips with size of 20 × 5 × 2 mm, tested with single cantilever bending mode and heated from −100 to 125 °C, at heating rate of 5 °C min−1 and frequency of 1 Hz in a nitrogen atmosphere.
The glass transition temperature of tri-block copolymers was also performed to on a differential scanning calorimetry (DSC)-SP thermal analyzer (Netzsch STA 409, Germany). The sample was heated from −80 °C to 125 °C at a heating rate of 10 °C min−1 under a steady flow of a nitrogen atmosphere.
Synthesis of PI
All the polymerization and reactions were carried out under Ar atmosphere and all the flasks were inflated by Ar after baked. The anionic homo-polymerization of Ip (20 mL) was performed in a three-necked flask at 50 °C with n-BuLi (2.4 mmol) as an initiator and CH (150 mL) as a solvent. Different amounts of THF (0, 0.48, 0.24, 0.096, 0.048 mmol, respectively) was added as the polar regulator before reaction. Along with the polymerization proceeding, a small quantity of polymeric sample was drawn out by an injector at the reaction time of 5, 10, 15, 20, 30 and 40 minutes, respectively, and terminated by methanol immediately. At last, all the polymers were obtained by drying in the vacuum oven at 50 °C for 12 h.Synthesis of PS-b-PI-b-PMMA
The reaction was conducted in a 500 mL reaction kettle, whose gas tight test and removing impurities should be done before the polymerization as commonly introduced in the anionic polymerization. The purified CH solvent (400 mL) and n-BuLi (5 mL) were injected into the kettle and kept stirring for 2 hours in order to eliminate the impurities further. Afterward, the solution in the kettle was drawn out. Then, another purified CH (400 mL) and the trace amounts of THF (nTHF/nn-BuLi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were injected in the kettle and stirred. The polymerization of St (15 mL) in this kettle was initiated by n-BuLi in CH (0.5 mL) mentioned above at 50 °C. After 30 minutes, Ip (40 mL) was added in and the polymerization was carried out for another 1 hour, then DPE (0.5 mL) was added subsequently. MMA (15 mL) was injected in after the solution was cooled down to 0 °C. The polymerization of MMA was carried out for 45 minutes and terminated by methanol (Scheme 1). The final polymer was precipitated out with methanol and dried in vacuum oven at 50 °C for 24 h. A series of experiments were conducted in the same steps with injecting different amounts of monomers.
10) were injected in the kettle and stirred. The polymerization of St (15 mL) in this kettle was initiated by n-BuLi in CH (0.5 mL) mentioned above at 50 °C. After 30 minutes, Ip (40 mL) was added in and the polymerization was carried out for another 1 hour, then DPE (0.5 mL) was added subsequently. MMA (15 mL) was injected in after the solution was cooled down to 0 °C. The polymerization of MMA was carried out for 45 minutes and terminated by methanol (Scheme 1). The final polymer was precipitated out with methanol and dried in vacuum oven at 50 °C for 24 h. A series of experiments were conducted in the same steps with injecting different amounts of monomers.
Results and discussion
The effect of THF in the anionic polymerization of PI
THF is a common polar regulator in anionic polymerization, which can be utilized to accelerate the reaction process. Its effect on the reaction rate in the polymerization of Ip is shown in Fig. 1, where the r means the molar ratio of THF/n-BuLi. It was revealed that comparing with the polymerization without THF, even quite a little THF could bring remarkable acceleration in the polymerization of Ip and it became more remarkable after 10 minutes. At 50 °C, the polymerization conversion of PI without THF was around 8%, in contrast, a high conversion of 88% was observed in the polymerization with 0.48 mmol THF (nTHF/nn-BuLi = 1/5) at 10 min. Furthermore, the complete conversion time was shortened from more than 40 minutes (r = 0) to 20 minutes (r = 1/5).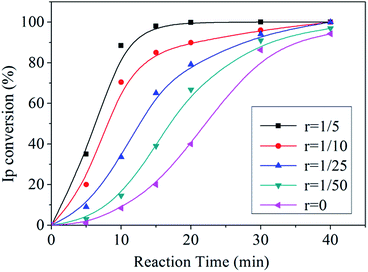 | ||
| Fig. 1 Time-conversion curves of the polymerization of PI with different molar ratios of THF/n-BuLi (r) at 50 °C. | ||
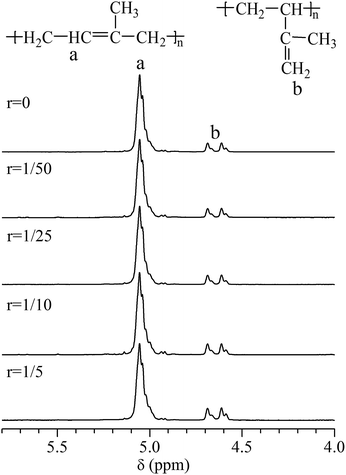
THF as the polar regulator has another effect on the microstructure of conjugated diolefine formed during anionic polymerization. For example, the content of 1,4-addition structure will be decreased following the increasing of THF during the anionic polymerization of PI. The 1H-NMR spectra of the polymers produced in the anionic polymerization adding THF were shown in Fig. 2.
There are three types of microstructure in PI: 1,4-, 1,2- and 3,4-addition microstructure, respectively. The peak (a) at δ 4.95–5.14 indicates the existence of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in 1,4-addition microstructure and the peak (b) at δ 4.56–4.71 corresponds to the protons of –C
C– in 1,4-addition microstructure and the peak (b) at δ 4.56–4.71 corresponds to the protons of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 in 3,4-addition microstructure. Based on the 1H-NMR spectra, the compositions of PI were concluded in Table 1 through calculating the integral area ratio of relevant characteristic peaks. It was confirmed that with increasing of added THF, there was a growing trend in the content percentage of 3,4-addition structure. The percentage of 1,4-addition structure, in contrast, reduced slightly and there was a mutation between 1/10 and 1/5 of “r”. Furthermore, there was no 1,2-addition structure all the way.
CH2 in 3,4-addition microstructure. Based on the 1H-NMR spectra, the compositions of PI were concluded in Table 1 through calculating the integral area ratio of relevant characteristic peaks. It was confirmed that with increasing of added THF, there was a growing trend in the content percentage of 3,4-addition structure. The percentage of 1,4-addition structure, in contrast, reduced slightly and there was a mutation between 1/10 and 1/5 of “r”. Furthermore, there was no 1,2-addition structure all the way.
The formation of 1,2-addition structure, as stated by Cheng's work,34 might be unable to occur until the value of “r” reached to 25.
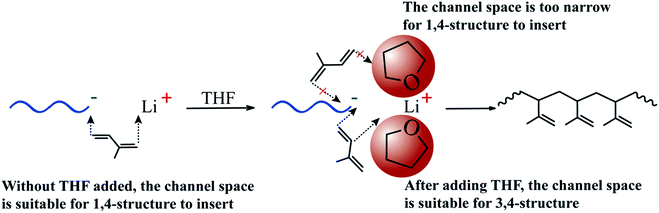
Our laboratory's previous studies35 proposed a new explanation about the formation mechanism of different microstructures in PI, called “Ion Pair Channel Idea”. It stated that when n-BuLi is used as the initiator, the polymerization rate and molecular structure of the products are determined by the channel space between the ion pairs. Naturally, when the channel space is smaller than the requirement for the monomers to insert in, the polymerization cannot occur. However, in contrast, when the channel space is much larger than that for the monomers to insert in, the polymerization also is not easy to occur, because the too large channel space between the ion pairs is unfavorable for the electric charge on the inserted monomers to migrate. Therefore, when the channel space is compatible with the requirement for the certain conformation of monomers to insert in, the polymerization rate of the configuration formed from this conformation would be maximum. The THF molecules could associate with Li+ due to electronegative oxygen atom, as a result, the channel between the ion pairs was blocked partly (see Fig. 3). For this reason, the increase of 3,4-structure and the decrease of 1,4-structure are very natural. Li et al. primely explained the reason of formed microstructure of PI in the anionic polymerization process with the “Ion Pair Channel Idea”.35 However, they didn't adopt THF but the lithium phenolate.
In order to make PS-b-PI-b-PMMA become a kind of ideal thermoplastic elastomer, high content of 1,4-addition PI is expected. Meanwhile, for shorting the reaction time of first two blocks, trace amounts of THF could be added with little effect to the structure of PI blocks. Therefore, adding trace amounts of THF (nTHF/nn-BuLi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) into polymerization system was appropriate.
10) into polymerization system was appropriate.
Polymerization and characterizations of PS-b-PI-b-PMMA
PMMA is a kind of polar polymer which is insoluble in nonpolar solvent, therefore, the polymerization of PMMA in CH is a precipitation polymerization. That is why polar solvent or nonpolar/polar mixed solvent was used in almost every previous study of anionic polymerization of copolymer contain PMMA block. However, the polar solvents may contain more impurities which is unfavourable to anionic polymerization, thus the side reactions caused by the carbonyl in PMMA are more likely to occur in polar solvent.In our work, the anionic polymerization of MMA was conducted in a nonpolar solvent CH at 0 °C. The usage of nonpolar solvent is different from others' studies. Therefore, the mild conditions enable the polymerization to carry out in the industry conditions. Because of the good solubility of long chains of PS-b-PI in CH, the chains of PS-b-PI-b-PMMA formed after addition of MMA can be almost completely dissolved as well. Furthermore, the electromagnetic stirring made the MMA monomers homogeneously disperse in CH and the narrow MWD copolymer was obtained. Fortunately, because CH was utilized instead of any other polar solvent, the side reactions of MMA could be controlled even the reaction temperature was elevated to 0 °C. On the other hand, the trace amounts of THF helped to accelerate the polymerization.
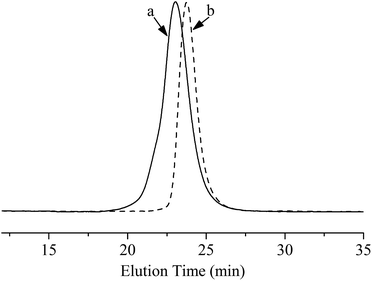
The results of polymerization of PS-b-PI-b-PMMA were summarized in Table 2. The MWD of the final tri-block copolymer extended to 1.21 was slightly higher than that of the PS-b-PI copolymer before MMA added (1.03). However, considering that the polymerization does not need any ligand for stable and the reaction temperature could be raised up to 0 °C, the MWD of PS-b-PI-b-PMMA was rather narrow. The Mn of copolymer from GPC was rather close to the calculated ones and a special note is that the yield reached up to 100%, which could not be achieved in many previous researches in the area of anionic polymerization of MMA at the 0 °C. Furthermore, it was clearly indicated from Table 2 that the Mn of PMMA block was about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600. On the other hand, the reason why the Mn of final copolymer was a little higher than the calculated, one was that there still existed the trace impurities derived from the solvent and the after added monomers, which could kill the reactive species in the system, even though all reaction agents were purified strictly. The initiation efficiency of 86.9% can be figured out. According to the GPC traces in Fig. 4, the MWDs of both PS-b-PI-b-PMMA and its precursor PS-b-PI were in the good narrow distribution. The results indicated that the side reactions of MMA were well inhibited and a homogeneous and ideal PS-b-PI-b-PMMA was obtained.
600. On the other hand, the reason why the Mn of final copolymer was a little higher than the calculated, one was that there still existed the trace impurities derived from the solvent and the after added monomers, which could kill the reactive species in the system, even though all reaction agents were purified strictly. The initiation efficiency of 86.9% can be figured out. According to the GPC traces in Fig. 4, the MWDs of both PS-b-PI-b-PMMA and its precursor PS-b-PI were in the good narrow distribution. The results indicated that the side reactions of MMA were well inhibited and a homogeneous and ideal PS-b-PI-b-PMMA was obtained.
| Sample | Mn/×10−4 | ||||
|---|---|---|---|---|---|
| Obsedb | Calcd | MWDb | Yield | Effc (%) | |
a Synthesized by anionic polymerization with trace amount of THF (nTHF/nn-BuLi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10).b Determined by GPC in THF elusion.c Initiator efficiency (I*) = Mn(calcd)/Mn(obsed), where Mn(calcd) = MW(monomer) × [monomer]/[initiator] × conversion% + MW of chain end groups. 10).b Determined by GPC in THF elusion.c Initiator efficiency (I*) = Mn(calcd)/Mn(obsed), where Mn(calcd) = MW(monomer) × [monomer]/[initiator] × conversion% + MW of chain end groups. |
|||||
| PS-b-PI | 3.91 | 3.40 | 1.03 | ∼100% | 87.0 |
| PS-b-PI-b-PMMA | 5.27 | 4.58 | 1.21 | ∼100% | 86.9 |
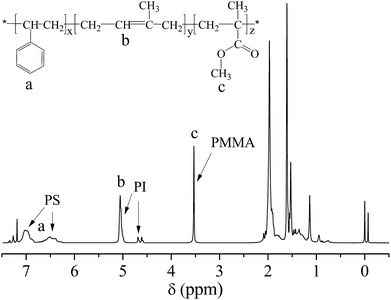
1H-NMR of PS-b-PI-b-PMMA was revealed in Fig. 5, in which there were a broad signal at δ 6.3–7.1 (a) corresponding to the aromatic protons (C6H5–), some peaks at δ 5.13 and δ 4.6–4.8 (b) assigned to the olefinic protons of 1,4-addition (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and 3,4-addition (–C
C–) and 3,4-addition (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) of PI, and a signal at δ 3.6 (c) attributing to the protons of the methyl connected with the ester group in PMMA (–O–CH3). Calculating the ratio of the integral areas of these characteristic peaks, we figured out the mole ratio of St, Ip and MMA (nSt
CH2) of PI, and a signal at δ 3.6 (c) attributing to the protons of the methyl connected with the ester group in PMMA (–O–CH3). Calculating the ratio of the integral areas of these characteristic peaks, we figured out the mole ratio of St, Ip and MMA (nSt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nIp
nIp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nMMA) is 19
nMMA) is 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21, which is very approximate to the theoretical value (19.5
21, which is very approximate to the theoretical value (19.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59.5
59.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21). The result confirmed almost no side reaction existed during the process of anionic polymerization of MMA and the conversion of St, Ip and MMA monomers were complete. Based on nSt
21). The result confirmed almost no side reaction existed during the process of anionic polymerization of MMA and the conversion of St, Ip and MMA monomers were complete. Based on nSt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nIp = 19
nIp = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, the Mn of PS block is about 12
60, the Mn of PS block is about 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 while Mn of PI block is about 26
760 while Mn of PI block is about 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340. Obviously, the Mn of PMMA block (13
340. Obviously, the Mn of PMMA block (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) is larger than that of PS block.
600) is larger than that of PS block.
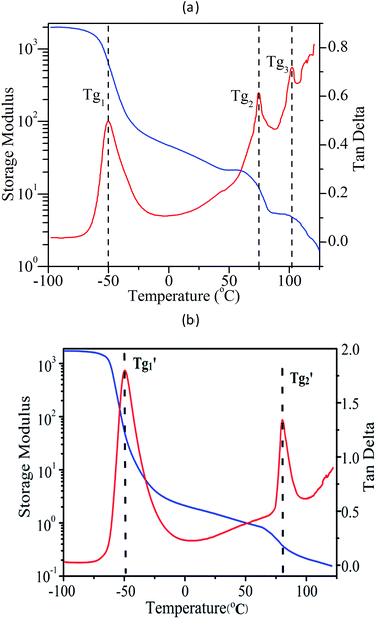
Fig. 6(a) depicted the dynamic mechanical behaviour of the synthesized PS-b-PI-b-PMMA tri-block copolymer. The storage modulus and loss tangent clearly indicated three glass transitions. Of course, Tg1 (−50 °C) was referred to the glass transition temperature of PI block. Furthermore, because the Tg of PS equals approximately to that of PMMA and is close to Tg3, Tg (103 °C) may belong either to PS block or to PMMA block. However, the glass transition temperature of polymers follows the eqn (1):
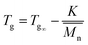 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) is larger than that of PS block (12
600) is larger than that of PS block (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760), so that Tg2 (75 °C) should belong to PS block and Tg3 (103 °C) to the PMMA block. To discriminate the two different Tgs further, the di-block copolymers PS-b-PI was synthesized in exactly the same conditions as the PS-b-PI-b-PMMA mentioned above before adding of MMA monomer. Its DMA results were shown in Fig. 6(b), two glass transition temperatures (T′g1 = −49.3 °C; T′g2 = 81.2 °C) were clear indicated. By comparison of the DMA results of PS-b-PI-b-PMMA and PS-b-PI, it is obvious that the Tg2 belonged to PS block and Tg3 was the glass transition temperature of PMMA block.
760), so that Tg2 (75 °C) should belong to PS block and Tg3 (103 °C) to the PMMA block. To discriminate the two different Tgs further, the di-block copolymers PS-b-PI was synthesized in exactly the same conditions as the PS-b-PI-b-PMMA mentioned above before adding of MMA monomer. Its DMA results were shown in Fig. 6(b), two glass transition temperatures (T′g1 = −49.3 °C; T′g2 = 81.2 °C) were clear indicated. By comparison of the DMA results of PS-b-PI-b-PMMA and PS-b-PI, it is obvious that the Tg2 belonged to PS block and Tg3 was the glass transition temperature of PMMA block.
These results not only clearly demonstrated the micro-phase separation existing in PS-b-PI-b-PMMA, but also suggested that the molecular weight of PMMA block was large enough to surpass the length of segment and to possess the properties of PMMA.
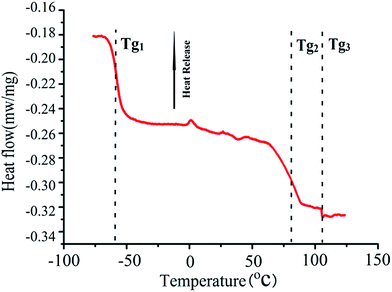
The thermodynamics properties of PS-b-PI-b-PMMA tri-block copolymers was also studied by DSC showed in Fig. 7. Heat flow was legible and negative value during the rise processing of temperatures, which means the change being heat-absorbing. Since DSC measurements showed three glass transition temperatures (Tg1 = −57.7 °C; Tg2 = 80.2 °C; Tg3 = 105.4 °C) which were corresponded to the results of DMA, it suggested the presences of different phases in tri-block copolymers. Combined with the DMA measurements, we were clear that the length of PMMA block has ensured the PMMA properties to exist in copolymers according to the appearance of Tg3 (105.4 °C) which belonged to PMMA block.
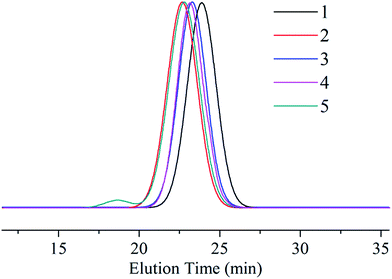
To confirm the synthesis method are controlled, we varied the last PMMA block lengths and a series of tri-block copolymers were shown in Table 3. The MWDs were stable at 1.2–1.3 and yields nearly hit 100% until the contents of PMMA block came to 30%. Meanwhile, the MWDs increased a little along with the ratio of PMMA however the ratios of three blocks calculated by 1H-NMR spectra were still corresponded to the designed ones. The broaden of MWDs may be caused by two aspects: the side reactions of MMA and the raise of temperature which were both attributed to the increase of MMA monomers. We compared the GPC traces of 5 samples (Fig. 8) to analyse the effect of MMA's amount in anionic polymerization and found out that those two tri-block copolymers were all in the good narrow distribution. However, a small peak found in the left of the spectra of sample 5 showed that the high molecular weight polymer was product in the tri-block copolymerization.
| Sample | Ratio (wt%) | Conversion of MMA (%) | MWDb | Mn/×10−4b | WtSt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wtIp wtIp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wtMMA wtMMA |
|
|---|---|---|---|---|---|---|
| Calcd | Obsedc | |||||
a Synthesized in the same way with trace amounts of THF (nTHF/nn-BuLi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10).b Determined by GPC in THF elusion.c Calculated by 1H-NMR spectra. 10).b Determined by GPC in THF elusion.c Calculated by 1H-NMR spectra. |
||||||
| 1 | 16 | ∼100 | 1.22 | 4.11 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 2 | 20 | ∼100 | 1.21 | 6.32 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
19.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.5 20.5 |
| 3 | 25.5 | ∼100 | 1.21 | 5.27 | 25.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.5 25.5 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.5 49.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.5 25.5 |
| 4 | 28 | ∼100 | 1.26 | 5.85 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.5 45.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.5 27.5 |
| 5 | 30 | ∼100 | 1.28 | 6.23 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.5 41.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.5 29.5 |
Speculated on the mechanism of side reactions, the formation of that polymer which we inferred was shown in Fig. 9. Indeed, this part polymer was too rare to affect the properties of tri-block copolymers and that was why we would ignore it.
Conclusions
In summary, the anionic polymerization of well-defined PS-b-PI-b-PMMA tri-block copolymer was conducted in CH solvent with trace amounts of THF as a polar regulator, and the reaction temperature of MMA was elevated to 0 °C. The measurement results demonstrated that a homogeneous and ideal PS-b-PI-b-PMMA tri-block copolymer was obtained. Specifically, GPC data and 1H-MNR spectra revealed that the MWD of the final copolymer was rather narrow and Mn of PS, PI and PMMA were 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760, 26
760, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 and 13
340 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 respectively, which was approximate to the calculated values. After adding trace amounts of THF, the reaction rate was remarkably accelerated and meanwhile high content of 1,4-addition structure of PI was guaranteed, herein, the “Ion Pair Channel Idea” can be used to explain the microstructure forming mechanism of PI. Furthermore, determined by DMA and DSC, the micro-phase separation clearly existed in synthesized PS-b-PI-b-PMMA and it was figured out that the Mn of PMMA block was large enough to possess the properties of PMMA. Varying the lengths of last PMMA blocks, we confirmed that until the content of PMMA block raised up to 30%, the structure of tri-block copolymers was well-defined. Accordingly, this new method with appropriate temperature rather than −78 °C, a faster reaction rate, fewer side reactions and a definite structure provides the possibility to realize the anionic polymerization of copolymers containing polar PMMA block at a commercial scale.
600 respectively, which was approximate to the calculated values. After adding trace amounts of THF, the reaction rate was remarkably accelerated and meanwhile high content of 1,4-addition structure of PI was guaranteed, herein, the “Ion Pair Channel Idea” can be used to explain the microstructure forming mechanism of PI. Furthermore, determined by DMA and DSC, the micro-phase separation clearly existed in synthesized PS-b-PI-b-PMMA and it was figured out that the Mn of PMMA block was large enough to possess the properties of PMMA. Varying the lengths of last PMMA blocks, we confirmed that until the content of PMMA block raised up to 30%, the structure of tri-block copolymers was well-defined. Accordingly, this new method with appropriate temperature rather than −78 °C, a faster reaction rate, fewer side reactions and a definite structure provides the possibility to realize the anionic polymerization of copolymers containing polar PMMA block at a commercial scale.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (50933002, 51373052).References
- D. Baskaran and A. H. E. Müller, Prog. Polym. Sci., 2007, 2, 173–219 CrossRef.
- Y. Ren, Q. Gao, C. Zhou, Z. Wei, Y. Zhang and Y. Li, RSC Adv., 2015, 35, 27421–27430 RSC.
- A. Mavroudis and N. Hadjichristidis, Macromolecules, 2006, 39(2), 535–540 CrossRef CAS.
- S. Ito, R. Goseki, I. Manners, T. Ishizone and A. Hirao, Macromol. Chem. Phys., 2015, 14, 1523–1533 CrossRef.
- H. F. Fei, W. Xie, Q. Wang, X. Gao, T. Hu, Z. Zhang and Z. Xie, RSC Adv., 2014, 99, 56279–56287 RSC.
- Y. Matsuo, R. Konno, T. Ishizone, R. Goseki and A. Hirao, Polymers, 2013, 3, 1012–1040 CrossRef.
- Y. Deng, Chem. Ind. Eng. Prog., 2005, 2, 212–215 Search PubMed.
- K. Knoll and N. Niessner, Macromol. Symp., 1998, 132, 231–243 CrossRef CAS.
- J. Wang, D. Shan, W. Liu and A. Zheng, Polym. Mater. Sci. Eng., 2015, 10, 99–104 Search PubMed.
- X. Yuan, J. Wang, D. Shan and A. Zheng, Polym. Mater. Sci. Eng., 2014, 8, 1–6 Search PubMed.
- D. Shan, L. Yang, J. Wang and A. Zheng, Chemical Journal of Chinese Universities, 2014, 12, 2698–2705 Search PubMed.
- J. Wang, B. Chen, S. Tang and A. Zheng, Chin. J. Polym. Sci., 2015, 8, 1096–1103 CrossRef.
- Y. Duan, M. Thunga, R. Schlegel, K. Schneider, E. Rettler, R. Weidisch, H. W. Siesler, M. Stamm, J. W. Mays and N. Hadjichristidis, Macromolecules, 2009, 42(12), 4155–4164 CrossRef CAS.
- D. Baskaran, Prog. Polym. Sci., 2003, 28, 521–581 CrossRef CAS.
- J. S. Kim, J. O. Kweon, J. H. Lee and S. T. Noh, Macromol. Res., 2015, 1, 100–110 CrossRef.
- S. Y. Lee and T. Y. Chang, Eur. Polym. J., 2011, 47, 800–804 CrossRef CAS.
- S. Tanaka, R. Goseki, T. Ishizone and A. Hirao, Macromolecules, 2014, 47, 2333–2339 CrossRef CAS.
- D. Baskaran and S. Sivaram, Macromolecules, 1997, 30, 1550–1555 CrossRef CAS.
- A. Zheng, J. Zhang, Y. Guan, F. Hu, D. Wei and S. Wang, 8546503 B2, USA, 2013.
- B. Chen, J. Wang, M. Shu, B. Zou and A. Zheng, Chinese Journal of Chemistry, 2014, 32, 1128–1134 CrossRef CAS.
- R. Zhuang and A. H. E. Müller, Macromolecules, 1995, 28, 8035–8042 CrossRef CAS.
- O. W. Webster, W. R. Hertler, D. Y. Sogah, W. B. Farnham and T. V. RajanBabu, J. Am. Chem. Soc., 1983, 105, 5706–5708 CrossRef CAS.
- F. Bandermann, D. Beckelmann, D. Broska, A. Fieberg, T. Roloff and D. Wolters, Macromol. Chem. Phys., 1995, 196, 2335–2347 CrossRef CAS.
- M. T. Reetz and R. Ostarek, J. Chem. Soc., Chem. Commun., 1988, 213–215 RSC.
- L. Yi, X. Zhan, F. Chen, B. Jiang and B. Chen, Chemical Journal of Chinese Universities, 2007, 28(12), 2393–2397 CAS.
- H. Ruckdäschel, J. K. W. Sandler, V. Altstädt, C. Rettig, H. Schmalz and V. Abetz, Polymer, 2006, 47, 2772–2790 CrossRef.
- C. Auschra and R. Stadler, Macromolecules, 1993, 26, 6364–6377 CrossRef CAS.
- X. Lv, G. Jin, Q. Liu and X. Yang, J. Beijing Univ. Chem. Technol., 2000, 27(3), 29–32 Search PubMed.
- W. Wang, R. Schlegel, B. T. White and K. Williams, Macromolecules, 2016, 49(7), 2646–2655 CrossRef CAS.
- M. Schlosser, Organometallics in Organic Synthesis, Wiley, New York, 1994, p. 172 Search PubMed.
- H. Iatrou, J. W. Mays and N. Hadjichristidis, Macromolecules, 1998, 31, 6697–6701 CrossRef CAS.
- Y. Lee, C. Chang and C. Kao, Langmuir, 2010, 26(6), 4196–4206 CrossRef CAS PubMed.
- M. Su, B. Chen, B. Zou, F. Chen, A. Zheng and Y. Guan, Acta Polym. Sin., 2015, 7, 835–844 Search PubMed.
- J. Cheng, C. He and G. Jin, China Elastomerics, 1998, 2, 12–16 Search PubMed.
- T. Li, B. Chen, S. Wu, Y. Guan and A. Zheng, Acta Polym. Sin., 2014, 9, 1204–1211 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |