Open Access Article
Open Access ArticleCopper salt-catalyzed formation of a novel series of triazole–spirodienone conjugates with potent anticancer activity†
Linghui Gua,
Peng Wanga,
Qiu Zhongbc,
Yuxing Denga,
Jiangping Xiea,
Fei Liua,
Fan Xiaod,
Shilong Zhengbc,
Yue Chene,
Guangdi Wang*bc and
Ling He*a
aKey Laboratory of Drug-Targeting and Drug-Delivery Systems of the Ministry of Education, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China
bRCMI Cancer Research Center, Xavier University of Louisiana, New Orleans, LA 70125, USA
cDepartment of Chemistry, Xavier University of Louisiana, New Orleans, LA 70125, USA
dJiangxi Provincial People's Hospital, Nanchang, 330006, China
eDepartment of Nuclear Medicine Affiliated Hospital, Luzhou Medical College, No. 25 Taiping Street, Luzhou, 646000, P. R. China
First published on 30th January 2017
Abstract
Copper salt-catalyzed oxidative amination resulted in the formation of a novel series of triazole–spirodienone conjugates, 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-3,8-diones and 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-8-ones. A single crystal of compound 1p among them was grown and analyzed by X-ray crystallography. These compounds were evaluated for their antiproliferative activities against MDA-MB-231, HeLa, A549 and MCF-7 cell lines. Most of them showed moderate to high anticancer potency in the four cancer cell lines. The discovery of the triazole–spirodienone conjugates as cytotoxic agents against cancer cells may open up a new field in which these novel small molecules could be further explored as promising anticancer agents.
Introduction
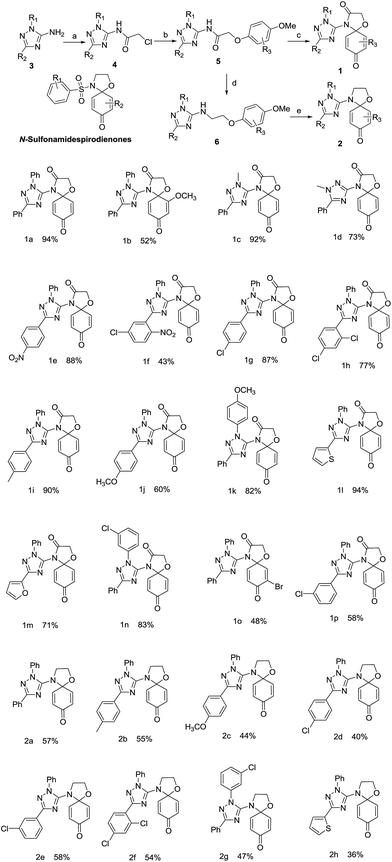
More than one hundred drugs have been approved by the United States Food and Drug Administration (USFDA) for clinical treatment of cancers over the last half century, yet the search for new chemical entities as potential anticancer agents continues in the hope that improved pharmaceutical profiles may come from hitherto unknown molecular structures that encode the keys to greater efficacy and more manageable adverse side effects. Among numerous drug discovery approaches such as high-throughout screening, molecular modeling, and natural compound mimetics, one method may prove effective that takes pharmacophores important in existing anticancer agents on which to rationally design modified structures that may confer a better therapeutic index.Quinone is a common moiety of numerous biologically active molecules, including natural and synthetic anticancer drugs such as doxorubicin,1 mitoxantrone,2 andaunorubicin3 and mitomycin C.4 The role of the quinone moiety appears to be important in endowing bioactivities in the molecules ranging from simple small quinones to large complex quinoid systems.5–9 Thus the utility of quinone-containing structures has been extensively explored in search of anticancer drug candidates, leading to an ever increasing library of new promising quinones with anticancer activities.10–16 We have previously obtained some quinone derivatives and conjugates as anticancer agents.17,18 In another previous work on quinones,19 we found that N-sulfonamide spirodienone derivatives (Fig. 1) demonstrated moderate cytotoxic activities against HCT-8, Bel-7402, BGC-823, A549 and A2780 cell lines with IC50 values in the range of 1.93–51 μM.
The introduction of a 1,2,4-trizole20,21 ring into molecules has been reported to significantly increase anticancer activity or improve the bioavailability and overall pharmacological profile of a drug candidate.22–26 Indeed, clinically proven anticancer drugs27,28 such as letrozole, vorozole, and anastrozole all contain a 1,2,4-triazole nucleus which is stable to metabolism and acts as an important pharmacophore at the active site of receptors as hydrogen bond acceptor or donor. We hypothesize that conjugating quinones with a 1,2,4-triazole nucleus may generate novel molecular entities with desirable anticancer activities. Therefore, N-sulfonamide-spiro-dienones were chosen as the lead compound and triazole–spirodienone conjugates were designed by replacing the sulfonyl functional group with a 1,2,4 (the relative position of ring nitrogen)-triazole nucleus (Fig. 1). Herein, we report the synthesis and anticancer evaluation of the designed triazole–spirodienone conjugates: 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-3,8-diones (1) and 4-triazolyl-1-oxa-4-azaspiro[4,5]-deca-6,9-dien-8-ones (2).
Results and discussion
Chemical synthesis
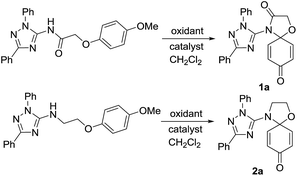
The target conjugates were prepared following the synthetic scheme as depicted in Fig. 1. The starting materials 1,2,4-triazoles (3) were prepared in an efficient one-pot procedure including the cyanoimidation of aldehydes and cyclization.29 Following acylation of 3 to form 1,3-substituted-2-chloro-N-(1H-1,2,4-trizol-5-yl)acetamides (4), the sodium phenoates attacked 4 to provide N-(1,3-disubstituent-1H-1,2,4-triazol-5-yl)-2-phenoxyacetamides (5). The reduction of 5 gave 1,3-disubstituent-N-(2-phenoxyethyl)-1H-1,2,4-triazol-5-amines (6). Subsequently, the key step is the oxidative amination reaction19,30,31 of 5 and 6 leading to their corresponding 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-3,8-dione (1) and 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-8-one (2) in moderate to high yields.The formations reaction of 1a and 2a were taken as an example for optimization of reaction conditions of the oxidative amination using the previous work as ref. 19 and 31 The results (Table 1) show that copper(II), rhodium(II) and ruthenium(II) could be used as a catalyst for the amination of aromatic ether 5 and 6. Other metal complexes, such as those of Mn(II), Co(II) and Fe(II) displayed poor catalytic activities. At same time, we found that the yields increased inconspicuously when the loading of [Cu(CH3CN)4]ClO4 and Cu(CF3SO2)2 increased from 5% to 20%. In addition, PhI(OAc)2 and PhI(CF3CO2)2 were employed effectively as oxidant for the oxidative amination of amides (5) and amines (6). Finally, the optimized conditions for the formation of triazole–spirodienones 1a and 2a are in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 of substrate
0.1 of substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhI(CF3CO2)2
PhI(CF3CO2)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu[(CH3CN)4]ClO4 for 1a or (Cu(CF3SO2)2) for 2a at room temperature. Next, the reaction conditions of 1a and 2a were expanded to the formation reactions of all triazole–spirodienones 1 and 2, respectively. Thus, twenty four triazole–spirodienone 1 and 2 were obtained in 36–94% yield (Fig. 1) from corresponding 2-phenoxy-ethyl-acet-N-2,3,5-triazoleamides and 2-phenoxy-ethyl-ethyl-N-2,3,5-triazoleamines.
Cu[(CH3CN)4]ClO4 for 1a or (Cu(CF3SO2)2) for 2a at room temperature. Next, the reaction conditions of 1a and 2a were expanded to the formation reactions of all triazole–spirodienones 1 and 2, respectively. Thus, twenty four triazole–spirodienone 1 and 2 were obtained in 36–94% yield (Fig. 1) from corresponding 2-phenoxy-ethyl-acet-N-2,3,5-triazoleamides and 2-phenoxy-ethyl-ethyl-N-2,3,5-triazoleamines.
| Entry | Catalyst | Oxidant | Yieldb (%) | |
|---|---|---|---|---|
| 1a | 2a | |||
| a Substrate(1 mmol), oxidant (2.5 mmol), Al2O3(2.5 mmol); CH2Cl2 as solvent, room temperature, 4 Å molecular sieve.b Isolated yield based on the amount of compounds 1a and 2a consumed. | ||||
| 1 | — | PhI(CF3CO2)2 | — | — |
| 2 | 10% CuCl2 | PhI(CF3CO2)2 | 32 | Trace |
| 3 | 10% CuBr2 | PhI(CF3CO2)2 | 32 | Trace |
| 4 | 10% Cu(CF3SO3)2 | PhI(CF3CO2)2 | 49 | 57 |
| 5 | 10% Cu(acac)2 | PhI(CF3CO2)2 | 36 | 28 |
| 6 | 10% Cu(OAC)2 | PhI(CF3CO2)2 | 30 | 22 |
| 7 | 10% Cu(CF3COCH2COCF3)2 | PhI(CF3CO2)2 | 36 | 29 |
| 8 | 10% Cu[(CH3CN)4]ClO4 | PhI(CF3CO2)2 | 94 | 40 |
| 9 | 5% Rh2(OAc)4 | PhI(CF3CO2)2 | 33 | Trace |
| 10 | 5% Ru(TTP)CO | PhI(CF3CO2)2 | 44 | Trace |
| 11 | 10% Cu(CF3SO3)2 | PhI(OAc) 2 | 35 | 51 |
| 12 | 10% Cu(CF3SO3)2 | PhI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
27 | Trace |
| 13 | 10% Cu[(CH3CN)4]ClO4 | PhI(OAc) 2 | 45 | 47 |
| 14 | 10% Cu[(CH3CN)4]ClO4 | PhI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
30 | Trace |
In entry, the effects of the substituents on the oxidative amidation reaction were noticed that the electron-donating groups sped the oxidative amination reaction of the aromatic ether derivatives (5 and 6) and the electron-withdrawing substituents resulted in moderate yields of the reaction, thereby, the reaction rates rely on the easy of oxidation of the substrate and the yields of triazole–spirodienone (1 and 2) are linked with the activity of the nitrogen sources, especially, the effect of N–H dissociation energies of the nitrogen source.
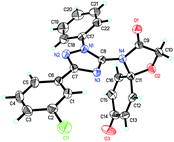
To confirm the structures of triazole spirodienone conjugates, a single crystal of 1p was grown and analyzed by X-ray crystallography (the detailed crystal data are provided in the ESI†). The ORTEP structure plot of 1p is displayed in Fig. 2.
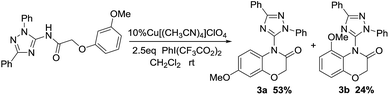
The effect of substituent was investigated using different substituted aromatic ether derivatives as substrates under strictly identical condition. Interestingly, we notice that the reaction of these methoxy group substituent in the meta-position gave only two corresponding six-membered heterocyclic products 3a and 3b in 53% and 24% yield respectively (Scheme 1).
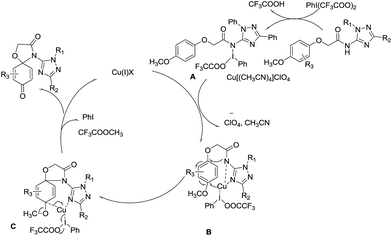
A mechanism is proposed in Scheme 2 for the oxidative amination reaction of the aromatic ether with β-NH–imidazole based on the mechanisms proposed originally for the amination by Antonchick32 and Chiba.33 Initially ditrifluoro-acetoxyiodobenzene reacts with the amide to give intermediate A, which is then transformed into intermediate B through an oxidative insertion of N–I bond by copper(II). The reductive elimination of intermediate C releases copper(I) salt, trifluoroacetic acid and iodobenzene, leading to final product 4-(1,3-diphenyl-1H-1,2,4-triazol-5-yl)-1-oxa-4-azaspiro[4.5]-deca-6,9-diene-3,8-dione (1). And the 4-(1,3-diphenyl-1H-1,2,4-triazol-5-yl)-1-oxa-4-azaspiro[4.5]deca-6,9-diene-8-one (2) was obtained in similar mechanisms.
Antiproliferation activity against a panel of cancer cell lines
All of triazole–spirodienone conjugates (1 and 2) were examined for in vitro antiproliferation activities against a triple negative human breast cancer cell line, MDA-MB-231, an ER + human epithelial mammary carcinoma cell line, MCF-7, an invasive cervical cancer cell line, HeLa, and a metastatic non-small cell lung cancer cell line, A549. Table 2 displayed the IC50 values of 1 and 2 against these four cell lines. Moderate to high antiproliferative activities were observed in most of 1 and 2 derivatives, with IC50 values ranging from low nanomolar concentration to micromolar concentration. The triazole–spirodienone conjugates inhibited cancer cell growth in a dose-dependent manner in all four cancer cell lines tested, and appear to exert a greater inhibitory effect on MDA-MB-231 cells in particular. Notably, 4-triazolyl-1-oxa-4-azaspiro[4,5]-deca-6,9-dien-3,8-diones (1) were generally stronger cell growth inhibitors than the corresponding 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-8-ones (2). The only structural difference between 1 and 2 is the extra carbonyl group on 3-position of the spirodienone ring of 1, suggesting that introduction of a 3-one functional group at the spiro-ring increased the antiproliferation activity against cancer cells.| Compds | IC50 (nM) | |||
|---|---|---|---|---|
| MDA-MB-231 | HeLa | A549 | MCF-7 | |
| 1a | 20.3 | 93.9 | 288 | 40.7 |
| 1b | 16.8 | 12.2 | 69.0 | 231 |
| 1c | 17.1 | 95.5 | 395 | 111 |
| 1d | 4.56 | 21.0 | 4.18 | 62.1 |
| 1e | 67.8 | 326 | 1800 | 31.4 |
| 1f | 6.44 | 17.5 | 457 | 16.2 |
| 1g | 73.7 | 123 | 485 | 315 |
| 1h | 270 | 308 | 399 | 397 |
| 1i | 1.37 | 62.9 | 203 | 43.3 |
| 1j | 22.2 | 25.2 | 266 | 116 |
| 1k | 27.7 | 10.0 | 268 | 88.8 |
| 1l | 113 | 727 | 346 | 245 |
| 1m | 123 | 134 | 340 | 763 |
| 1n | 157 | 107 | 484 | 1080 |
| 1o | 453 | 381 | 31.1 | 465 |
| 1p | 0.80 | 45.3 | 25.2 | 4.11 |
| 2a | 470 | 123 | 357 | 694 |
| 2b | 555 | 126 | 698 | 442 |
| 2c | 60.6 | 976 | 465 | 843 |
| 2d | 170 | 979 | 809 | 846 |
| 2e | 309 | 753 | 1800 | 1530 |
| 2f | 684 | 996 | 1010 | 476 |
| 2g | 332 | 84.9 | 511 | 1100 |
| 2h | 141 | 1240 | 1190 | 442 |
Cytotoxicity towards MCF-10A normal mammary epithelial cells
Seven of the most promising triazole–spirodienone conjugates (1a, 1d–f, and 1i–k) were selected for further evaluation of toxicity in normal mammary epithelial cells, MCF-10A. At 10 μM, all the compounds were found to be significantly toxic towards MCF-10A cells, with toxicities moderating at a lower dosage of 1 μM. At both doses of 1 and 10 μM, doxorubicin, a widely used anticancer drug in clinic, was found to be much more toxic to normal cells than any of the selected triazole–spirodienone conjugates (the detailed data see ESI†). This observation is encouraging because the novel structural motif of triazole–spirodienone conjugates may offer a better separation of desired tumor toxicity and toxicity to normal cells.Apoptosis of HeLa cells
We performed a caspase 3/7 activation assay for compounds 1b, 1i, 1f and 1p using fluorescence microscopy. HeLa cells were treated with four selected triazole–spirodienone conjugates at 1.0 μM or vehicle for 24 hours. In this assay, fluorescence signal was related to the degree of caspase 3/7 activation and hence apoptosis induced by the treatment. As shown in Fig. 3, untreated cells showed minimal fluorescence, while treated cells exhibited significant increase in fluorescence (shown in green) indicating the prevalence of apoptosis of HeLa cells. These observations strongly suggest that triazole–spirodienone conjugates inhibited HeLa cells proliferation by triggering apoptosis.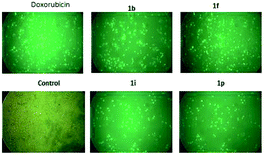 | ||
| Fig. 3 Images of apoptosis induced by triazole–spirodienones conjugates, doxorubicin and vehicle control in HeLa cell lines. | ||
Conclusions
Copper salt-catalyzed oxidative amination resulted in the formation of novel triazole–spirodienone conjugates, 4-triazolyl-1-oxa-4-aza-spiro[4,5]deca-6,9-dien-3,8-diones (1) and 4-triazolyl-1-oxa-4-aza-spiro[4,5]deca-6,9-dien-8-ones (2) which were evaluated for their antiproliferative activities against MDA-MB-231, HeLa, A549 and MCF-7 cell lines. Most of them showed moderate to high anticancer potency in the four cancer cell lines. Moreover, 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-3,8-diones (1) were found to exhibit superior anticancer activities compared to 4-triazolyl-1-oxa-4-azaspiro[4,5]deca-6,9-dien-8-ones (2). The cytotoxicities of 1a, 1d–f, and 1i–k toward non-cancerous cells (MCF-10A) were measurable, but much less severe than that of doxorubicin, a known and widely used chemotherapy agent. Our study represents the first report on the preparation and biological evaluation of the novel triazole–spirodienone conjugates as potential anticancer agents. The analogs (1) are promising lead compounds on which further optimization of structure–activity relationships may lead to therapeutically useful new chemical entities for clinical treatment of cancer. Preliminary study on the mode of action suggests that the triazole–spirodienone conjugates inhibited cancer cell proliferation by triggering apoptosis, with more in-depth mechanistic studies under way in our laboratories.Acknowledgements
This work was supported by the Sichuan University-Lu Zhou Strategic Cooperation Projects (No. 2013CDLZ-S18) (Ling He) and the NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595-07 (G. Wang).References
- O. Tacar, P. Sriamornsak and C. R. Dass, J. Pharm. Pharmacol., 2013, 65, 157–170 CrossRef CAS PubMed.
- C. Parker, R. Waters, C. Leighton, J. Hancock, R. Sutton, A. V. Moorman, P. Ancliff, M. Morgan, A. Masurekar, N. Goulden, N. Green, T. Révész, P. Darbyshire, S. Love and V. Saha, Lancet, 2010, 376, 2009–2017 CrossRef CAS.
- C. Tan, H. Tasaka, K.-P. Yu, M. L. Murphy and D. Karnofsky, Cancer, 1967, 20, 333–353 CrossRef CAS PubMed.
- M. Tomasz and C. Mitomycin, Chem. Biol., 1995, 2, 575–579 CrossRef CAS PubMed.
- N. R. Bachur, S. L. Gordon, M. V. Gee and H. Kon, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 954–957 CrossRef CAS.
- M. T. Hoyt, R. Palchaudhuri and P. J. Hergenrother, Invest. New Drugs, 2011, 29, 562–573 CrossRef CAS PubMed.
- G. Kaur, R. P. Cholia, A. K. Mantha and R. Kumar, J. Med. Chem., 2014, 57, 10241–10256 CrossRef CAS PubMed.
- N. R. Bachur, S. L. Gordon and M. V. Gee, Cancer Res., 1978, 38, 1745–1750 CAS.
- J. S. Bair, R. Palchaudhuri and P. J. Hergenrother, J. Am. Chem. Soc., 2010, 132, 5469–5478 CrossRef CAS PubMed.
- I. Gomez-Monterrey, P. Campiglia, C. Aquino, A. Bertamino, I. Granata, A. Carotenuto, D. Brancaccio, P. Stiuso, I. Scognamiglio and M. R. Rusciano, J. Med. Chem., 2011, 54, 4077–4091 CrossRef CAS PubMed.
- M. H. El-Dakdouki, N. Adamski, L. Foster, M. P. Hacker and P. W. Erhardt, J. Med. Chem., 2011, 54, 8224–8227 CrossRef CAS PubMed.
- L. Trzoss, T. Fukuda, L. V. Costa-Lotufo, P. Jimenez, J. J. La Clair and W. Fenical, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 14687–14692 CrossRef CAS PubMed.
- P. Morales, D. Vara, M. Goméz-Cañas, M. C. Zúñiga, C. Olea-Azar, P. Goya, J. Fernández-Ruiz, I. Díaz-Laviada and N. Jagerovic, Eur. J. Med. Chem., 2013, 70, 111–119 CrossRef CAS PubMed.
- A. A. Vieira, I. R. Brandão, W. O. Valença, C. A. de Simone, B. C. Cavalcanti, C. Pessoa, T. R. Carneiro, A. L. Braga and E. N. da Silva, Eur. J. Med. Chem., 2015, 101, 254–265 CrossRef CAS PubMed.
- E. N. da Silva Jr, B. C. Cavalcanti, T. T. Guimarães, M. d. C. F. R. Pinto, I. O. Cabral, C. Pessoa, L. V. Costa-Lotufo, M. O. de Moraes, C. K. Z. de Andrade, M. R. dos Santos, C. A. de Simone, M. O. F. Goulart and A. V. Pinto, Eur. J. Med. Chem., 2011, 46, 399–410 CrossRef PubMed.
- C. Grasso, L. Larsen, M. McConnell, R. A. J. Smith and M. V. Berridge, J. Med. Chem., 2013, 56, 3168–3176 CrossRef CAS PubMed.
- X. Li, S.-L. Zheng, X. Li, J.-L. Li, O. Qiang, R. Liu and L. He, Eur. J. Med. Chem., 2012, 54, 42–48 CrossRef CAS PubMed.
- L.-M. Zhao, F.-Y. Ma, H.-S. Jin, S. Zheng, Q. Zhong and G. Wang, Eur. J. Med. Chem., 2015, 102, 303–309 CrossRef CAS PubMed.
- L. He, Y. Chen, J. Xie and J. Li, CN102285934, 2013.
- Z.-K. Chen, Q.-Q. Yan, Z.-X. Liu, Y.-M. Xu and Y.-H. Zhang, Angew. Chem., Int. Ed., 2013, 52, 13324–13328 CrossRef CAS PubMed.
- Z.-K. Chen, Q.-Q. Yan, H. Yi, Z.-X. Liu, A.-W. Lei and Y.-H. Zhang, Chem.–Eur. J., 2014, 20, 13692–13697 CrossRef CAS PubMed.
- Y.-P. Hou, J. Sun, Z.-H. Pang, P.-C. Lv, D.-D. Li, L. Yan, H.-J. Zhang, E. X. Zheng, J. Zhao and H.-L. Zhu, Bioorg. Med. Chem., 2011, 19, 5948–5954 CrossRef CAS PubMed.
- F. Xu, Y. Jia, Q. Wen, X. Wang, L. Zhang, Y. Zhang, K. Yang and W. Xu, Eur. J. Med. Chem., 2013, 64, 377–388 CrossRef CAS PubMed.
- J. Lee, S. J. Kim, H. Choi, Y. H. Kim, I. T. Lim, H.-m. Yang, C. S. Lee, H. R. Kang, S. K. Ahn, S. K. Moon, D.-H. Kim, S. Lee, N. S. Choi and K. J. Lee, J. Med. Chem., 2010, 53, 6337–6354 CrossRef CAS PubMed.
- R. Romagnoli, P. G. Baraldi, O. Cruz-Lopez, C. Lopez Cara, M. D. Carrion, A. Brancale, E. Hamel, L. Chen, R. Bortolozzi, G. Basso and G. Viola, J. Med. Chem., 2010, 53, 4248–4258 CrossRef CAS PubMed.
- R. Kaur, A. R. Dwivedi, B. Kumar and V. Kumar, Anti-Cancer Agents Med. Chem., 2016, 16, 465–489 CrossRef CAS PubMed.
- R. J. Santen, H. Brodie, E. R. Simpson, P. K. Siiteri and A. Brodie, Endocr. Rev., 2009, 30, 343–375 CrossRef CAS PubMed.
- P. Goss, Breast Cancer Res. Treat., 1998, 49, S59–S65 CrossRef PubMed.
- P. Yin, W.-B. Ma, Y. Chen, W.-C. Huang, Y. Deng and L. He, Org. Lett., 2009, 11, 5482–5485 CrossRef CAS PubMed.
- T. Dohi, A. Maruyama, Y. Minamitsuji, N. Takenaga and Y. Kita, Chem. Commun., 2007, 12, 1224–1226 RSC.
- Y.-X. Deng, J.-P. Xie, W.-W. Zhang, P. Yin, J. Yu and L. He, Chem.–Eur. J., 2012, 18, 1077–1082 CrossRef CAS PubMed.
- A. P. Antonchick, R. Samanta, K. Kulikov and J. Lategahn, Angew. Chem., Int. Ed., 2011, 50, 8605–8608 CrossRef CAS PubMed.
- S. Chiba, L. Zhang and J.-Y. Lee, J. Am. Chem. Soc., 2010, 132, 7266–7267 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1486522. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra24764d |
| This journal is © The Royal Society of Chemistry 2017 |