DOI:
10.1039/C6RA24734B
(Paper)
RSC Adv., 2017,
7, 1671-1686
Towards macrocyclic ionic liquids: novel ammonium salts based on tetrasubstituted p-tert-butylthiacalix[4]arenes†
Received
4th October 2016
, Accepted 28th November 2016
First published on 5th January 2017
Abstract
Water-insoluble ionic liquids based on p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim with amide and quaternary ammonium groups containing alkyl, phenyl, ester, phthalimide, glycine, alanine and glycylglycine groups in cone and 1,3-alternate conformations were synthesized. It was established that macrocycles containing quaternary ammonium fragments with alkyl, phenyl and ester groups at the nitrogen atom in cone conformation melt lower by 8–31 °C than 1,3-alternate stereoisomers. It was shown that the introduction of the bis(trifluoromethylsulfonyl)imide anion as a counterion in the structure of quaternary ammonium salts based on thiacalix[4]arenes led to a substantial decrease in the melting point of the above salts.
Introduction
Synthesis of ionic liquids for extraction and determination of organic compounds is one of the promising areas of investigation in modern organic chemistry.1–7 Water-insoluble solvents have recently been widely used for extraction and separation of biologically significant compounds but they have some technological disadvantages and do not often meet modern environmental standards. The replacement of such solvents is an important applied task. One of the possible solutions to this problem is the use of ionic liquids, e.g., molten salts that are liquids at temperatures below 100 °C. Ionic liquids are mostly non-flammable, synthetically accessible and have negligible vapor pressure; selection of their cations and anions allows adjusting their properties over a wide range. Unique combinations of hydrophobicity and ionic nature, thermal stability and high electrical conductivity of ionic liquids offer new opportunities in the field of organic and analytical chemistry, catalysis and electrochemistry.8–12 The development of approaches to creation of new high-performance systems for the extraction and separation of various compounds based on ionic liquids and functionalized macrocycles, e.g., cyclodextrins, cucurbit[n]urils, (thia)calix[n]arenes, pillar[n]arenes, crown ethers is of great interest.13–17
There are two basic approaches to the creation of such systems: (1) synthesis of the macrocyclic compounds soluble in ionic liquids, and (2) design of ionic liquids containing macrocyclic fragment as their cation or anion (Fig. 1).
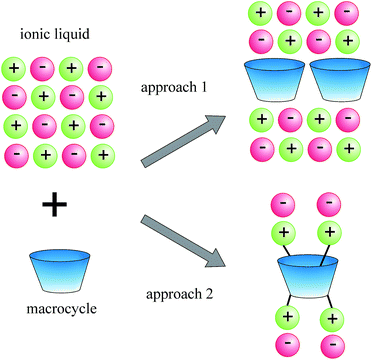 |
| | Fig. 1 Two basic approaches to the creation of macrocyclic ionic liquids based on ionic liquids and macrocyclic compounds. | |
Despite large synthetic and conformational diversity of (thia)calix[4]arenes as molecular building platform,18–24 there are only few examples of the synthesis of ionic liquids based on them.7,25 It should be noted that the macrocyclic ionic liquids described in the literature7,25,26 belong to the pyridinium and imidazolium derivatives. Synthesis of ionic liquids based on quaternary ammonium salt is one of the most important tasks in modern organic chemistry.27 Ease of synthesis, low cost and non-toxicity are the advantages of quaternary ammonium fragments. According to the examples in the literature of chemistry of ionic liquids28–34 and (thia)calixarenes,1–7,35 we can assume that the introduction of quaternary ammonium fragments at the lower rim of p-tert-butylthiacalix[4]arene may be promising for the synthesis of macrocyclic ionic liquids capable to the molecular recognition of target species.
In this work, the synthesis of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim with quaternary ammonium groups in cone and 1,3-alternate conformations as potential ionic liquids is described.
Results and discussion
Synthesis of p-tert-butylthiacalix[4]arenes containing quaternary ammonium groups
To synthesize ionic liquids based on the p-tert-butylthiacalix[4]arenes containing quaternary ammonium groups, the reaction of the compounds 1–4 with different alkylating reagents in acetonitrile under reflux has been studied. Alkyl iodides and alkyl bromides were selected as highly reactive alkylating agents. Iodomethane and iodoethane as two simplest homologues were chosen. Based on these compounds, we can verify previously described in the literature suggestion36 that the increase in the length of the alkyl substituent led to decrease of the melting point. According to the literature,36 it was also assumed that the introduction of planar π-aromatic ring systems and the ester groups at the lower rim of the macrocycle could result in the synthesis of tetraalkylammonium derivatives of p-tert-butylthiacalix[4]arene in cone and 1,3-alternate conformations with low melting points. Thus, benzyl bromide, ethyl bromoacetate and pentyl bromoacetate were used from this consideration. It is interesting to note that in case of iodomethane and iodoethane the reaction was carried out at room temperature because alkyl iodides are more reactive than alkyl bromides.
It was found that the reactivity of the macrocycles 2 and 4 containing tertiary amino groups with ethyl substituents at the lower rim was lower than the reactivity of the thiacalix[4]arenes 1 and 3 with tertiary amino groups with methyl substituents. Probably, this is due to steric hindrance at the amino nitrogen atom. The increase of reaction time from 8 to 48 hours led to the compounds 5–9, 14–18, 22–26 and 31–35 with 85–98% yields (Scheme 1). It should be noted that all the synthesized macrocycles 5–9, 14–18, 22–26 and 31–35 are water-soluble. The solubility of the thiacalix[4]arenes 5–9 and 14–18 in cone conformation was much higher than that of the 1,3-alternate stereoisomers 22–26 and 31–35.
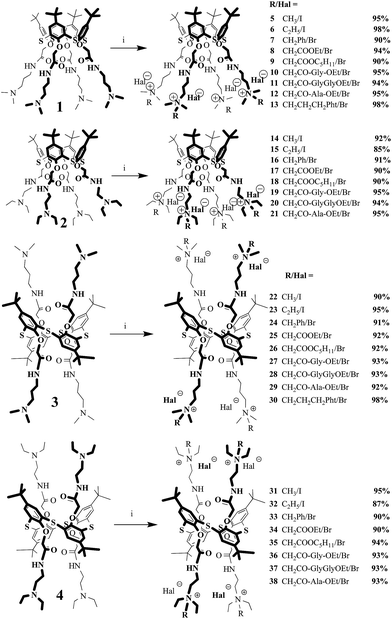 |
| | Scheme 1 Reagents and conditions: i – R-Hal, CH3CN, reflux. | |
To synthesize ionic liquids able to recognize various substrates, stating from low molecular compounds to biomacromolecules, synthesis of the quaternary ammonium salts based on p-tert-butylthiacalix[4]arenes containing peptide and phthalimide fragments has been performed. N-Bromoacetyl-glycine ethyl ester, N-bromoacetyl-glycylglycine ethyl ester, N-bromoacetyl-L-alanine ethyl ester and N-(3-bromopropyl)phthalimide were chosen as alkylating reagents. The presence of α-amino acid groups in the structure of p-tert-butylthiacalix[4]arenes is necessary for increasing the efficiency of interaction of the macrocycles with biomacromolecules (proteins and DNA) based on the formation of hydrogen bonds. The introduction of phthalimide groups in the structure of macrocycles is also of great interest because the compounds with phthalimide fragments can be used as intercalators for interaction with DNA or for detection of proteins by hydrophobic interactions.37
The interaction of ethyl esters of N-bromoacetyl-glycine, N-bromoacetyl-glycylglycine and N-bromoacetyl-alanine with aminothiacalix[4]arenes 1–4 containing methyl and ethyl groups at the nitrogen atom in cone and 1,3-alternate conformations was studied earlier. Quaternary ammonium salts 10–12, 19–21, 27–29 and 36–38 with amino acid and peptide groups were obtained38 (Scheme 1).
It is interesting to note that the reaction of the amines 1–4 with N-(3-bromopropyl)phthalimide in acetonitrile under 8 h reflux resulted in formation of the products only in the case of the compounds 1 and 3 containing tertiary amino groups with N,N-dimethyl substituents at the lower rim in cone and 1,3-alternate conformations. The compounds 13 and 30 were synthesized with excellent yields38 (Scheme 1). According to the 1H NMR spectroscopy, the mixture of differently substituted products difficult for separation was obtained for the macrocycles 2 and 4 containing tertiary amine groups with N,N-diethyl substituents at the lower rim. The increase of the reaction time up to 40 hours did not lead to the formation of the target products. Probably, reactivity of the macrocycles 2 and 4 is reduced by steric hindrance in tertiary amino groups with N,N-diethyl substituents against that of the compounds 1 and 3 containing tertiary amino groups with methyl substituents.
Melting point is one of main characteristics of ionic liquids (see Table 1 for the synthesized macrocycles 5–21 (cone) and 22–38 (1,3-alternate)). Conformation changes and length of the alkyl substituents led to a slight decrease in the melting point is agreement with the literature.36 Introduction of benzyl and ester groups in the structure of macrocycles decreased the melting points by 30–40 °C, in contrast to 5–7 °C predicted in the literature.36 However, main goal has not been achieved. All the obtained salts 5–21 (cone) and 22–38 (1,3-alternate) melt above 100 °C except the macrocycle 35 containing pentyl acetate fragment.
Table 1 Melting points (°C) of the macrocycles 5–21 (cone) and 22–38 (1,3-alternate)
| R/Hal− |
NH(CH2)3N+(CH3)2R |
NH(CH2)2N+(C2H5)2R |
| Cone |
1,3-Alternate |
Cone |
1,3-Alternate |
| CH3/I |
192 (5) |
197 (22) |
159 (14) |
156 (31) |
| C2H5/I |
165 (6) |
215 (23) |
163 (15) |
173 (32) |
| CH2Ph/Br |
135 (7) |
150 (24) |
139 (16) |
131 (33) |
| CH2COOC2H5/Br |
112 (8) |
123 (25) |
118 (17) |
115 (34) |
| CH2COOC5H11/Br |
106 (9) |
103 (26) |
135 (18) |
88 (35) |
| CH2CO-Gly-OEt/Br |
114 (10) |
112 (27) |
111 (19) |
114 (36) |
| CH2CO-GlyGlyOEt/Br |
113 (11) |
120 (28) |
120 (20) |
124 (37) |
| CH2CO-Ala-OEt/Br |
116 (12) |
118 (29) |
116 (21) |
118 (38) |
| CH2CH2CH2Pht/Br |
152 (13) |
154 (30) |
— |
— |
Synthesis of ionic liquids based on p-tert-butylthiacalix[4]arenes containing alkyl, ester, aromatic, peptide and phthalimide fragments
As shown in the literature39 replacement of the halide ions by bis(trifluoromethylsulfonyl)imide ions considerably decreased melting point. This can be explained by the fact that the increase in the size of anions decreased symmetry of the molecule obtained39 (Fig. 2). Thus, the study of the interaction of the compounds 5–38 with lithium bis(trifluoromethylsulfonyl)imide in water at room temperature was next step of the work (Scheme 2, Fig. 2).
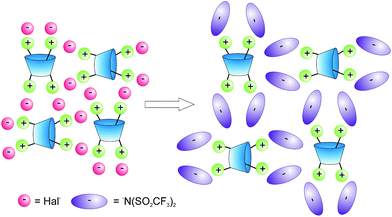 |
| | Fig. 2 Possible scheme of the changes of crystal packing of the p-tert-butylthiacalix[4]arene ammonium salts at the replacement of halide ions by bis(trifluoromethylsulfonyl)imide ions. | |
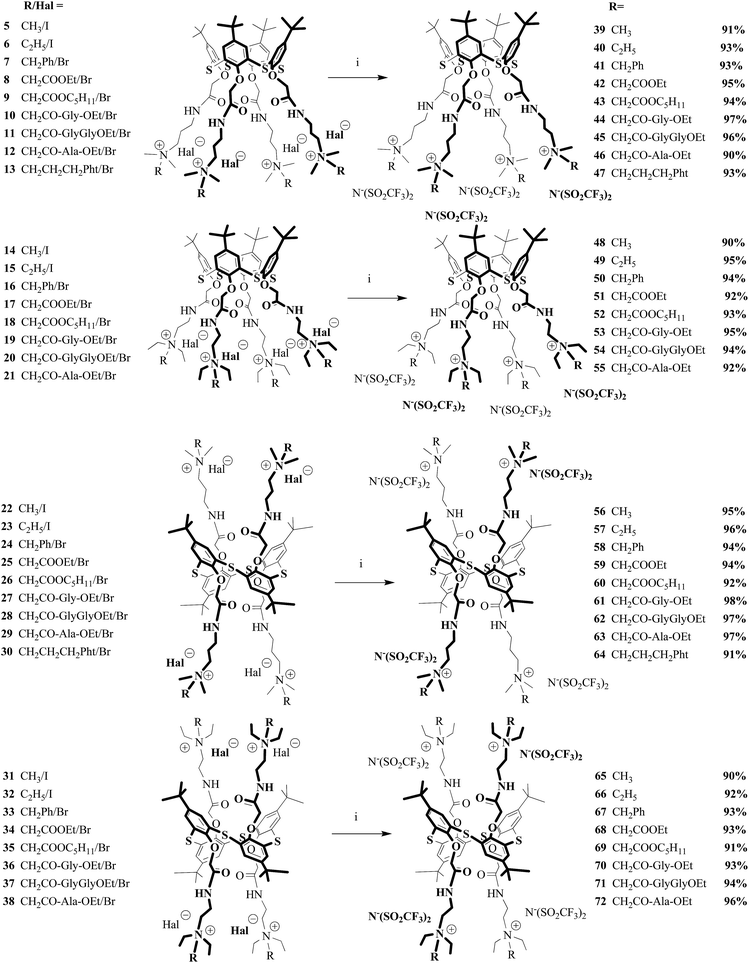 |
| | Scheme 2 Reagents and conditions: i – Li+N−(SO2CF3)2, H2O. | |
The structure and composition of the synthesized compounds 39–72 were determined by the 1H and 13C NMR, IR spectroscopy, mass spectrometry and elemental analysis. The 13C NMR spectrum of the compounds 39–72 exhibits a quartet at 120 ppm; these signals correspond to anion −N(SO2CF3)2 (Fig. S67–S100, ESI†).
The configuration of p-tert-butylthiacalix[4]arenes can be studied by two-dimensional NMR spectroscopy. However, the configuration of the compounds 5–72 can be also determined by one-dimensional 1H NMR spectroscopy based on specific proton signals.
The conformational differentiation of the cone and 1,3-alternate stereoisomers of the p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim can be recognized by chemical shifts of the tert-butyl group, aromatic ring, oxymethylene and amide protons in the 1H NMR spectra (Table S1, ESI†). In the compounds 22–38 and 56–72 in the 1,3-alternate conformation, the protons of the –OCH2– and amide groups are located in the shielded zone of neighboring aromatic rings of the macrocycle, and their signals in the 1H NMR spectrum are recorded at stronger fields (3.96–4.20 and 8.01–8.38 ppm, respectively) than those of the macrocycles 5–21 and 39–55 in cone conformation (4.79–5.03 and 8.23–9.13 ppm, respectively). The chemical shifts of the aromatic protons depend less on the conformation of the macrocyclic cavity. They drift by only 0.19–0.25 ppm upfield from cone 5–21 and 39–55 (7.30–7.45 ppm) to 1,3-alternate 22–38 and 56–72 (7.59–7.61 ppm) stereoisomers. This is an evidence of the shielding effect of neighboring aryl fragments in the cone stereoisomer on the aryl protons of macrocycle ring. The protons of the tert-butyl groups of the cone stereoisomers 5–21 and 39–55 were found at a stronger field (1.07–1.12 ppm) against corresponding proton signals of the 1,3-alternate stereoisomers 22–38 and 56–72 (1.19–1.22 ppm). This effect is probably due to the spatial location of the tert-butyl groups of the 1,3-alternate stereoisomer shielded by neighboring fragments of the macrocycle.
It should be noted that the proton signals in the 1H NMR spectra of the quaternary ammonium salts 5–38 (Table S1, Fig. S1–S16, ESI†) containing halide anions, and the proton signals of initial salts 39–72 (Table S1, Fig. S17–S50, ESI†) containing bis(trifluoromethylsulfonyl)imide anions have identical multiplicity and exert very similar chemical shifts. It can be explained that these compounds are able to form solvent-separated ion pairs in solution.
Melting points of the synthesized thiacalix[4]arenes 39–72 are presented in Table 2. One can see (Tables 1 and 2) that the replacement of halide ions by bis(trifluoromethylsulfonyl)imide ions leads to significant decrease in the melting points of the thiacalix[4]arenes studied. All the synthesized macrocycles 39–72 containing bis(trifluoromethylsulfonyl)imide anions melt below 100 °C, except the product 56 (Table 2).
Table 2 Melting points (°C) of the macrocycles 39–55 (cone) and 56–72 (1,3-alternate)
| R |
NH(CH2)3N+(CH3)2R*N(SO2CF3)2− |
NH(CH2)2N+(C2H5)2R*N(SO2CF3)2− |
| Cone |
1,3-Alternate |
Cone |
1,3-Alternate |
| CH3 |
87 (39) |
106 (56) |
72 (48) |
83 (65) |
| C2H5 |
71 (40) |
96 (57) |
68 (49) |
79 (66) |
| CH2Ph |
56 (41) |
87 (58) |
60 (50) |
76 (67) |
| CH2COOC2H5 |
45 (42) |
53 (59) |
43 (51) |
58 (68) |
| CH2COOC5H11 |
35 (43) |
47 (60) |
39 (52) |
49 (69) |
| CH2CO-Gly-OEt |
63 (44) |
73 (61) |
66 (53) |
62 (70) |
| CH2CO-GlyGlyOEt |
69 (45) |
76 (62) |
64 (54) |
64 (71) |
| CH2CO-Ala-OEt |
56 (46) |
60 (63) |
63 (55) |
62 (72) |
| CH2CH2CH2Pht |
83 (47) |
87 (64) |
— |
— |
One can see (Table 2) that stereoisomerism of the macrocycles 39–43, 48–52, 56–60, 65–69 has an impact on their melting points. In the case of the cone (39–43, 48–52) stereoisomers, their melting points are lower by 8–31 °C against those of the thiacalix[4]arenes (56–60, 65–69) in 1,3-alternate conformation. It is well known that packing density of the molecules in the crystal lattice is a major factor affecting the melting point of the substance. More symmetrical molecules have denser packing in crystal and higher melting point. Obviously, molecular symmetry of the cone (39–43, 48–52) stereoisomers results in maximal spatial separation of the bulk lipophilic tert-butyl and charged ammonium groups and hence in decrease of the packing density and appropriate reduction of their melting point. On the other hand, in the case of the symmetric 1,3-alternate (56–60, 65–69) stereoisomers that show higher melting points, alternation of tert-butyl and ammonium groups at adjacent aryl fragments led to denser packing of the molecules. However, the introduction of additional amide groups with amino acid residues (Gly, Ala) in the structure of thiacalix[4]arene compared to the macrocycles 39–43, 48–52, 56–60, 65–69 decreased influence of the macrocycle configuration on their melting points. Obviously, peptide groups able to form hydrogen bonds contribute to the formation of denser packing of the molecules in the crystal. It can be assumed that two opposite factors influence melting points of the thiacalix[4]arenes depending on the structure of macrocycles, i.e., conformation (melting points of the stereoisomers decrease in the range: 1,3-alternate, cone) and presence of the proton-donating (–NH) and proton-accepting (carbonyl) groups (melting points are increased due to the formation of associates). This results in the fact that melting points of the macrocycles (44–47, 53–55, 61–64, 70–72) in cone and 1,3-alternate conformations are slightly different.
Increasing length of the alkyl substituent of the macrocycle by one CH2– group leads to decrease of the melting point by 8–9 °C in good agreement with the literature.36 It should also be noted that the compound 43 containing pentoxy carbonylmethylene groups at the lower rim in cone conformation has the lowest melting point (35 °C). This corresponds closely to the hypothesis about the influence of the ester groups on the melting points of the target products.
Thermal stability and ionic conductivity have been established for the compound 43 with the lowest melting point. Thermal stability of the compound 43 toward pyrolysis was investigated by thermogravimetric analysis (Fig. S201, ESI†). The 5 wt% loss temperature (T5) of the compound 43 under nitrogen was equal to 293.5 °C indicating its high thermal stability. It is known that macrocyclic ionic liquids have lower ionic conductivity in comparison with their non-macrocyclic analogues.26 The ionic conductivity of the compound 43 was evaluated by ac impedance spectroscopy. The ionic conductivity of compound 43 in the bulk state at 324 K was found to be 6.00 × 10−7 S × cm−1 (Fig. 3) corresponded to moderate ionic conductivity. Fig. 3 shows low- (120 Hz) and high-frequency (500 kHz) electrical conductivity exhibiting exponential increase with the temperature. The activation energy for the high-frequency conductivity EA1 = 0.69 eV is approx. twofold less than that of the low-frequency conductivity (EA2 = 1.24 eV). This behavior of the activation energy was also observed for other organic semiconductors42 and is usually associated with the processes of the hopping of charge carriers in an inhomogeneous conducting medium. This model supposes the current in organic semiconductors generated by hopping carriers between polyconjugated areas from one to another limited by dielectric barrier created by disordered (non-conjugated) structure. Small activation energy values are typical for the occurrence of carriers within interface area and manifest themselves in the measurements at a high frequency, while measuring at the DC and low frequencies give substantially higher values of the activation energy associated probably with the above barrier hopping between coupling fragments.
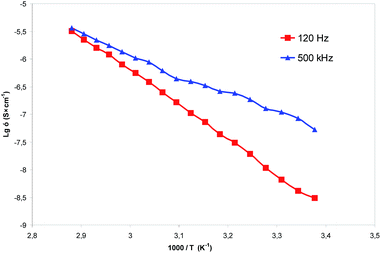 |
| | Fig. 3 Ionic conductivity of the thiacalix[4]arene cone 43. | |
Conclusions
Thus, water-soluble and water-insoluble p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim with amide and quaternary ammonium groups with alkyl, ester, amino acid, peptide and phthalimide fragments in cone and 1,3-alternate conformation were synthesized. The structure and composition of the synthesized macrocycles were determined by the physical methods, i.e., the 1H and 13C NMR, IR spectroscopy, MALDI TOF and ESI mass spectrometry and elemental analysis. It was found that the replacement of halide ions in the synthesized macrocycles by bis(trifluoromethylsulfonyl)imide ions led to water-insoluble salts with melting points below 100 °C. It was shown that the macrocycles containing quaternary ammonium fragments with alkyl, phenyl and ester groups at the nitrogen atom in cone conformation melt lower by 8–31 °C than 1,3-alternate stereoisomers. Macrocyclic water-insoluble ionic liquids synthesized in this work showed high thermal stability and moderate ionic conductivity. These salts can be used in the sensor assemblies for the molecular recognition of the target substrates, e.g., biomacromolecules and cations of heavy and transition metals.
Experimental
General
The 1H and 13C NMR spectra of compounds (3–5% solution in CDCl3, (CD3)2SO) were recorded on 400 MHz and 100 MHz Bruker Avance 400 spectrometer using CDCl3 and (CD3)2SO as internal standard.
The IR spectra were recorded on Spectrum 400 (Perkin Elmer) IR spectrometer. The IR spectra from 4000 to 400 cm−1 were considered in this analysis. The spectra were measured with 4 cm−1 resolution and 14 scans co-addition.
Elemental analysis was performed on Perkin-Elmer 2400 Series II instruments.
Mass spectra (MALDI-TOF) were recorded on Ultraflex III mass spectrometer in the 4-nitroaniline matrix.
Mass spectra (ESI) were recorded on an AmaZonX mass spectrometer (Bruker Daltonik GmbH, Germany). The drying gas was nitrogen at 300 °C. The capillary voltage was 4.5 kV. The samples were dissolved in acetonitrile (concentration ∼ 10−6 g ml−1).
Melting points were determined using Boetius Block apparatus. The purity of the compounds was monitored by melting, boiling points, 1H NMR and thin layer chromatography (TLC) on 200 μm UV 254 silica gel plate using UV-light (254 nm).
Conductivity measurements were performed on the RLC-meter E7-20 in “sandwich” type cell at frequencies of 120 Hz and 500 kHz at temperatures range from room temperature to 74 °C. The sample size was 1 cm2, thickness – 4 mm.
In this work, the following reagents and solvents were used: acetonitrile (chemical pure), benzyl bromide (chemical pure), lithium bis(trifluoromethansulfonyl)imide (Acros Organic), N-(3-bromopropyl)phthalimide (Acros Organic), distilled water, iodomethane (Acros Organic), iodoethane (Acros Organic), ethyl bromoacetate (Acros Organic), pentyl bromoacetate (chemical pure).
N-Bromoacetyl-glycine ethyl ester, N-bromoacetyl-glycylglycine ethyl ester, N-bromoacetyl-L-alanine ethyl ester were synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethylaminopropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene (cone 1) was synthesized according to the literature procedure.40
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethylaminoethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene (cone 2) was synthesized according to the literature procedure.40
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethylaminopropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate 3) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethylaminoethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene (1,3-alternate 4) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′,3′-trimethyl)-ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (cone 5) was synthesized according to the literature procedure.40
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′3′-dimethyl-3′-ethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (cone 6) was synthesized according to the literature procedure.41
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-benzyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 7) was synthesized according to the literature procedure.40
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(ethoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 8) was synthesized according to the literature procedure.41
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 10) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 11) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 12) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{3′′-propylphthalimide}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 13) was synthesized according to the literature procedure.41
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′-methyl-2′,2′-diethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (cone 14) was synthesized according to the literature procedure.40
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-{(ethoxycarbonylmethyl)amidocarbonylmethyl})ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 19) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 20) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 21) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 27) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 28) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 29) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-{(ethoxycarbonylmethyl)amidocarbonylmethyl})ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 36) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 37) was synthesized according to the literature procedure.38
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 38) was synthesized according to the literature procedure.38
General procedure for the synthesis of compounds 9, 15–18, 22–26, 31–35
The compounds 1–4 (0.10 g, 0.08 × 10−3 mol) were dissolved in 2 ml of acetonitrile in the round bottom flask equipped with magnetic stirrer and a reflux condenser. Iodomethane, iodoethane, ethyl bromoacetate, pentyl bromoacetate, or benzyl bromide (0.32 × 10−3 mol) was added. The reaction mixture was refluxed for 48 h. The solvent was removed under reduced pressure. The precipitate was dried under reduced pressure over phosphorus pentoxide.
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(pentoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 9). Yield: 0.12 g (90%), mp 106 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 0.87 (t, 3JHH = 6.9 Hz, 12H, CH2CH3), 1.08 (s, 36H, (CH3)3C), 1.27–1.31 (m, 16H, O(CH2)2CH2C2H5, O(CH2)3CH2CH3), 1.60 (m, 8H, OCH2CH2C3H7), 1.95 (m, 8H, –NCH2CH2CH2NH), 3.22–3.25 (s, 32H, (CH3)2N+, –NCH2CH2CH2NH), 3.60 (m, 8H, NCH2CH2CH2NH), 4.14 (t, 3JHH = 6.9 Hz, 8H, OCH2C4H9), 4.51 (s, 8H, N+CH2CO), 4.82 (s, 8H, OCH2CO), 7.39 (s, 8H, ArH), 8.56 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.23, 164.79, 157.94, 146.70, 134.42, 128.06, 74.25, 65.74, 62.44, 60.58, 51.01, 35.25, 33.90, 30.67, 27.45, 27.33, 22.53, 21.68, 13.82. El. anal. calcd for C96H156Br4N8O16S4: C 54.23%, H 7.40%, N 5.27%, S 6.03%. Found: C 54.48%, H 7.64%, N 5.52%, S 6.24%. MS (ESI): calcd for [M − 2Br−]2+ m/z = 983.2, [M − 3Br−]3+ m/z = 628.8, [M − 4Br−]4+ m/z = 451.6, found m/z = 983.4, 628.7, 451.6. IR νmax: 1663, 1742 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3349 (NH).
O), 2956, 3349 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′,2′-triethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (cone 15). Yield: 0.10 g (85%), mp 163 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.41 (t, 3JHH = 7.1 Hz, 36H, CH3CH2–), 3.57 (q, 3JHH = 7.1 Hz, 24H, –CH2CH3), 3.66 (m, 8H, –NCH2CH2NH), 3.94 (m, 8H, NCH2CH2NH), 4.99 (s, 8H, OCH2CO), 7.34 (s, 8H, ArH), 8.82 (t, 3JHH = 5.9 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 169.76, 157.13, 147.73, 134.91, 128.17, 73.99, 55.82, 54.15, 34.31, 33.68, 31.11, 8.29. El. anal. calcd for C80H132I4N8O8S4: C 48.78%, H 6.75%, N 5.69%, S 6.51%. Found: C 48.59%, H 6.66%, N 5.51%, S 6.42%. MS (ESI): calcd for [M − 2I−]2+ m/z = 858.0, [M − 3I−]3+ m/z = 529.7, [M − 4I−]4+ m/z = 365.5, found m/z = 857.9, 529.6, 365.4. IR νmax: 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3317 (NH).
O), 2955, 3317 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-benzyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 16). Yield: 0.12 g (91%), mp 139 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.10 (s, 36H, (CH3)3C), 1.44 (t, 3JHH = 6.7 Hz, 24H, CH3CH2–), 3.50 (q, 3JHH = 6.7 Hz, 16H, –CH2CH3), 3.63 (m, 8H, –NCH2CH2NH), 4.10 (m, 8H, NCH2CH2NH), 4.92 (s, 8H, N+CH2Ph), 5.03 (s, 8H, OCH2CO), 7.32 (s, 8H, ArH), 7.36–7.40 (m, 8H, Ar′H), 7.64 (m, 12H, Ar′H), 9.08 (t, 3JHH = 5.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.89, 157.44, 147.45, 134.85, 132.89, 130.61, 129.38, 128.30, 127.09, 73.98, 62.04, 55.80, 53.91, 34.27, 33.72, 31.11, 8.70. El. anal. calcd for C100H140Br4N8O8S4: C 59.16%, H 6.95%, N 5.52%, S 6.32%. Found: C 59.35%, H 6.72%, N 5.23%, S 6.47%. MS (ESI): calcd for [M − 2Br−]2+ m/z = 935.2, [M − 3Br−]3+ m/z = 596.8, [M − 4Br−]4+ m/z = 427.6, found m/z = 935.4, 596.7, 427.7. IR νmax: 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2960, 3320 (NH).
O), 2960, 3320 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(ethoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 17). Yield: 0.12 g (90%), mp 118 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.10 (s, 36H, (CH3)3C), 1.29 (t, 3JHH = 7.1 Hz, 12H OCH2CH3), 1.46 (t, 3JHH = 7.0 Hz, 24H, CH3CH2–), 3.88–4.03 (m, 32H, –CH2CH3, –NCH2CH2NH, 8H, NCH2CH2NH), 4.24 (q, 3JHH = 7.1 Hz, 8H, OCH2CH3). 4.75 (s, 8H, N+CH2CO), 4.97 (s, 8H, OCH2CO), 7.33 (s, 8H, ArH), 9.03 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.97, 164.50, 157.34, 147.56, 134.84, 128.27, 73.95, 62.90, 57.49, 56.71, 56.23, 34.26, 33.71, 31.08, 14.02, 8.56. El. anal. calcd for C88H140Br4N8O16S4: C 52.48%, H 6.53%, N 5.86%, S 6.70%. Found: C 52.65%, H 6.71%, N 6.01%, S 6.27%. MS (ESI): calcd for [M − 3Br−]3+ m/z = 591.3, [M − 4Br−]4+ m/z = 423.5, found m/z = 591.5, 423.5. IR νmax: 1666, 1740 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3322 (NH).
O), 2958, 3322 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(pentoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (cone 18). Yield: 0.11 g (90%), mp 135 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 0.88 (t, 3JHH = 6.8 Hz, 12H, O(CH2)4CH3), 1.09 (s, 36H, (CH3)3C), 1.26–1.33 (m, 16H, O(CH2)3CH2CH3, O(CH2)2CH2C2H5), 1.44 (t, 3JHH = 7.1 Hz, 24H, CH3CH2N+), 1.63 (m, 8H, OCH2CH2C3H7), 3.88–3.97 (m, 32H, –N+CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.14 (t, 3JHH = 6.8 Hz, 8H, OCH2C4H9), 4.70 (s, 8H, N+CH2CO), 4.93 (s, 8H, OCH2CO), 7.30 (s, 8H, ArH), 9.01 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.88, 164.55, 157.40, 147.51, 134.82, 128.22, 73.90, 66.91, 57.63, 56.76, 55.91, 34.25, 33.81, 31.09, 27.97, 27.75, 22.90, 13.91, 8.78. El. anal. calcd for C100H164Br4N8O16S4: C 55.04%, H 7.57%, N 5.13%, S 5.88%. Found: C 54.99%, H 7.53%, N 4.98%, S 5.63%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 465.6, found m/z = 465.6. IR νmax: 1670, 1741 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3320 (NH).
O), 2956, 3320 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′,3′-trimethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (1,3-alternate 22). Yield: 0.12 g (90%), mp 197 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.91 (m, 8H, –NCH2CH2CH2NH), 3.17 (s, 36H, (CH3)3N+), 3.32 (m, 8H, –NCH2CH2CH2NH), 3.60 (m, 8H, NCH2CH2CH2NH), 3.98 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.01 (t, 3JHH = 5.2 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.37, 157.02, 146.08, 132.95, 127.61, 70.96, 63.23, 52.23, 35.81, 33.89, 30.78, 22.86. El. anal. calcd for C72H116I4N8O8S4: C 46.55%, H 6.29%, N 6.03%, S 6.90%. Found: C 46.40%, H 5.89%, N 5.60%, S 6.00%. MS (MALDI TOF): calcd for [M − I−]+ m/z = 1730.7, found m/z = 1730.2. IR νmax: 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3292 (NH).
O), 2955, 3292 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-ethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (1,3-alternate 23). Yield: 0.14 g (95%), mp 215 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.24 (t, 3JHH = 7.1 Hz, 12H, N+CH2CH3), 1.88 (m, 8H, –NCH2CH2CH2NH), 3.01 (s, 24H, (CH3)2N+), 3.19 (m, 8H, –NCH2CH2CH2NH), 3.29 (m, 8H, NCH2CH2CH2NH), 3.35 (q, 3JHH = 7.2 Hz, 8H, N+CH2CH3), 3.99 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.03 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.37, 157.06, 146.04, 133.00, 127.56, 70.84, 60.23, 58.59, 49.60, 35.80, 33.88, 30.78, 22.44, 7.84. El. anal. calcd for C76H124I4N8O8S4: C 47.70%, H 6.53%, N 5.86%, S 6.70%. Found: C 47.53%, H 6.52%, N 5.56%, S 6.64%. MS (MALDI TOF): calcd for [M − I−]+ m/z = 1786.8, found m/z = 1786.5. IR νmax: 1653 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3335 (NH).
O), 2958, 3335 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-benzyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 24). Yield: 0.12 g (91%), mp 150 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (s, 36H, (CH3)3C), 2.05 (m, 8H, –NCH2CH2CH2NH), 3.00 (s, 24H, (CH3)2N+), 3.21 (m, 8H, –NCH2CH2CH2NH), 3.37 (m, 8H, NCH2CH2CH2NH), 4.01 (s, 8H, OCH2CO), 4.60 (s, 8H, N+CH2Ph), 7.52 (m, 12H, Ar′H), 7.59 (m, 8H, Ar′H), 7.60 (s, 8H, ArH), 8.10 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.44, 157.03, 146.06, 133.12, 132.95, 130.29, 128.89, 128.00, 127.57, 70.90, 65.91, 61.12, 49.22, 35.86, 33.87, 30.79, 22.63. El. anal. calcd for C96H132Br4N8O8S4: C 58.41%, H 6.74%, N 5.68%, S 6.50%. Found: C 58.75%, H 6.62%, N 6.01%, S 6.37%. MS (MALDI TOF): calcd for [M − Br−]+ m/z = 1245.7, found m/z = 1245.8. IR νmax: 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3287 (NH).
O), 2955, 3287 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(ethoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 25). Yield: 0.13 g (92%), mp 123 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (s, 36H, (CH3)3C), 1.25 (t, 3JHH = 7.1 Hz, 12H, OCH2CH3) 1.91 (m, 8H, –NCH2CH2CH2NH), 3.17 (m, 8H, –NCH2CH2CH2NH), 3.22 (s, 24H, (CH3)2N+), 4.00 (s, 8H, OCH2CO), 4.55 (m, 8H, NCH2CH2CH2NH), 4.24 (q, 3JHH = 7.2 Hz, 8H, N+CH2CH3). 4.47 (s, 8H, N+CH2CO), 7.60 (s, 8H, ArH), 8.05 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.39, 164.85, 157.40, 146.19, 133.26, 127.82, 70.89, 62.30, 62.00, 60.54, 51.12, 35.69, 33.85, 30.77, 22.44, 13.85. El. anal. calcd for C84H132Br4N8O16S4: C 51.53%, H 6.80%, N 5.72%, S 6.55%. Found: C 51.49%, H 6.58%, N 5.55%, S 6.64%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 409.6, found m/z = 409.5. IR νmax: 1666, 1742 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3300 (NH).
O), 2958, 3300 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(pentoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 26). Yield: 0.13 g (92%), mp 103 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.87 (t, 3JHH = 6.9 Hz, 12H CH2CH3), 1.20 (s, 36H, (CH3)3C), 1.27–1.33 (m, 16H, O(CH2)2CH2C2H5, O(CH2)3CH2CH3), 1.62 (m, 8H, OCH2CH2C3H7), 1.92 (m, 8H, –NCH2CH2CH2NH), 3.18 (m, 8H, –NCH2CH2CH2NH), 3.35 (s, 24H, (CH3)2N+), 3.56 (m, 8H, NCH2CH2CH2NH), 3.98 (s, 8H, OCH2CO), 4.17 (t, 3JHH = 6.6 Hz, 4H, OCH2C4H9) 4.50 (s, 8H, N+CH2CO), 7.60 (s, 8H, ArH), 8.06 (t, 3JHH = 5.4 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.37, 164.77, 157.10, 146.01, 133.03, 127.54, 70.94, 65.80, 62.36, 60.53, 51.12, 35.70, 33.84, 30.78, 27.44, 27.36, 22.55, 21.66, 13.79. El. anal. calcd for C96H156Br4N8O16S4: C 54.23%, H 7.40%, N 5.27%, S 6.03%. Found: C 54.12%, H 7.54%, N 5.38%, S 5.96%. MS (ESI): calcd for [M − 2Br−]2+ m/z = 983.2, [M − 3Br−]3+ m/z = 628.8, [M − 4Br−]4+ m/z = 451.6, found m/z = 982.4, 628.9, 451.6. IR νmax: 1665, 1742 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2954, 3300 (NH).
O), 2954, 3300 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′-methyl-2′,2′-diethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (1,3-alternate 31). Yield: 0.13 g (95%), mp 156 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.22 (s, 36H, (CH3)3C), 1.27 (t, 3JHH = 6.5 Hz, 24H, (CH3CH2–)), 2.98 (s, 12H, (CH3)N+), 3.21 (m, 8H, –NCH2CH2NH), 3.39 (q, 3JHH = 6.5 Hz, 16H, –CH2CH3), 3.49 (m, 8H, NCH2CH2NH), 4.05 (s, 8H, OCH2CO), 7.61 (s, 8H, ArH), 8.18 (t, 3JHH = 5.5 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.03, 156.80, 146.08, 132.95, 127.51, 76.13, 70.51, 65.99, 62.12, 56.83, 56.16, 47.02, 33.91, 32.13, 30.87, 26.70, 25.36, 7.46. El. anal. calcd for C76H124I4N8O8S4: C 47.70%, H 6.53%, N 5.86%, S 6.70%. Found: C 47.54%, H 5.93%, N 6.16%, S 6.17%. MS (ESI): calcd for [M − 2I−]2+ m/z = 829.3, [M − 3I−]3+ m/z = 510.3, [M − 4I−]4+ m/z = 351.2, found m/z = 829.3, 510.6, 351.4. IR νmax: 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3300 (NH).
O), 2955, 3300 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′,2′-triethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetraiodide (1,3-alternate 32). Yield: 0.11 g (87%), mp 173 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.22 (s, 36H, (CH3)3C), 1.23 (t, 3JHH = 7.1 Hz, 36H, CH3CH2–), 3.15 (m, 8H, –NCH2CH2NH), 3.31 (q, 3JHH = 7.1 Hz, 24H, –CH2CH3), 3.47 (m, 8H, NCH2CH2NH), 4.03 (s, 8H, OCH2CO), 7.61 (s, 8H, ArH), 8.13 (t, 3JHH = 5.3 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.98, 156.74, 146.14, 132.94, 127.58, 70.46, 53.53, 52.46, 33.91, 31.84, 30.84, 7.04. El. anal. calcd for C80H132I4N8O8S4: C 48.78%, H 6.75%, N 5.69%, S 6.51%. Found: C 49.02%, H 6.58%, N 5.73%, S 6.37%. MS (ESI): calcd for [M − 4I−]4+ m/z = 365.6, found m/z = 365.5. IR νmax: 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3261 (NH).
O), 2955, 3261 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(benzyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 33). Yield: 0.12 g (90%), mp 131 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.19 (s, 36H, (CH3)3C), 1.39 (t, 3JHH = 7.1 Hz, 24H, CH3CH2–), 3.16 (m, 8H, NCH2CH2NH), 3.25 (q, 3JHH = 7.1 Hz, 16H, –CH2CH3), 3.62 (m, 8H, NCH2CH2NH), 4.20 (s, 8H, OCH2CO), 4.60 (s, 8H, N+CH2Ph), 7.53 (m, 8H, Ar′H), 7.58 (m, 12H, Ar′H), 7.60 (s, 8H, ArH), 8.38 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.07, 157.35, 145.80, 133.44, 132.63, 130.46, 129.15, 127.49, 127.18, 70.84, 60.55, 54.14, 53.19, 33.87, 32.07, 30.89, 7.57. El. anal. calcd for C100H140Br4N8O8S4: C 59.16%, H 6.95%, N 5.52%, S 6.32%. Found: C 58.95%, H 7.05%, N 5.28%, S 6.13%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 427.6, found m/z = 427.3. IR νmax: 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2954, 3194 (NH).
O), 2954, 3194 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(ethoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 34). Yield: 0.12 g (90%), mp 115 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.22 (s, 36H, (CH3)3C), 1.26 (t, 3JHH = 7.1 Hz, 12H, OCH2CH3), 1.28 (t, 3JHH = 7.0 Hz, 24H, CH3CH2–), 3.45–3.65 (m, 32H, –CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.04 (s, 8H, OCH2CO), 4.24 (q, 3JHH = 7.1 Hz, 8H, OCH2CH3), 4.48 (s, 8H, N+CH2CO), 7.60 (s, 8H, ArH), 8.27 (t, 3JHH = 4.9 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.03, 164.24, 156.99, 145.96, 133.08, 127.38, 70.57, 62.13, 55.83, 55.54, 55.08, 33.86, 32.00, 30.87, 13.76, 7.41. El. anal. calcd for C88H140Br4N8O16S4: C 52.48% H 6.53%, N 5.86%, S 6.70%. Found: C 52.64%, H 6.73%, N 6.06%, S 6.33%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 423.2, [M − 3Br−]3+ m/z = 591.4, [M − 2Br−]2+ m/z = 927.0, found m/z = 423.5, 591.5, 926.8. IR νmax: 1669, 1741 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2957, 3187 (NH).
O), 2957, 3187 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(pentoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 35). Yield: 0.14 g (94%), mp 88 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.87 (t, 3JHH = 6.6 Hz, 12H, –O(CH2)4CH3), 1.21 (s, 36H, (CH3)3C), 1.25–1.32 (m, 16H, O(CH2)3CH2CH3, O(CH2)2CH2C2H5), 1.28 (t, 3JHH = 7.0 Hz, 24H, (CH3CH2N+–)), 1.63 (m, 8H, OCH2CH2C3H7), 3.45–3.63 (m, 32H, –N+CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.08 (s, 8H, OCH2CO), 4.18 (t, 3JHH = 6.4 Hz, 8H, OCH2C4H9), 4.51 (s, 8H, N+CH2CO), 7.60 (s, 8H, ArH), 8.29 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.93, 164.44, 157.02, 145.20, 133.11, 127.37, 70.58, 65.69, 55.87, 55.52, 55.05, 33.86, 31.53, 30.87, 27.43, 27.35, 21.66, 13.80, 7.95. El. anal. calcd for C100H164Br4N8O16S4: C 55.04%, H 7.57%, N 5.13%, S 5.88%. Found: C 54.96%, H 7.68%, N 5.11%, S 5.94%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 465.6, found m/z = 465.6. IR νmax: 1669, 1741 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3300 (NH).
O), 2955, 3300 (NH).
Procedure for the synthesis of compound 30
The compound 3 (0.10 g, 0.08 × 10−3 mol) was dissolved in 10 ml of acetonitrile in the round bottom flask equipped with magnetic stirrer and a reflux condenser. N-(3-Bromopropyl)phthalimide (0.32 × 10−3 mol) was added. The reaction mixture was refluxed for 8 h. The solvent was removed under reduced pressure. The precipitate was dried under reduced pressure over phosphorus pentoxide.
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{3′′-propylphthalimide}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetrabromide (1,3-alternate 30). Yield: 0.14 g (98%), mp 154 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (s, 36H, (CH3)3C), 1.88 (m, 8H, CH2CH2CH2Pht), 2.06 (m, 8H, NHCH2CH2CH2N+), 3.00 (s, 24H, (CH3)2N+), 3.17 (m, 8H, NHCH2CH2CH2N+) 3.30 (m, 8H, CH2CH2CH2Pht), 3.56 (m, 8H, NHCH2CH2CH2N+), 3.70 (m, 8H, CH2CH2CH2Pht), 3.99 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 7.82–7.89 (m, 16H, Pht), 8.05 (t, 3JHH = 5.3 Hz, 4H, NHCH2CH2CH2N+). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.94, 167.21, 146.00, 134.42, 134.15, 131.66, 127.64, 123.10, 122.96, 58.56, 49.88, 36.15, 34.69, 33.85, 31.89, 31.10, 30.55, 21.60, 20.73. El. anal. calcd for C112H144Br4N12O16S4: C 56.94%, H 6.14%, N 7.12%, S 5.43%, Br 13.53%. Found: C 56.91%, H 5.85%, N 6.04%, S 5.46%, Br 13.65%. MS (ESI): calcd for [M − 4Br−]4+ m/z = 510.7, found m/z = 510.6. IR νmax: 1667, 1705 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3315 (N-H).
O), 2956, 3315 (N-H).
General procedure for the synthesis of compounds 39–72
The compounds 5–38 (0.10 g) were dissolved in 2 ml of water in the round bottom flask equipped with magnetic stirrer. Lithium bis(trifluoromethylsulfonyl)imide was added in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 taken for each ammonium group. The reaction mixture was stirred for 24 h. The precipitate was filtered and dried under reduced pressure over phosphorus pentoxide.
1 taken for each ammonium group. The reaction mixture was stirred for 24 h. The precipitate was filtered and dried under reduced pressure over phosphorus pentoxide.
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′,3′-trimethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 39). Yield: 0.09 g (91%), mp 87 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.08 (s, 36H, (CH3)3C), 1.89 (m, 8H, –NCH2CH2CH2NH), 3.05 (s, 36H, (CH3)3N+), 3.25 (m, 8H, –NCH2CH2CH2NH), 3.27 (m, 8H, NCH2CH2CH2NH), 4.82 (s, 8H, OCH2CO), 7.40 (s, 8H, ArH), 8.49 (t, 3JHH = 5.2 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.26, 157.99, 146.80, 134.42, 128.02, 119.96 (q), 74.22, 63.40, 52.29, 35.38, 33.91, 30.69, 22.83. El. anal. calcd for C80H116F24N12O24S12: C 38.89%, H 4.73%, N 6.80%, S 15.57%. Found: C 39.03%, H 5.02%, N 6.53%, S 15.12%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2188.5, found m/z = 2189.4. IR νmax: 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3327 (NH).
O), 2965, 3327 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-ethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 40). Yield: 0.10 g (93%), mp 71 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.08 (s, 36H, (CH3)3C), 1.22 (t, 3JHH = 7.0 Hz, 12H, N+CH2CH3) 1.87 (m, 8H, –NCH2CH2CH2NH), 2.97 (s, 24H, (CH3)2N+), 3.23 (m, 8H, –NCH2CH2CH2NH), 3.29 (q, 3JHH = 7.2 Hz, 8H, N+CH2CH3), 3.32 (m, 8H, NCH2CH2CH2NH), 4.82 (s, 8H, OCH2CO), 7.40 (s, 8H, ArH), 8.49 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.24, 157.94, 146.78, 134.43, 127.99, 119.67 (q), 74.17, 60.33, 58.65, 49.53, 35.39, 33.91, 30.67, 22.35, 7.74. El. anal. calcd for C84H124F24N12O24S12: C 39.93%, H 4.95%, N 6.65%, S 15.23%. Found: C 39.37%, H 5.00%, N 6.76%, S 14.89%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2244.6, found m/z = 2244.8. IR νmax: 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3328 (NH).
O), 2965, 3328 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-benzyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 41). Yield: 0.10 g (93%), mp 56 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.10 (s, 36H, (CH3)3C), 2.23 (m, 8H, –NCH2CH2CH2NH), 2.98 (s, 24H, (CH3)2N+), 3.50 (m, 8H, –NCH2CH2CH2NH), 3.53 (m, 8H, NCH2CH2CH2NH), 4.42 (s, 8H, N+CH2CO), 4.91 (s, 8H, OCH2CO), 7.35 (s, 8H, ArH), 7.43 (m, 20H, Ar′H), 8.29 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.24, 157.30, 147.80, 134.97, 132.85, 131.06, 129.43, 127.87, 126.49, 119.94 (q), 74.26, 68.37, 63.07, 49.46, 36.20, 34.38, 31.04, 23.01. El. anal. calcd for C104H132F24N12O24S12: C 45.01%, H 4.79%, N 6.06%, S 13.87%. Found: C 45.30%, H 4.69%, N 5.84%, S 14.04%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2492.6, found m/z = 2493.3. IR νmax: 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2961, 3328 (NH).
O), 2961, 3328 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(ethoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 42). Yield: 0.11 g (95%), mp 45 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.30 (t, 3JHH = 7.1 Hz, 12H, OCH2CH3), 2.14 (m, 8H, –NCH2CH2CH2NH), 3.34 (s, 24H, (CH3)2N+), 3.47 (m, 8H, –NCH2CH2CH2NH), 3.72 (m, 8H, NCH2CH2CH2NH), 4.23 (s, 8H, N+CH2CO), 4.26 (q, 3JHH = 7.0 Hz, 8H, N+CH2CH3), 4.89 (s, 8H, OCH2CO), 7.35 (s, 8H, ArH), 8.23 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.12, 164.07, 157.27, 147.82, 134.87, 119.67 (q), 74.26, 64.50, 62.95, 56.57, 51.42, 35.93, 34.28, 31.05, 29.81, 22.82, 13.71. El. anal. calcd for C92H132F24N12O32S12: C 40.05%, H 4.82%, N 6.09%, S 13.95%. Found: C 39.66%, H 4.81%, N 5.77%, S 13.66%. MS (MALDI TOF): calcd for [M− N−(SO2CF3)2]+ m/z = 2478.7, found m/z = 2480.0. IR νmax: 1669, 1747 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3328 (NH).
O), 2965, 3328 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(pentoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 43). Yield: 0.12 g (94%), mp 35 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.87 (t, 3JHH = 6.7 Hz, 12H, O(CH2)4CH3), 1.08 (s, 36H, (CH3)3C), 1.24–1.36 (m, 16H, O(CH2)3CH2CH3, O(CH2)2CH2C2H5), 1.57 (m, 8H, OCH2CH2C3H7), 1.94 (m, 8H, –NCH2CH2CH2NH), 3.16 (s, 24H, (CH3)2N+), 3.49 (m, 8H, –NCH2CH2CH2NH), 3.54 (m, 8H, NCH2CH2CH2NH), 4.14 (t, 3JHH = 6.5 Hz, 8H, OCH2C4H9), 4.42 (s, 8H, N+CH2CO), 4.79 (s, 8H, OCH2CO), 7.39 (s, 8H, ArH), 8.47 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.26, 164.71, 158.00, 146.79, 134.42, 127.99, 119.66 (q), 74.21, 65.76, 62.35, 60.58, 51.11, 35.22, 33.89, 30.64, 27.43, 27.34, 22.49, 21.66, 13.77. El. anal. calcd for C104H156F24N12O32S12: C 42.67%, H 5.37%, N 5.74%, S 13.15%. Found: C 42.58%, H 5.36%, N 5.55%, S 13.00%. MS (ESI): calcd for [M − 2N−(SO2CF3)2]2+ m/z = 1183.4, [M − 3N−(SO2CF3)2]3+ m/z = 695.0, [M − 4N−(SO2CF3)2]4+ m/z = 451.6, found m/z = 1183.0, 695.3, 451.5. IR νmax: 1669, 1747 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3329 (NH).
O), 2962, 3329 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 44). Yield: 0.13 g (97%), mp 63 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.26 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 2.17 (m, 8H, NHCH2CH2CH2N+), 3.31 (s, 12H, (CH3)2N+), 3.47 (m, 8H, NHCH2CH2CH2N+), 3.62 (m, 8H, NHCH2CH2CH2N+), 3.97 (d, 3JHH = 5.5 Hz, 8H, NHCH2CO), 4.10 (s, 8H, N+CH2CO), 4.15 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.89 (s, 8H, OCH2CO), 7.35 (s, 8H, ArH), 7.77 (t, 3JHH = 5.8 Hz, 4H, NHCH2CH2CH2N+), 8.23 (br. s, 4H, NHCH2CO). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.35, 168.48, 162.93, 157.12, 147.89, 135.02, 127.69, 119.75 (q), 74.26, 64.84, 62.56, 61.60, 51.98, 41.34, 35.99, 34.34, 31.04, 22.96, 14.03. El. anal. calcd for C100H144F24N16O36S12: C 40.21%, H 4.86%, N 7.50%, S 12.88%. Found: C 40.12%, H 5.12%, N 7.53%, S 12.47%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 715.5, [M − 4N−(SO2CF3)2]4+ m/z = 466.6, found m/z = 715.3, 466.6. IR νmax: 1670, 1743 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3354 (N-H).
O), 2966, 3354 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 45). Yield: 0.14 g (96%), mp 69 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.08 (s, 36H, (CH3)3C), 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.93 (m, 8H, NHCH2CH2CH2N+), 3.16 (s, 12H, (CH3)2N+), 3.24 (m, 8H, NHCH2CH2CH2N+), 3.47 (m, 8H, NHCH2CH2CH2N+), 3.86 (d, 3JHH = 5.7 Hz, 8H, NHCH2CO), 3.87 (d, 3JHH = 6.1 Hz, 8H, NHCH2CO), 4.06 (s, 8H, N+CH2CO), 4.09 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.80 (s, 8H, OCH2CO), 7.40 (s, 8H, ArH), 8.50 (br. s, 16H, NHCH2CH2CH2N+, NHCH2CO), 8.82 (t, 3JHH = 5.9 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 169.75, 168.51, 163.04, 157.72, 146.88, 134.43, 127.82, 119.48 (q), 73.34, 62.73, 62.02, 60.50, 54.78, 51.23, 41.53, 40.59, 35.36, 33.85, 30.68, 22.52, 13.97. El. anal. calcd for C108H156F24N20O40S12: C 40.34%, H 4.89%, N 8.71%, S 11.97%. Found: C 41.00%, H 4.71%, N 7.58%, S 12.41%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 523.7, found m/z = 523.6. IR νmax: 1663 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3342 (N-H).
O), 2965, 3342 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 46). Yield: 0.12 g (90%), mp 56 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.08 (s, 36H, (CH3)3C), 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.31 (d, 3JHH = 7.3 Hz, 12H, CH3CH), 1.92 (m, 8H, NHCH2CH2CH2N+), 3.16 (s, 12H, (CH3)2N+), 3.23 (m, 8H, NHCH2CH2CH2N+), 3.48 (m, 8H, NHCH2CH2CH2N+), 4.06 (s, 8H, N+CH2CO), 4.09 (m, 8H, CH3CH2O–), 4.26 (m, 4H, CH3CH), 4.80 (s, 8H, OCH2CO), 7.39 (s, 8H, ArH), 8.50 (t, 3JHH = 5.4 Hz, 4H, NHCH2CH2CH2N+), 9.02 (d, 3JHH = 6.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 171.45, 167.98, 163.20, 158.28, 146.99, 134.03, 127.79, 119.49 (q), 74.03, 62.43, 61.69, 60.83, 51.16, 47.75, 35.12, 33.52, 30.36, 22.74, 16.63, 13.51. El. anal. calcd for C104H152F24N16O36S12: C 41.05%, H 5.03%, N 7.36%, S 12.64%. Found: C 40.51%, H 4.69%, N 6.16%, S 13.32%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 480.5. IR νmax: 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3353 (N-H).
O), 2966, 3353 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{3′′-propylphthalimide}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 47). Yield: 0.13 g (93%), mp 83 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.07 (s, 36H, (CH3)3C), 1.88 (m, 8H, CH2CH2CH2Pht), 2.05 (m, 8H, NHCH2CH2CH2N+), 2.98 (s, 24H, (CH3)2N+), 3.22–3.30 (m, 16H, NHCH2CH2CH2N, CH2CH2CH2Pht), 3.37 (m, 8H, NHCH2CH2CH2N+), 3.67 (t, 3JHH = 5.9 Hz, 8H, CH2CH2CH2Pht), 4.80 (s, 8H, OCH2CO), 7.38 (s, 8H, ArH), 7.80–7.88 (m, 16H, Pht), 8.50 (t, 3JHH = 5.2 Hz, 4H, NHCH2CH2CH2N+). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.22, 168.01, 158.00, 146.77, 134.42, 131.65, 128.03, 123.03, 119.52 (q), 74.24, 61.22, 60.96, 50.07, 35.37, 34.58, 33.91, 30.68, 22.44, 21.64. El. anal. calcd for C120H144F24N16O32S12: C 45.56%, H 4.59%, N 7.08%, S 12.16%. Found: C 46.16%, H 4.45%, N 6.61%, S 12.79%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 480.6. IR νmax: 1668, 1708, 1772 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2963, 3334 (N-H).
O), 2963, 3334 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′-methyl-2′,2′-diethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 48). Yield: 0.12 g (90%), mp 72 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.32 (t, 3JHH = 7.2 Hz, 24H, CH3CH2–), 3.03 (s, 12H, (CH3)N+), 3.38 (q, 3JHH = 7.2 Hz, 16H, –CH2CH3), 3.48 (t, 3JHH = 6.3 Hz, 8H, –NCH2CH2NH), 3.85 (m, 8H, NCH2CH2NH), 4.91 (s, 8H, OCH2CO), 7.36 (s, 8H, ArH), 8.36 (br. s, 8H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.70, 156.74, 148.21, 135.01, 127.79, 119.72 (q), 73.88, 59.11, 56.99, 47.52, 34.35, 32.94, 31.02, 7.62. El. anal. calcd for C84H124F24N12O24S12: C 39.93%, H 4.95%, N 6.65%, S 15.23%. Found: C 40.05%, H 5.06%, N 6.78%, S 15.02%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 562.0, found m/z = 561.9. IR νmax: 1667 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3315 (NH).
O), 2965, 3315 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′,2′-triethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 49). Yield: 0.12 g (95%), mp 68 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.30 (t, 3JHH = 7.1 Hz, 36H, CH3CH2–), 3.33 (q, 3JHH = 7.1 Hz, 24H, –CH2CH3), 3.40 (m, 8H, –NCH2CH2NH), 3.77 (m, 8H, NCH2CH2NH), 4.91 (s, 8H, OCH2CO), 7.36 (s, 8H, ArH), 8.40 (br. s, 8H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.75, 156.70, 148.16, 135.01, 127.83, 119.75 (q), 73.85, 55.03, 53.51, 34.34, 32.66, 31.02, 7.28. El. anal. calcd for C88H132F24N12O24S12: C 40.92%, H 5.15%, N 6.51%, S 14.90%. Found: C 41.11%, H 5.13%, N 6.58%, S 14.67%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 365.6, [M − 3N−(SO2CF3)2]3+ m/z = 580.7, found m/z = 365.7, 580.5. IR νmax: 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2963, 3314 (NH).
O), 2963, 3314 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-benzyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 50). Yield: 0.12 g (94%), mp 60 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.10 (s, 36H, (CH3)3C), 1.39 (t, 3JHH = 7.1 Hz, 24H, (CH3CH2–)), 3.24 (q, 3JHH = 6.9 Hz, 16H, –CH2CH3), 3.39 (t, 3JHH = 7.1 Hz, 8H, –NCH2CH2NH), 3.93 (m, 8H, NCH2CH2NH), 4.43 (s, 8H, N+CH2Ph), 4.95 (s, 8H, OCH2CO), 7.35 (s, 8H, ArH), 7.41–7.46 (m, 20H, Ar′H). 8.51 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.90, 156.78, 148.04, 135.0, 132.36, 131.06, 129.62, 127.82, 126.19, 119.74 (q), 73.85, 61.76, 55.18, 53.70, 34.18, 32.78, 31.02, 7.83. El. anal. calcd for C108H140F24N12O24S12: C 45.82%, H 4.98%, N 5.94%, S 13.59%. Found: C 45.86%, H 5.23%, N 6.12%, S 13.44%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 427.6, found m/z = 427.4. IR νmax: 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3330 (NH).
O), 2967, 3330 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(ethoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 51). Yield: 0.12 g (92%), mp 43 °C. 1H NMR (400 MHz, 298 K, CDCl3) δ: 1.11 (s, 36H, (CH3)3C), 1.25 (m, 12H, OCH2CH3), 1.33 (t, 3JHH = 7.2 Hz, 24H, CH3CH2–), 3.66 (m, 16H, –CH2CH3), 3.73 (m, 8H, –NCH2CH2NH), 3.83 (m, 8H, NCH2CH2NH), 4.14 (m, 8H, OCH2CH3), 4.19 (s, 8H, N+CH2CO), 4.90 (s, 8H, OCH2CO), 7.36 (s, 8H, ArH), 8.36 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, CDCl3) δ: 169.90, 163.93, 156.72, 148.19, 142.94, 139.0, 135.06, 127.73, 119.89 (q), 73.89, 62.97, 56.99, 55.74, 34.40, 32.92, 31.02, 13.68, 7.60. El. anal. calcd for C96H140F24N12O32S12: C 40.96%, H 5.01%, N 5.97%, S 13.67%. Found: C 41.11%, H 4.89%, N 6.01%, S 13.45%. MS (ESI): calcd for [M − 2N−(SO2CF3)2]2+ m/z = 1127.3, [M − 3N−(SO2CF3)2]3+ m/z = 657.6, [M − 4N−(SO2CF3)2]4+ m/z = 423.6, found m/z = 1126.9, 657.6, 423.5. IR νmax: 1669, 1748 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3328 (NH).
O), 2962, 3328 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(pentoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 52). Yield: 0.13 g (93%), mp 39 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.86 (t, 3JHH = 6.8 Hz, 12H, O(CH2)4CH3), 1.08 (s, 36H, (CH3)3C), 1.21–1.32 (m, 16H, O(CH2)3CH2CH3, O(CH2)2CH2C2H5), 1.26 (t, 3JHH = 7.1 Hz, 24H, CH3CH2N+), 1.57 (m, 8H, OCH2CH2C3H7), 3.52–3.60 (m, 32H, –N+CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.10 (t, 3JHH = 6.8 Hz, 8H, OCH2C4H9), 4.44 (s, 8H, N+CH2CO), 4.85 (s, 8H, OCH2CO), 7.40 (s, 8H, ArH), 8.78 (t, 3JHH = 5.1 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.96, 164.72, 157.65, 147.04, 134.59, 127.87, 119.26 (q), 74.22, 65.87, 55.90, 55.42, 55.98, 33.91, 32.14, 30.61, 27.40, 27.31, 21.66, 13.77, 7.36. El. anal. calcd for C108H164F24N12O32S12: C 43.48%, H 5.54%, N 5.63%, S 12.90%. Found: C 43.52%, H 5.63%, N 5.55%, S 12.72%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 465.6, found m/z = 465.8. IR νmax: 1672, 1746 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3313 (NH).
O), 2962, 3313 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-{(ethoxycarbonylmethyl)amidocarbonylmethyl})ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 53). Yield: 0.13 g (95%), mp 66 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.07 (s, 36H, (CH3)3C), 1.28 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.28 (t, 3JHH = 7.0 Hz, 24H, CH3CH2N+), 3.52 (q, 3JHH = 7.0 Hz, 8H, CH3CH2N+), 3.55 (m, 8H, NHCH2CH2N+), 3.62 (m, 8H, NHCH2CH2N+), 3.93 (d, 3JHH = 5.6 Hz, 8H, NHCH2CO), 4.10 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.11 (s, 8H, N+CH2CO), 4.83 (s, 8H, OCH2CO), 7.39 (s, 8H, ArH), 8.76 (t, 3JHH = 5.7 Hz, 4H, NHCH2CH2N+), 9.06 (t, 3JHH = 5.6 Hz, 4H, NHCH2CO). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 169.06, 168.99, 163.58, 157.58, 146.81, 134.56, 127.96, 119.43 (q), 74.14, 60.78, 56.34, 56.08, 55.09, 40.67, 34.04, 32.18, 30.55, 13.98, 7.35. El. anal. calcd for C108H160F24N16O36S12: C 41.85%, H 5.20%, N 7.23%, S 12.42%. Found: C 42.11%, H 4.92%, N 7.33%, S 12.12%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 480.6. IR νmax: 1180 (COC), 1676 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3353 (N-H).
O), 2965, 3353 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 54). Yield: 0.14 g (94%), mp 64 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.07 (s, 36H, (CH3)3C), 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.28 (t, 3JHH = 6.7 Hz, 24H, CH3CH2N+), 3.50–3.58 (m, 24H, CH3CH2N+, NHCH2CH2N+), 3.62 (m, 8H, NHCH2CH2N+), 3.84 (d, 3JHH = 5.9 Hz, 8H, NHCH2CO), 3.87 (d, 3JHH = 6.0 Hz, 8H, NHCH2CO), 4.08 (s, 8H, N+CH2CO), 4.10 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O), 4.83 (s, 8H, OCH2CO), 7.45 (s, 8H, ArH), 8.50 (t, 3JHH = 5.9 Hz, 4H, NHCH2CH2N+), 8.74 (br. s, 4H, CONH), 8.87 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 170.10, 169.34, 168.80, 163.67, 158.08, 147.57, 135.05, 128.46, 119.57 (q), 74.54, 60.93, 56.99, 56.49, 55.58, 41.87, 41.05, 34.41, 32.68, 31.14, 14.41, 7.89. El. anal. calcd for C112H164F24N20O40S12: C 41.12%, H 5.05%, N 8.56%, S 11.76%. Found: C 40.99%, H 5.07%, N 8.66%, S 11.54%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 810.3, [M − 4N−(SO2CF3)2]4+ m/z = 537.6, found m/z = 810.3, 537.8. IR νmax: 1182 (COC), 1665 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3332 (N-H).
O), 2966, 3332 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (cone 55). Yield: 0.12 g (92%), mp 63 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.08 (s, 36H, (CH3)3C), 1.18 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.30 (d, 3JHH = 7.3 Hz, 12H, CH3CH), 1.31 (t, 3JHH = 7.2 Hz, 24H, CH3CH2N+), 3.48–3.61 (m, 24H, NHCH2CH2N+, N+CH2CH3), 3.63 (m, 8H, NHCH2CH2N+), 4.09 (s, 8H, N+CH2CO), 4.11 (m, 8H, CH3CH2O–), 4.27 (m, 4H, CH3CH), 4.84 (s, 8H, OCH2CO), 7.40 (s, 8H, ArH), 8.81 (br. s, 4H, NHCH2CH2N+), 9.23 (d, 3JHH = 6.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 171.74, 168.94, 162.97, 157.56, 146.83, 134.55, 127.82, 119.46 (q), 73.98, 60.75, 56.15, 55.17, 55.07, 47.64, 33.95, 32.19, 30.55, 16.56, 13.96, 7.35. El. anal. calcd for C108H160F24N16O36S12: C 41.85%, H 5.20%, N 7.23%, S 12.42%. Found: C 42.01%, H 5.17%, N 7.43%, S 12.17%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 494.6, found m/z = 494.3. IR νmax: 1183 (COC), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3347 (N-H).
O), 2966, 3347 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′,3′-trimethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 56). Yield: 0.11 g (95%), mp 106 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.90 (m, 8H, –NCH2CH2CH2NH), 3.06 (s, 36H, (CH3)3N+), 3.18 (m, 8H, –NCH2CH2CH2NH), 3.31 (m, 8H, NCH2CH2CH2NH), 4.00 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.03 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.09, 157.35, 145.86, 133.12, 127.57, 119.75 (q), 70.93, 63.31, 52.37, 35.76, 33.95, 30.58, 22.94. El. anal. calcd for C80H116F24N12O24S12: C 38.89%, H 4.73%, N 6.80%, S 15.57%. Found: C 39.03%, H 4.91%, N 6.73%, S 15.72%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2190.4, found m/z = 2190.1. IR νmax: 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3313 (NH).
O), 2966, 3313 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-ethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 57). Yield: 0.12 g (96%), mp 96 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.24 (t, 3JHH = 7.0 Hz, 12H, N+CH2CH3), 1.87 (m, 8H, –NCH2CH2CH2NH), 2.99 (s, 24H, (CH3)2N+), 3.19 (m, 8H, –NCH2CH2CH2NH), 3.24 (m, 8H, NCH2CH2CH2NH), 3.33 (q, 3JHH = 7.1 Hz, 8H, N+CH2CH3), 4.00 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.03 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.18, 157.05, 145.99, 133.07, 127.45, 119.90 (q), 73.72, 60.28, 58.67, 49.44, 35.92, 33.85, 30.75, 22.43, 7.76. El. anal. calcd for C84H124F24N12O24S12: C 39.93%, H 4.95%, N 6.65%, S 15.23%. Found: C 39.74%, H 5.10%, N 6.78%, S 15.25%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2244.6, found m/z = 2244.8. IR νmax: 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3310 (NH).
O), 2966, 3310 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(3′,3′-dimethyl-3′-benzyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 58). Yield: 0.11 g (94%), mp 87 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (s, 36H, (CH3)3C), 2.04 (m, 8H, –NCH2CH2CH2NH), 2.97 (s, 24H, (CH3)2N+), 3.22 (m, 8H, –NCH2CH2CH2NH), 3.27 (m, 8H, NCH2CH2CH2NH), 4.03 (s, 8H, OCH2CO), 4.53 (s, 8H, N+CH2CO), 7.51–7.57 (m, 20H, Ar′H), 7.60 (s, 8H, ArH), 8.07 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.62, 157.18, 145.98, 133.09, 132.91, 130.34, 128.93, 127.91, 127.49, 119.34 (q), 71.01, 66.16, 61.13, 49.32, 35.85, 33.85, 30.76, 22.63. El. anal. calcd for C104H132F24N12O24S12: C 45.01%, H 4.79%, N 6.06%, S 13.87%. Found: C 44.96%, H 4.99%, N 5.90%, S 13.62%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2495.8, found m/z = 2495.3. IR νmax: 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3374 (NH).
O), 2965, 3374 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(ethoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 59). Yield: 0.11 g (94%), mp 53 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (s, 36H, (CH3)3C), 1.25 (t, 3JHH = 7.1 Hz, 12H, OCH2CH3) 1.92 (m, 8H, –NCH2CH2CH2NH), 3.18 (m, 8H, –NCH2CH2CH2NH), 3.20 (s, 24H, (CH3)2N+), 3.51 (m, 8H, NCH2CH2CH2NH), 4.00 (s, 8H, OCH2CO), 4.23 (q, 3JHH = 7.2 Hz, 8H, N+CH2CH3), 4.42 (s, 8H, N+CH2CO), 7.59 (s, 8H, ArH), 8.02 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.40, 164.74, 157.11, 146.00, 132.97, 127.54, 119.46 (q), 70.93, 62.32, 61.86, 60.54, 51.21, 35.68, 33.83, 30.73, 22.52, 13.75. El. anal. calcd for C92H132F24N12O32S12: C 40.05%, H 4.82%, N 6.09%, S 13.95%. Found: C 39.75%, H 5.19%, N 5.85%, S 13.66%. MS (MALDI TOF): calcd for [M − N−(SO2CF3)2]+ m/z = 2476.6, found m/z = 2476.9. IR νmax: 1666, 1748 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3316 (NH).
O), 2969, 3316 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-(pentoxycarbonylmethyl)ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 60). Yield: 0.13 g (92%), mp 47 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.88 (t, 3JHH = 6.9 Hz, 12H, CH2CH3), 1.20 (s, 36H, (CH3)3C), 1.22–1.30 (m, 16H, O(CH2)2CH2C2H5, O(CH2)3CH2CH3), 1.62 (m, 8H, OCH2CH2C3H7), 1.92 (m, 8H, –NCH2CH2CH2NH), 3.19 (s, 24H, (CH3)2N+), 3.30 (m, 8H, NCH2CH2CH2NH), 3.53 (m, 8H, –NCH2CH2CH2NH), 3.99 (s, 8H, OCH2CO), 4.19 (t, 3JHH = 6.5 Hz, 8H, OCH2C4H9), 4.48 (s, 8H, N+CH2CO), 7.61 (s, 8H, ArH), 8.06 (br. s, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.36, 164.75, 157.12, 145.98, 133.05, 127.54, 119.58 (q), 70.94, 65.79, 62.31, 60.46, 51.20, 35.67, 33.82, 30.73, 27.42, 27.36, 22.53, 21.66, 13.78. El. anal. calcd for C104H156F24N12O32S12: C 42.67%, H 5.37%, N 5.74%, S 13.15%. Found: C 42.55%, H 5.44%, N 5.53%, S 12.89%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 695.0, [M − 4N−(SO2CF3)2]4+ m/z = 451.6, found m/z = 695.0, 451.5. IR νmax: 1667, 1747 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3312 (NH).
O), 2962, 3312 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 61). Yield: 0.13 g (98%), mp 73 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.20 (s, 36H, (CH3)3C), 1.93 (m, 8H, NHCH2CH2CH2N+), 3.18 (s, 12H, (CH3)2N+), 3.22 (m, 8H, NHCH2CH2CH2N+), 3.50 (m, 8H, NHCH2CH2CH2N+), 3.96 (d, 3JHH = 5.7 Hz, 8H, NHCH2CO), 3.99 (s, 8H, OCH2CO), 4.10 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.12 (s, 8H, N+CH2CO), 7.59 (s, 8H, ArH), 8.03 (t, 3JHH = 5.4 Hz, 4H, NHCH2CH2CH2N+), 9.04 (t, 3JHH = 5.7 Hz, 4H, NHCH2CO). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 170.79, 169.34, 167.78, 163.90, 157.56, 147.15, 131.57, 128.09, 119.34 (q), 71.56, 62.25, 61.13, 60.21, 51.80, 43.30, 40.99, 31.01, 22.80, 14.39. El. anal. calcd for C100H144F24N16O36S12: C 40.21%, H 4.86%, N 7.50%, S 12.88%. Found: C 40.43%, H 4.97%, N 7.34%, S 12.56%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 466.6, found m/z = 466.6. IR νmax: 1180 (COC), 1673 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2968, 3374 (N-H).
O), 2968, 3374 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 62). Yield: 0.14 g (97%), mp 76 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.18 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.20 (s, 36H, (CH3)3C), 1.93 (m, 8H, NHCH2CH2CH2N+), 3.18 (s, 12H, (CH3)2N+), 3.19 (m, 8H, NHCH2CH2CH2N+), 3.49 (m, 8H, NHCH2CH2CH2N+), 3.86 (br. s, 8H, NHCH2CO), 3.87 (br. s, 8H, NHCH2CO), 3.97 (s, 8H, OCH2CO), 4.08 (s, 8H, N+CH2CO), 4.09 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 7.59 (s, 8H, ArH), 8.02 (t, 3JHH = 4.9 Hz, 4H, NHCH2CH2CH2N+), 8.50 (t, 3JHH = 5.7 Hz, 4H, CONH), 8.86 (t, 3JHH = 5.4 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 170.14, 168.82, 167.93, 157.53, 146.55, 133.46, 128.09, 119.32 (q), 71.39, 63.14, 62.53, 60.97, 51.76, 42.03, 41.12, 36.29, 34.34, 31.22, 22.96, 14.52. El. anal. calcd for C108H156F24N20O40S12: C 40.34%, H 4.89%, N 8.71%, S 11.97%. Found: C 40.70%, H 5.05%, N 8.92%, S 11.66%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 791.3, [M − 4N−(SO2CF3)2]4+ m/z = 523.5, found m/z = 791.3, 523.6. IR νmax: 1182 (COC), 1663 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3313 (N-H).
O), 2966, 3313 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 63). Yield: 0.13 g (97%), mp 60 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.18 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.20 (s, 36H, (CH3)3C), 1.33 (d, 3JHH = 7.3 Hz, 12H, CH3CH), 1.93 (m, 8H, NHCH2CH2CH2N+), 3.18 (s, 12H, (CH3)2N+), 3.19 (m, 8H, NHCH2CH2CH2N+), 3.51 (m, 8H, NHCH2CH2CH2N+), 4.01 (s, 8H, OCH2CO), 4.09 (s, 8H, N+CH2CO), 4.10 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.30 (m, 4H, CH3CH), 7.59 (s, 8H, ArH), 8.03 (t, 3JHH = 4.9 Hz, 4H, NHCH2CH2CH2N+), 9.04 (d, 3JHH = 6.5 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 172.05, 166.94, 162.72, 157.53, 145.93, 133.83, 127.27, 119.53 (q), 70.65, 62.43, 61.15, 60.62, 51.16, 47.99, 35.62, 33.55, 30.37, 23.09, 16.65, 13.81. El. anal. calcd for C104H152F24N16O36S12: C 41.05%, H 5.03%, N 7.36%, S 12.64%. Found: C 39.86%, H 4.66%, N 6.96%, S 13.50%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 480.4. IR νmax: 1181 (COC), 1679 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3357 (N-H).
O), 2965, 3357 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(3′,3′-dimethyl-3′-{3′′-propylphthalimide}ammoniumpropyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 64). Yield: 0.13 g (91%), mp 87 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.88 (m, 8H, CH2CH2CH2Pht), 2.05 (m, 8H, NHCH2CH2CH2N+), 2.99 (s, 24H, (CH3)2N+), 3.17 (m, 8H, NHCH2CH2CH2N+), 3.29 (m, 8H, CH2CH2CH2Pht), 3.39 (m, 8H, NHCH2CH2CH2N+), 3.67 (t, 3JHH = 6.2 Hz, 8H, CH2CH2CH2Pht), 3.99 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 7.84–7.89 (m, 16H, Pht), 8.04 (t, 3JHH = 5.3 Hz, 4H, NHCH2CH2CH2N+). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 167.98, 167.32, 157.15, 146.11, 134.41, 133.07, 131.70, 127.54, 123.06, 119.32 (q), 71.01, 61.35, 61.12, 49.86, 35.79, 34.56, 33.84, 30.74, 22.48, 21.65. El. anal. calcd for C120H144F24N16O32S12: C 45.56%, H 4.59%, N 7.08%, S 12.16%. Found: C 45.45%, H 4.26%, N 7.01%, S 12.45%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 480.5. IR νmax: 1668, 1708, 1771 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3314 (N-H).
O), 2967, 3314 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′-methyl-2′,2′-diethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 65). Yield: 0.12 g (90%), mp 83 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.22 (s, 36H, (CH3)3C), 1.26 (t, 3JHH = 7.1 Hz, 24H, (CH3CH2–)), 3.02 (s, 12H, (CH3)N+), 3.21 (m, 8H, –NCH2CH2NH), 3.37 (q, 3JHH = 7.1 Hz, 16H, –CH2CH3), 3.48 (m, 8H, NCH2CH2NH), 4.05 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.17 (t, 3JHH = 5.6 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.02, 156.89, 146.04, 132.99, 127.49, 117.85 (q), 70.54, 56.87, 56.16, 46.96, 33.88, 32.13, 30.84, 7.39. El. anal. calcd for C84H124F24N12O24S12: C 39.93%, H 4.95%, N 6.65%, S 15.23%. Found: C 40.02%, H 4.99%, N 6.48%, S 15.03%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 351.5, found m/z = 351.3. IR νmax: 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3375 (NH).
O), 2969, 3375 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′,2′-triethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 66). Yield: 0.11 g (92%), mp 79 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.23 (t, 3JHH = 7.0 Hz, 36H, CH3CH2–), 3.14 (m, 8H, –NCH2CH2NH), 3.31 (m, 24H, –CH2CH3), 3.45 (m, 8H, NCH2CH2NH), 4.04 (s, 8H, OCH2CO), 7.60 (s, 8H, ArH), 8.14 (t, 3JHH = 5.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.05, 154.01, 145.92, 133.26, 127.55, 119.67 (q), 70.51, 53.57, 52.46, 33.88, 31.83, 30.84, 7.04. El. anal. calcd for C88H132F24N12O24S12: C 40.92%, H 5.15%, N 6.51%, S 14.90%. Found: C 41.23%, H 5.13%, N 6.71%, S 15.07%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 365.5, found m/z = 365.4. IR νmax: 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2964, 3374 (NH).
O), 2964, 3374 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-benzyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 67). Yield: 0.12 g (93%), mp 76 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.19 (s, 36H, (CH3)3C), 1.39 (t, 3JHH = 7.0 Hz, 24H, (CH3CH2–)), 3.15 (m, 8H, –NCH2CH2NH), 3.25 (q, 3JHH = 7.0 Hz, 16H, –CH2CH3), 3.61 (m, 8H, NCH2CH2NH), 4.20 (s, 8H, OCH2CO), 4.58 (s, 8H, N+CH2Ph), 7.50–7.56 (m, 20H, Ar′H), 7.61 (s, 8H, ArH), 8.34 (t, 3JHH = 5.8 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.05, 153.77, 145.78, 133.41, 132.68, 130.43, 129.12, 119.49 (q), 70.82, 60.56, 54.12, 53.17, 33.84, 32.04, 30.85, 11.11, 7.51. El. anal. calcd for C108H140F24N12O24S12: C 45.82%, H 4.98%, N 5.94%, S 13.59%. Found: C 46.07%, H 5.28%, N 5.99%, S 13.35%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 427.2, found m/z = 427.4. IR νmax: 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3393 (NH).
O), 2966, 3393 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(ethoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 68). Yield: 0.12 g (93%), mp 58 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.21 (s, 36H, (CH3)3C), 1.26 (t, 3JHH = 7.1 Hz, 12H, OCH2CH3), 1.28 (t, 3JHH = 7.0 Hz, 24H, CH3CH2–), 3.45–3.60 (m, 32H, –CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.08 (s, 8H, OCH2CO), 4.24 (q, 3JHH = 7.1 Hz, 8H, OCH2CH3), 4.46 (s, 8H, N+CH2CO), 7.61 (s, 8H, ArH), 8.22 (t, 3JHH = 4.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.04, 164.63, 157.00, 145.99, 133.07, 127.40, 119.46 (q), 70.60, 62.13, 55.83, 55.53, 55.08, 33.85, 31.98, 30.85, 13.73, 7.36. El. anal. calcd for C96H140F24N12O32S12: C 40.96%, H 5.01%, N 5.97%, S 13.67%. Found: C 41.12%, H 4.95%, N 5.99%, S 13.55%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 423.6, found m/z = 423.5. IR νmax: 1670, 1747 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3374 (NH).
O), 2967, 3374 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-(pentoxycarbonylmethyl)ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 69). Yield: 0.11 g (91%), mp 49 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 0.87 (t, 3JHH = 6.6 Hz, 12H, –O(CH2)4CH3), 1.21 (s, 36H, (CH3)3C), 1.27–1.35 (m, 16H, O(CH2)3CH2CH3, OCH2CH2C3H7), 1.28 (t, 3JHH = 7.0 Hz, 24H, (CH3CH2N+–)), 1.65 (m, 8H, O(CH2)2CH2C2H5), 3.44–3.65 (m, 32H, –N+CH2CH3, –NCH2CH2NH, –NCH2CH2NH), 4.07 (s, 8H, OCH2CO), 4.19 (q, 3JHH = 6.5 Hz, 8H, OCH2C4H9), 4.48 (s, 8H, N+CH2CO), 7.69 (s, 8H, ArH), 8.22 (t, 3JHH = 5.1 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 168.01, 164.77, 156.91, 146.00, 133.00, 127.45, 119.47 (q), 70.55, 65.90, 55.78, 55.42, 54.99, 33.84, 31.96, 30.83, 27.41, 27.35, 21.66, 13.79, 7.34. El. anal. calcd for C108H164F24N12O32S12: C 43.48%, H 5.54%, N 5.63%, S 12.90%. Found: C 43.71%, H 5.52%, N 5.77%, S 12.68%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 465.6, found m/z = 465.5. IR νmax: 1671, 1746 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2961, 3373 (NH).
O), 2961, 3373 (NH).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[(N-(2′,2′-diethyl-2′-{(ethoxycarbonylmethyl)amidocarbonylmethyl})ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 70). Yield: 0.13 g (93%), mp 62 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.20 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.21 (s, 36H, (CH3)3C), 1.31 (t, 3JHH = 7.0 Hz, 24H, CH3CH2N+), 3.48 (m, 8H, NHCH2CH2N+), 3.53–3.59 (m, 16H, CH3CH2N+, NHCH2CH2N+), 3.96 (d, 3JHH = 5.7 Hz, 8H, NHCH2CO), 4.05 (s, 8H, OCH2CO), 4.12 (q, 3JHH = 7.0 Hz, 8H, CH3CH2O–), 4.11 (s, 8H, N+CH2CO), 7.60 (s, 8H, ArH), 8.22 (t, 3JHH = 5.4 Hz, 4H, NHCH2CH2N+), 9.10 (t, 3JHH = 5.7 Hz, 4H, NHCH2CO). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 169.05, 168.06, 163.55, 156.85, 145.92, 132.80, 127.65, 119.46 (q), 70.12, 60.85, 56.37, 55.87, 55.10, 40.71, 33.84, 31.95, 30.82, 14.00, 7.36. El. anal. calcd for C108H160F24N16O36S12: C 41.85%, H 5.20%, N 7.23%, S 12.42%. Found: C 41.99%, H 5.31%, N 7.24%, S 12.05%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 480.6, found m/z = 481.0. IR νmax: 1180 (COC), 1685 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3364 (N-H).
O), 2967, 3364 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{([ethoxycarbonylmethyl]amidocarbonylmethyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 71). Yield: 0.14 g (94%), mp 64 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.21 (s, 36H, (CH3)3C), 1.30 (t, 3JHH = 7.1 Hz, 24H, CH3CH2N+), 3.44 (m, 8H, NHCH2CH2N+), 3.51–3.58 (m, 24H, CH3CH2N+, NHCH2CH2N+), 3.87 (br. s, 8H, NHCH2CO), 3.88 (br. s, 8H, NHCH2CO), 4.04 (s, 8H, OCH2CO), 4.08 (q, 3JHH = 7.1 Hz, 8H, CH3CH2O–), 4.09 (s, 8H, N+CH2CO), 7.59 (s, 8H, ArH), 8.21 (br. t, 4H, NHCH2CH2N+), 8.51 (t, 3JHH = 5.7 Hz, 4H, CONH), 8.90 (t, 3JHH = 5.4 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 170.14, 168.84, 168.43, 163.49, 157.59, 146.49, 131.42, 127.97, 119.60 (q), 72.17, 60.98, 57.08, 56.37, 55.59, 41.92, 41.13, 34.35, 32.47, 31.21, 14.47, 7.90. El. anal. calcd for C112H164F24N20O40S12: C 41.12%, H 5.05%, N 8.56%, S 11.76%. Found: C 41.07%, H 5.15%, N 8.49%, S 11.92%. MS (ESI): calcd for [M − 3N−(SO2CF3)2]3+ m/z = 810.3, [M − 4N−(SO2CF3)2]4+ m/z = 537.6, found m/z = 810.0, 537.7. IR νmax: 1181 (COC), 1666 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2968, 3363 (N-H).
O), 2968, 3363 (N-H).
5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(2′,2′-diethyl-2′-{(ethoxycarbonyl[S-methyl]methyl)amidocarbonylmethyl}ammoniumethyl)carbomoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] (1,3-alternate 72). Yield: 0.13 g (96%), mp 62 °C. 1H NMR (400 MHz, 298 K, DMSO-d6) δ: 1.19 (t, 3JHH = 7.1 Hz, 12H, CH3CH2O–), 1.21 (s, 36H, (CH3)3C), 1.32 (d, 3JHH = 7.3 Hz, 12H, CH3CH), 1.34 (t, 3JHH = 7.2 Hz, 24H, CH3CH2N+), 3.49 (m, 8H, NHCH2CH2N+), 3.51–3.58 (m, 24H, N+CH2CH3, NHCH2CH2N+), 4.07 (s, 8H, OCH2CO), 4.09–4.11 (m, 16H, N+CH2CO, CH3CH2O–), 4.30 (m, 4H, CH3CH), 7.60 (s, 8H, ArH), 8.23 (t, 3JHH = 6.7 Hz, 4H, NHCH2CH2N+), 9.11 (d, 3JHH = 6.7 Hz, 4H, CONH). 13C NMR (100 MHz, 298 K, DMSO-d6) δ: 171.63, 167.99, 162.83, 156.91, 146.01, 133.00, 127.43, 119.48 (q), 70.54, 60.85, 56.36, 55.93, 55.17, 47.92, 33.84, 31.96, 30.83, 16.56, 13.97, 7.34. El. anal. calcd for C108H160F24N16O36S12: C 41.85%, H 5.20%, N 7.23%, S 12.42%. Found: C 42.08%, H 5.38%, N 6.97%, S 12.09%. MS (ESI): calcd for [M − 4N−(SO2CF3)2]4+ m/z = 494.6, found m/z = 494.6. IR νmax: 1181 (COC), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3361 (N-H).
O), 2969, 3361 (N-H).
Acknowledgements
The financial support of RFBR (16-33-60141 mol_a_dk) and the Program of the President of the Russian Federation for the State support of young Russian scientists-scholarships (CP-3597.2016.4) is gratefully acknowledged.
Notes and references
- K. Shimojo and M. Goto, Chem. Lett., 2004, 33, 320–321 CrossRef CAS.
- K. Shimojo and M. Goto, Anal. Chem., 2004, 76, 5039–5044 CrossRef CAS PubMed.
- N. Sieffert and G. Wipff, J. Phys. Chem. A, 2006, 110, 1106–1117 CrossRef CAS PubMed.
- N. Sieffert and G. Wipff, J. Phys. Chem. B, 2006, 110, 19497–19506 CrossRef CAS PubMed.
- T. Sun, Z. Wang and X. Shen, Inorg. Chim. Acta, 2012, 390, 8–11 CrossRef CAS.
- P. K. Mohapatra, P. Kandwal, M. Iqbal, J. Huskens, M. S. Murali and W. Verboom, Dalton Trans., 2013, 42, 4343–4347 RSC.
- F. Yang, F. Guo, Z. Jiao, C. Li and J. Ye, J. Iran. Chem. Soc., 2012, 9, 327–332 CrossRef CAS.
- B. Aitken, M. Lee, M. Hunley, H. Gibson and K. Wagener, Macromolecules, 2010, 43, 1699–1701 CrossRef CAS.
- J. Weng, C. Wang, H. Li and Y. Wang, Green Chem., 2006, 8, 96–99 RSC.
- J. Pernak, M. Smiglak, S. T. Griffin, W. L. Hough, T. B. Wilson, A. Pernak, J. Zabielska-Matejuk, A. Fojutowski, K. Kita and R. D. Rogers, Green Chem., 2006, 8, 798–806 RSC.
- X. L. Yuan, S. J. Zhang and X. M. Lu, J. Chem. Eng. Data, 2007, 52, 596–599 CrossRef CAS.
- S. Werner, M. Haumann and P. Wasserscheid, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 203–230 CrossRef CAS PubMed.
- Y. A. Gao, Z. H. Li, J. M. Du, B. X. Han, G. Z. Li, W. G. Hou, D. Shen, L. Q. Zheng and G. Y. Zhang, Chem.–Eur. J., 2005, 11, 5875–5880 CrossRef CAS PubMed.
- P. Montes-Navajas, A. Corma and H. J. Garcia, J. Mol. Catal. A: Chem., 2008, 279, 165–169 CrossRef CAS.
- N. Inazumi, S. Yamamoto and Y. Sueishi, J. Inclusion Phenom. Macrocyclic Chem., 2007, 59, 33–39 CrossRef CAS.
- D. N. Shurpik, L. S. Yakimova, I. K. Rizvanov, V. V. Plemenkov and I. I. Stoikov, Macroheterocycles, 2015, 8, 128–134 CrossRef.
- L. S. Yakimova, D. N. Shurpik, L. H. Gilmanova, A. R. Makhmutova, A. Rakhimbekova and I. I. Stoikov, Org. Biomol. Chem., 2016, 14, 4233–4238 CAS.
- R. V. Nosov and I. I. Stoikov, Macroheterocycles, 2014, 7, 345–350 CrossRef CAS.
- I. Spendlikova, J. John, V. Cuba, J. Jirasek and P. Lhotak, J. Radioanal. Nucl. Chem., 2015, 304, 257–262 CrossRef CAS.
- M. MacKova, M. Himl, J. Budka, M. Pojarova, I. Cisarova, V. Eigner, P. Curinova, H. Dvorakova and P. Lhotak, Tetrahedron, 2013, 69, 1397–1402 CrossRef CAS.
- D.-D. Zheng, D.-Y. Fu, Y. Wu, Y.-L. Sun, L.-L. Tan, T. Zhou, S.-Q. Ma, X. Zha and Y.-W. Yang, Chem. Commun., 2014, 50, 3201–3203 RSC.
- Y. Zhou, H. Li and Y.-W. Yang, Chin. Chem. Lett., 2015, 26, 825–828 CrossRef CAS.
- M. Yamada, Y. Ootashiro, Y. Kondo and F. Hamada, Tetrahedron Lett., 2013, 54, 1510–1514 CrossRef CAS.
- M. Yamada, M. Rajiv Gandhi, U. M. R. Kunda and F. Hamada, J. Inclusion Phenom. Macrocyclic Chem., 2016, 85, 1–18 CrossRef CAS.
- A. D. Iampolska, S. G. Kharchenko, Z. V. Voitenko, S. V. Shishkina, A. B. Ryabitskii and V. I. Kalchenko, Phosphorus Sulfur Relat. Elem., 2016, 191, 174–179 CrossRef CAS.
- T. Ogoshi, N. Ueshima, T. Yamagishi, Y. Toyota and N. Matsumi, Chem. Commun., 2012, 48, 3536–3538 RSC.
- A. Kokorin, Ionic liquids: theory, properties, new approaches, InTech, 2011 Search PubMed.
- F. Hurley and T. Wler, J. Electrochem. Soc., 1951, 98, 203–206 CrossRef CAS.
- J. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef PubMed.
- P. Wasserscheid and T. Welton, Ionic liquids in synthesis: second edition, John Wiley & Sons, Inc., 2009 Search PubMed.
- S. L. Craig, Angew. Chem., Int. Ed., 2009, 48, 2645–2647 CrossRef CAS PubMed.
- J. Lopes and A. Padua, J. Phys. Chem. B, 2006, 110, 3330–3335 CrossRef PubMed.
- J. H. Davis, K. J. Forrester and T. Merrigan, Tetrahedron Lett., 1988, 39, 8955–8958 CrossRef.
- K. Singh, D. G. Marangoni, J. G. Quinn and R. D. Singer, J. Colloid Interface Sci., 2009, 335, 105–111 CrossRef CAS PubMed.
- E. A. Andreyko, P. L. Padnya and I. I. Stoikov, J. Phys. Org. Chem., 2015, 28, 527–535 CrossRef CAS.
- Y. Huo, S. Xia, Y. Zhang and M. Peisheng, Ind. Eng. Chem. Res., 2009, 48, 2212–2217 CrossRef CAS.
- M. K. Rauf, R. Mushtaq, A. Badshah, R. Kingsford-Adaboh, J. J. E. K. Harrison and H. Ishida, J. Chem. Crystallogr., 2013, 43, 144–150 CrossRef CAS.
- P. L. Padnya, E. A. Andreyko, O. A. Mostovaya, I. Kh. Rizvanov and I. I. Stoikov, Org. Biomol. Chem., 2015, 13, 5894–5904 CAS.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS.
- E. A. Andreyko, P. L. Padnya, R. R. Daminova and I. I. Stoikov, RSC Adv., 2014, 4, 3556–3565 RSC.
- E. A. Andreyko, P. L. Padnya and I. I. Stoikov, Colloids Surf., A, 2014, 454, 74–83 CrossRef CAS.
- F. Gutmann and L. E. Lyons, Organic Semiconductors, Wiley, New York, 1967 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24734b |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article c,
Viktor V. Parfenovc,
Ildar Kh. Rizvanovd and
Ivan I. Stoikov
c,
Viktor V. Parfenovc,
Ildar Kh. Rizvanovd and
Ivan I. Stoikov *a
*a


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3349 (NH).
O), 2956, 3349 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3317 (NH).
O), 2955, 3317 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2960, 3320 (NH).
O), 2960, 3320 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3322 (NH).
O), 2958, 3322 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3320 (NH).
O), 2956, 3320 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3292 (NH).
O), 2955, 3292 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3335 (NH).
O), 2958, 3335 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3287 (NH).
O), 2955, 3287 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2958, 3300 (NH).
O), 2958, 3300 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2954, 3300 (NH).
O), 2954, 3300 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3300 (NH).
O), 2955, 3300 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3261 (NH).
O), 2955, 3261 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2954, 3194 (NH).
O), 2954, 3194 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2957, 3187 (NH).
O), 2957, 3187 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2955, 3300 (NH).
O), 2955, 3300 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2956, 3315 (N-H).
O), 2956, 3315 (N-H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 taken for each ammonium group. The reaction mixture was stirred for 24 h. The precipitate was filtered and dried under reduced pressure over phosphorus pentoxide.
1 taken for each ammonium group. The reaction mixture was stirred for 24 h. The precipitate was filtered and dried under reduced pressure over phosphorus pentoxide.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3327 (NH).
O), 2965, 3327 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3328 (NH).
O), 2965, 3328 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2961, 3328 (NH).
O), 2961, 3328 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3328 (NH).
O), 2965, 3328 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3329 (NH).
O), 2962, 3329 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3354 (N-H).
O), 2966, 3354 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3342 (N-H).
O), 2965, 3342 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3353 (N-H).
O), 2966, 3353 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2963, 3334 (N-H).
O), 2963, 3334 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3315 (NH).
O), 2965, 3315 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2963, 3314 (NH).
O), 2963, 3314 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3330 (NH).
O), 2967, 3330 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3328 (NH).
O), 2962, 3328 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3313 (NH).
O), 2962, 3313 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3353 (N-H).
O), 2965, 3353 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3332 (N-H).
O), 2966, 3332 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3347 (N-H).
O), 2966, 3347 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3313 (NH).
O), 2966, 3313 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3310 (NH).
O), 2966, 3310 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3374 (NH).
O), 2965, 3374 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3316 (NH).
O), 2969, 3316 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2962, 3312 (NH).
O), 2962, 3312 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2968, 3374 (N-H).
O), 2968, 3374 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3313 (N-H).
O), 2966, 3313 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965, 3357 (N-H).
O), 2965, 3357 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3314 (N-H).
O), 2967, 3314 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3375 (NH).
O), 2969, 3375 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2964, 3374 (NH).
O), 2964, 3374 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2966, 3393 (NH).
O), 2966, 3393 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3374 (NH).
O), 2967, 3374 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2961, 3373 (NH).
O), 2961, 3373 (NH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2967, 3364 (N-H).
O), 2967, 3364 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2968, 3363 (N-H).
O), 2968, 3363 (N-H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2969, 3361 (N-H).
O), 2969, 3361 (N-H).


